Abstract
Objectives
To describe the real-life use of temocillin for non-urinary tract infections, to assess its effectiveness in infections caused by ESBL-producing Enterobacterales, and to identify risk factors for treatment failure.
Method
Retrospective multicentric study in 14 tertiary care hospitals, including all patients who received at least one dose of temocillin for ESBL infections from 1 January 2016 to 31 December 2021 for non-urinary tract infections. Failure was a composite criterion defined within 28 day follow-up by persistence or reappearance of signs of infection, and/or switch to suppressive antibiotic treatment and/or death from infection. Logistic regression with univariable and multivariable analysis was performed to identify risks associated with failure.
Results
Data on 163 infection episodes were collected; 133 were due to ESBL-producing Enterobacterales and 128 were included in the effectiveness analysis. Median (IQR) age was 61 (53–70) years and 61.7% of patients were male. Main indications were lower respiratory tract infection (LRTI; 28.9%), intra-abdominal infections (IAI; 28.1%) and cutaneous infections (12.5%). The main bacteria involved were Klebsiella pneumoniae (48.4%), Escherichia coli (25.0%) and Enterobacter cloacae (24.2%). Polymicrobial infections occurred in 45.3% of cases. Temocillin was used as monotherapy in 86/128 (67.2%). Failure was found in 36/128 (28.1%) cases. In multivariable analysis, the only factor associated with failure was initial severity of the episode [adjusted OR 3.0 (95% CI: 1.06–8.69)].
Conclusions
During non-urinary tract infections, the main use of temocillin was for LRTIs and IAIs due to ESBL-producing E. coli and K. pneumoniae. The main risk factor for failure was initial severity of the disease.
Introduction
ESBL-producing Enterobacterales (ESBL-E) have spread worldwide and could be responsible for difficult-to-treat infection.1–3 Carbapenems are often used as first-line antibiotics against infections caused by ESBL-E.4,5 The increased use of carbapenems may lead to rising resistance to carbapenems among Gram-negative bacteria, which is associated with high costs and high mortality.6–8 For that reason, it is essential to consider non-carbapenem antibiotics.
Temocillin, a semisynthetic 6-α-methoxylpenicillin antibiotic derived from ticarcillin, has demonstrated stability against ESBL-, AmpC- and some KPC-producing Gram-negative bacteria.9–11 Temocillin has no activity against Gram-positive bacteria, anaerobes or non-fermenters like Pseudomonas aeruginosa and Acinetobacter baumanii.12 This narrow spectrum has minimized selection pressure on microbiota.13,14
Temocillin is used mainly for carbapenem-sparing in urinary tract infections (UTIs) with high clinical success rate.15–17 However, its use and efficacy for non-urinary tract infections is not well known.
We describe the use of temocillin in real-life settings and evaluate its effectiveness in treating non-urinary tract infections. Additionally, we aim to identify risk factors associated with treatment failure.
Materials and methods
A multicentre retrospective study was conducted in the greater Paris area, including all adult patients who received temocillin for non-urinary tract infections for at least 1 day from 1 January 2016 to 31 December 2021.
Data were collected from medical charts in 14 tertiary care hospitals. We collected demographic characteristics (age, sex, comorbidities, risk factors etc.), clinical, biological and microbiological data (clinical and severity signs, laboratory tests, organisms identified), therapeutic data (dosage of temocillin, other associated antibiotics), and adverse events and clinical outcome at Day 28 of the first temocillin dose and at the patient’s last visit.
Immunosuppression was defined as the presence of the following criteria: asplenia, neutropenia, agammaglobulinaemia, organ transplant, haematological malignancies, HIV infection with CD4 cell count below 200 cells/mm3, 20 mg of prednisolone equivalent over at least 3weeks, cancer chemotherapy or other immunosuppressive drugs (e.g. cyclophosphamide, azathioprine, cyclosporine etc.).
Neurological disease was defined as the presence of the following criteria: cerebral vascular disease, spinal cord injury, multiple sclerosis or Parkinson’s disease.
Bacterial strain and resistance mechanism (ESBL, AmpC) analyses and antibiotic susceptibility testing were performed using disc diffusion, and MICs were determined by broth microdilution, in the local laboratories of the centres, according to EUCAST and CLSI guidelines.18
Severe infection was defined as the need for hospitalization in an ICU. Failure was a composite criterion defined within a 28 day follow-up period by persistence or reappearance of signs of infection, and/or death from infection. Patients with missing data at Day 28 were excluded from this analysis.
Quantitative variables are presented as median (IQR), while qualitative variables are presented as number of occurrences and percentage. Enterobacterales were speciated and grouped as AmpC, ESBL or non-AmpC/non ESBL based on resistance patterns. Antibiotics received before temocillin were reviewed and analysed.
The distribution of categorial variables were compared using chi-squared tests; t-tests were used to compare the distribution of quantitative variables. A P value of <0.05 was considered statistically significant.
To identify risk factors associated with failure, a univariable analysis by logistic regression was performed, using demographic and medical characteristics, as well as all clinical and biological data. For patients requiring renal dosage adjustments, temocillin dosage used in the statistical analyses was the targeted dosage before reduction. A multivariable analysis by logistic regression was then performed using all variables from the univariable analysis that had a P value of <0.05. ORs were calculated from the univariate and multivariable analysis to quantify association with failure at Day 28 with 95% CIs.
All analyses were performed with R statistical software, version 4.3.1.
Ethics
Considering the retrospective study design, data collection from pre-existing medical records, and respect for the anonymity of the patients included (referred to as studies ‘Hors Loi Jardé’ in France), no ethical approval or administrative approval were necessary for this study. This study was approved by the French Data Protection Agency (CNIL), with the number 2225429 v 0.
Results
Baseline description
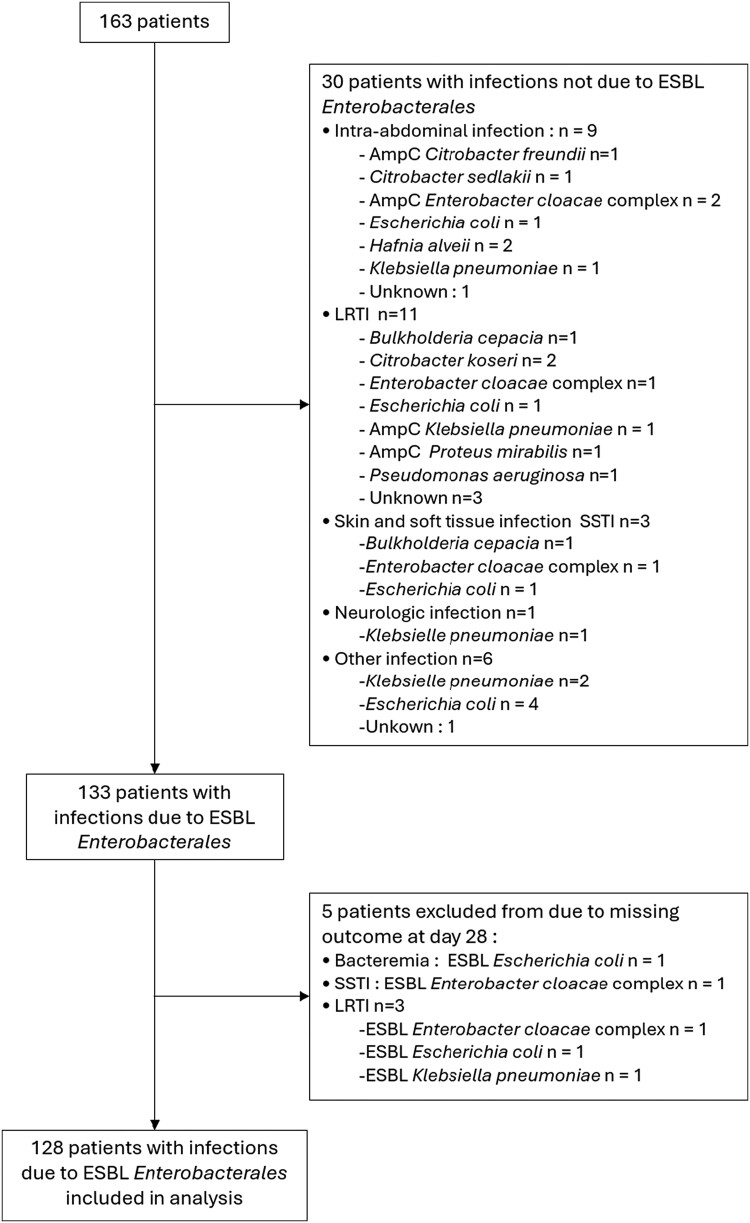
After exclusion of prophylactic treatment, 163 patients were treated with temocillin, and 133 of them presented with ESBL-producing Enterobacterales (ESBL-E) infections. Five patients were excluded from the effectiveness analysis due to missing data at Day 28 (see flow chart presented in Figure 1).
Figure 1.
Flow chart.
Male patients represented 61.7% (79/128) of the total study population. The median (IQR) age was 61 (53–70) years. The main sources of infections were lower respiratory tract infections (LRTIs), representing 28.9% (n = 37/128) of patients, followed by intra-abdominal infections (IAIs), representing 28.1% (n = 36/128). Forty-four patients (34.4%) presented with severe infection requiring hospitalization in an ICU (Table 1).
Table 1.
Population characteristics of patients treated with temocillin for non-urinary tract infections
| Total N = 128 |
Cure group N = 92 |
Failure group N = 36 |
P value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 61 (53–70) | 61 )54–69) | 63 (52–71) | 0.5 |
| Male patient, n (%) | 79 (61.7) | 54 (58.7) | 25 (69.4) | 0.3 |
| Hospital ward, n (%) | ||||
| ICU | 44 (34.4) | 23 (25.0) | 21 (58.3) | <0.001 |
| Medicine | 62 (48.4) | 50 (54.3) | 12 (33.3) | 0.032 |
| Surgery | 25 (19.5) | 22 (23.9) | 2 (8.3) | 0.046 |
| Comorbidities, n (%) | ||||
| Chronic respiratory failure | 9 (7.0) | 7 (7.6) | 2 (5.6) | >0.9 |
| Heart disease | 31 (24.2) | 20 (21.7) | 11 (30.6) | 0.3 |
| Chronic renal failure | 26 (20.3) | 19 (20.6) | 7 (19.4) | 0.9 |
| Liver failure | 20 (15.6) | 15 (16.3) | 5 (13.9) | 0.7 |
| Neurological disease | 13 (10.2) | 9 (9.8) | 4 (11.1) | 0.8 |
| Immunodepression | 52 (40.6) | 36 (39.1) | 16 (44.4) | 0.6 |
| AIDS | 6 (4.7) | 3 (3.3) | 3 (8.3) | 0.4 |
| Neutropenia <500 cells/mm3 | 2 (1.6) | 2 (2.2) | 0 (0.0) | >0.9 |
| Chemotherapy | 22 (17.5) | 16 (17.8) | 6 (16.7) | 0.9 |
| Immunosuppressive | 24 (18.8) | 17 (18.5) | 7 (19.4) | 0.9 |
| Corticosteroids >20 mg/L | 12 (9.4) | 8 (8.8) | 4 (11.1) | 0.7 |
| Diabetes mellitus | 39 (30.7) | 29 (31.9) | 10 (27.8) | 0.7 |
| Renal clearance (mL/min), median (IQR) | 82 (52–136) | 86 (54–141) | 75 (49–117) | 0.5 |
| Site of infection, n (%) | ||||
| LRTI | 37 (28.9) | 20 (21.7) | 17 (47.2) | 0.004 |
| IAI | 36 (28.1) | 30 (32.6) | 6 (16.7) | 0.071 |
| Intravascular device infection | 19 (14.8) | 15 (16.3) | 4 (11.1) | 0.3 |
| Skin and soft tissue infection | 16 (12.5) | 13 (14.1) | 3 (8.3) | 0.6 |
| Bone and joint infection | 7 (5.5) | 7 (7.7) | 0 (0.0) | 0.2 |
| Bloodstream infection | 4 (3.1) | 3 (3.3) | 1 (2.8) | >0.9 |
| Other type of infectiona | 3 (2.4) | 1 (1.1) | 2 (5.5) | >0.9 |
| Severity, n (%) | 46 (35.9) | 25 (27.2) | 21 (58.3) | <0.001 |
| Before temocillin treatment | ||||
| Surgical treatment, n (%) | 42 (32.8) | 35 (38.0) | 4 (19.4) | 0.044 |
| Number of antibiotic treatment lines, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 0.5 |
| Microbiology analysis, n (%) | ||||
| Polymicrobial infection | 58 (46.0) | 42 (42.2) | 16 (45.7) | >0.9 |
| ESBL-producing E. coli | 32 (25.0) | 21 (22.8) | 11 (30.6) | |
| ESBL-producing Enterobacter cloacae complex | 31 (24.2) | 23 (25.0) | 8 (22.2) | |
| ESBL-producing K. pneumoniae | 62 (48.4) | 46 (50) | 16 (44.4) | |
| Temocillin dosage, n (%) | 0.7 | |||
| Less than 4 g per day or equivalentb | 11 (9.3) | 7 (7.9) | 4 (13.8) | |
| 4 g per day or equivalentb | 32 (27.1) | 25 (28.1) | 7 (24.1) | |
| At least 6 g per day or equivalentb | 75 (63.6) | 57 (64.0) | 18 (62.1%) | |
| Treatment duration (days), median (IQR) | 7 (4–11) | 7 (4–13) | 7 (4–9) | 0.8 |
| Associated antibiotic, n (%) | 42 (33.1) | 30 (33.0) | 12 (33.3) | 0.7 |
aSurgical site infection (n = 2), wall abscess (n = 1).
bAccording to renal function.
Fifty-eight (46.0%) infections were polymicrobial and ESBL-producing Klebsiella pneumoniae was the most frequently isolated bacteria (48.4%; n = 62/128). Bacteria involved according to source are presented in Table 2. Prior to temocillin, 52 patients (40.6%) received carbapenems and 29 (22.6%) received piperacillin/tazobactam. Six patients (4.7%) received temocillin as first-line empirical treatment.
Table 2.
Microbiological results depending on type of infection
| Type of infection | n |
|---|---|
| LRTI | 37 |
| Monomicrobial | 16 |
| ESBL-producing E. coli | 6 |
| ESBL-producing K. pneumoniae | 8 |
| ESBL-producing Klebsiella aerogenes | 1 |
| ESBL-producing Klebsiella oxytoca | 1 |
| Polymicrobial | 21 |
| ESBL-producing E. coli + non-ESBL-producing Serratia marcescens | 1 |
| ESBL-producing E. coli + Gram positive bacteria | 2 |
| ESBL-producing K. pneumoniae + non-ESBL-producing E. coli | 1 |
| ESBL-producing K. pneumoniae + non-ESBL-producing E. cloacae complex | 2 |
| ESBL-producing K. pneumoniae + A. baumannii | 2 |
| ESBL-producing K. pneumoniae + Gram-positive bacteria | 4 |
| ESBL-producing E. cloacae complex + Candida albicans | 1 |
| ESBL-producing E. cloacae complex + Gram-positive bacteria | 4 |
| ESBL-producing E. cloacae complex + ESBL-producing K. oxytoca | 1 |
| ESBL-producing E. cloacae complex + P. aeruginosa | 2 |
| ESBL-producing E. cloacae complex + non-ESBL-producing Citrobacter koseri | 1 |
| IAI | 35 |
| Monomicrobial | 23 |
| ESBL-producing E. coli | 7 |
| ESBL-producing E. cloacae complex | 7 |
| ESBL-producing K. pneumoniae | 9 |
| Polymicrobial | 12 |
| ESBL-producing E. coli + ESBL-producing K. pneumoniae | 1 |
| ESBL-producing E. coli + non-ESBL-producing K. pneumoniae | 1 |
| ESBL-producing E. coli + ESBL-producing E. cloacae complex | 1 |
| ESBL-producing E. coli + Weissella confusa | 1 |
| ESBL-producing E. coli + AmpC-producing Enterobacter kobei | 1 |
| ESBL-producing E. coli + Gram-positive bacteria | 2 |
| ESBL-producing K. pneumoniae + non-ESBL-producing E. coli | 3 |
| ESBL-producing K. pneumoniae + non-ESBL-producing K. aerogenes | 1 |
| ESBL-producing K. pneumoniae + AmpC-producing Hafnia alvei | 1 |
| Skin and soft tissue infection | 16 |
| Monomicrobial | 9 |
| ESBL-producing E. coli | 1 |
| ESBL-producing K. pneumoniae | 5 |
| ESBL-producing E. cloacae | 3 |
| Polymicrobial | 7 |
| ESBL-producing C. koseri + non-ESBL-producing Proteus mirabilis | 1 |
| ESBL-producing K. pneumoniae + ESBL-producing E. cloacae | 2 |
| ESBL-producing K. pneumoniae + non-ESBL-producing P. mirabilis | 1 |
| ESBL-producing K. pneumoniae + Gram-positive bacteria | 2 |
| ESBL-producing K. pneumoniae + Stenotrophomonas maltophilia | 1 |
| Bone and joint infection | 7 |
| Monomicrobial | 4 |
| ESBL-producing K. pneumoniae | 3 |
| ESBL-producing E. cloacae | 1 |
| Polymicrobial | 3 |
| ESBL-producing E. cloacae + Gram-positive bacteria | 1 |
| ESBL-producing K. pneumoniae + Gram-positive bacteria | 2 |
| Other infections | 25 |
| Monomicrobial | 13 |
| ESBL-producing E. coli | 1 |
| ESBL-producing K. pneumoniae | 6 |
| ESBL-producing E. cloacae | 6 |
| Polymicrobial | 12 |
| ESBL-producing E. coli + non-ESBL-producing P. mirabilis | 1 |
| ESBL-producing E. coli + P. aeruginosa | 1 |
| ESBL-producing E. coli + ESBL-producing K. pneumoniae | 1 |
| ESBL-producing K. pneumoniae + non-ESBL-producing E. coli | 2 |
| ESBL-producing K. pneumoniae + non-ESBL-producing P. mirabilis | 1 |
| ESBL-producing K. pneumoniae + S. maltophilia | 1 |
| ESBL-producing E. cloacae + non-ESBL-producing K. pneumoniae | 1 |
| ESBL-producing E. cloacae + Gram-positive bacteria | 4 |
Median (IQR) duration of treatment was 7 (4–11) days. Seventy-five patients (63.6%) received 6 g of temocillin, associated in 42 cases (33.1%) with other antibiotics such as an anti-Gram-positive antibiotic (n = 12/128).
Follow-up at 28 days
Overall, failure was observed in 33 patients (28.1%) at Day 28.
Among patients treated with a temocillin dosage of ≥6 g per day, 57/75 (76.0%) had a favourable outcome at Day 28. Among these, 27/75 (36.0%) presented with LRTIs.
Among patients treated with a temocillin dosage lower than 6 g per day, 32/43 (74.4%) had a favourable outcome at Day 28. Among these, 11/43 (25.6%) presented with LRTIs.
In the multivariable analysis, the only risk factor for failure was severity of infection [adjusted OR (aOR): 3.0; 95% CI: 1.06–8.69; P = 0.039) (Table 3). Although not significant in multivariable analysis, the risk of failure tended to be high in LRTIs.
Table 3.
Multivariable regression factors associated with failure for 128 patients treated with temocillin for non-urinary tract infections
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Severe infection (ICU) | 4.81 (2.15–11.3) | <0.001 | 3.0 (1.06–8.69) | 0.039 |
| Hospitalization in medical ward | 0.42 (0.18–0.93) | 0.035 | 0.61 (0.21–1.73) | 0.3 |
| Hospitalization in surgical ward | 0.29 (0.07–0.91) | 0.057 | ||
| LRTI | 3.22 (1.42–7.39) | 0.005 | 1.26 (0.44–3.48) | 0.7 |
| Surgical treatment before temocillin | 0.39 (0.15–0.95) | 0.048 | 0.46 (0.15–1.32) | 0.2 |
Comorbidities, bacterial species and the dosage of temocillin seem to have had no impact on the outcome in the descriptive analysis (Table 1).
Adverse events
Only two Clostridioides difficile infections and one case of acute renal injury were reported as potential temocillin-related adverse events. No serious adverse drug reactions leading to discontinuation were observed.
Discussion
We conducted a large, multicentre cohort study to evaluate the real-life use of temocillin and its effectiveness in treating non-urinary tract infections caused by ESBL-E.
Temocillin is approved in Europe for treating bacteraemia, UTI and LRTI at a dosage of 2 g twice daily. It is currently available only in the UK, Belgium, Germany and France.9,15,19–22 Temocillin is particularly relevant for treating infections caused by resistant Gram-negative strains. A recent study found a 61.8% susceptibility rate among 400 isolates, including those producing ESBL, AmpC and KPC, using the BSAC breakpoint for systemic infections (≤8 mg/L).23
Our study confirmed temocillin’s effectiveness for treating non-urinary tract infections caused by ESBL-E. Furthermore, the study population frequently included patients with severe infections and many were immunocompromised. The main indications were LRTI and IAI, types of infections with limited data on temocillin’s effectiveness. Alexandre et al.24 found significantly lower clinical failure rates for UTIs compared with non-urinary tract infections (4.9% versus 26.7%; P = 0.001). Notably, clinical failure rates were significantly different between sepsis and severe sepsis or septic shock treated with temocillin (6.2% versus 25%; P = 0.011), although no significant variations were noted between different dosages or bacteria. These results are consistent with our data.
For LRTIs, clinical data on temocillin for both community-acquired and hospital-acquired pneumonia are limited, and information on epithelial lining fluid (ELF)/plasma penetration ratios is sparse.25,26 Temocillin is minimally effective against Gram-positive microorganisms and certain Gram-negative non-fermenters such as A. baumannii and P. aeruginosa. In vitro studies suggest that combination regimens may enhance its activity.27,28 In our study, about one-third of patients with LRTIs (12/37) received combination therapy with antibiotics like linezolid, vancomycin, quinolones, ceftazidime or trimethoprim/sulfamethoxazole.
A retrospective audit by Habayeb et al.29 compared piperacillin/tazobactam with amoxicillin plus temocillin in 192 cases of hospital-acquired pneumonia, and found no difference in clinical success rates between the two groups. However, patients treated with amoxicillin plus temocillin experienced significantly fewer episodes of diarrhoea and C. difficile infection.
In a study testing continuous infusion of 4 g per day of temocillin in ICU patients with nosocomial pneumonia, the drug maintained stability for 24 h and was compatible with concurrent administration of flucloxacillin and aminoglycosides. Despite achieving stable free serum concentrations above the breakpoint of 16 mg/L, the authors recommended considering a lower breakpoint of 8 mg/L due to individual variations in this population.30
For ventilator-associated pneumonia (VAP), temocillin is often administered via continuous infusion of 6 g daily, according to recent studies.31 Most patients in our study also received continuous or prolonged infusions of 6 g of temocillin daily.
For IAIs, pharmacokinetic/pharmacodynamic studies suggest that temocillin penetrates bile and peritoneal fluid at approximately 80%. Small case series have reported high clinical cure rates.17,19,32 In our study, more than a quarter of patients had IAI, which resulted in one of the highest success rates, even though it is not currently included in national guidelines.
A study on patients with peritonitis and intra-abdominal abscesses demonstrated the effectiveness of 1 g temocillin twice daily. This study included a broad range of bacteria susceptible to temocillin and found favourable outcomes in most cases, with no reported adverse reactions.33
In the peritoneal setting, temocillin rapidly penetrated, achieving a mean peritoneal concentration of 49.1 mg/L. This suggests that 1 g of temocillin administered twice daily reaches sufficiently high intraperitoneal levels to inhibit susceptible pathogens.34 Wittke et al.35 reported that a dosage of 2 g temocillin twice daily during biliary surgery showed good clinical effectiveness with few side effects. Regarding MDR microorganisms, Alexandre et al.19 demonstrated significant temocillin activity in peritoneal fluid, blood and spleen in a murine model infected with KPC-producing Escherichia coli. Furthermore, most patients with IAI in our study underwent surgery, contributing to the high rate of favourable outcomes in our cohort.
For bloodstream infections (BSIs), a study of 92 patients treated with temocillin for bacteraemia due to Enterobacterales reported an 84% cure rate, although no comparative studies with carbapenems have been published to our knowledge.36 The optimal dosage for this indication remains debated, with higher cure rates noted for 2 g twice daily versus less than 2 g twice daily, especially in the ESBL or derepressed AmpC subset.36
Regarding bone and joint infections and skin and soft tissue infections, data are currently limited. A single case report highlights temocillin’s potential in managing peripheral phlebitis associated with a K. pneumoniae infection complicating a psoas abscess caused by Staphylococcus aureus.37 Similarly, there is a paucity of studies on temocillin in osteoarticular contexts. Specifically, two cases documented its use in knee arthritis induced by Pantoea agglomerans and cervical osteomyelitis caused by Burkholderia cepacia.38,39 Additionally, some authors suggest to incorporate temocillin into antibiotic-loaded.40
In our study, temocillin was primarily used as a switch from first-line carbapenems, serving as a carbapenem-sparing treatment. There were few empirical uses in accordance with French guidelines due to its limited activity against Gram-positive bacteria and anaerobes. No significant differences in success rates were observed based on the causative microorganism. Few adverse events were reported, with only two cases of C. difficile infection, confirming temocillin’s safety.
Limitations
This study has several limitations. As a retrospective study, it is subject to potential selection bias and confounding factors. Additionally, no control group was included. We were also unable to provide detailed information regarding source control or the distribution of continuous versus intermittent temocillin administration. Most patients received carbapenem antibiotics before switching to temocillin, and approximately 33% received an additional antibiotic with temocillin, which introduces bias in evaluating temocillin’s efficacy but still reflects real-world use of the drug.
Conclusions
The primary use of temocillin for non-urinary tract infections was in the treatment of LRTIs caused by ESBL-producing K. pneumoniae. The main risk factor for treatment failure was the initial severity of the infections. Temocillin appears to be an effective option for treating non-urinary tract infections caused by susceptible pathogens and may serve as a viable alternative to carbapenems for eradicating ESBL-E. Its advantages include a favourable safety profile and a low incidence of adverse events. However, ongoing debates about clinical breakpoints and optimal dosages highlight the need for further clinical studies to better define its role and efficacy.
Acknowledgements
Members of the Paris Temocillin Study Group
Aurélien DINH, Christel MAMONA, Clara DURAN, Hugues MICHELON, Frédérique BOUCHAND (Raymond-Poincaré Hospital, AP-HP, Paris Saclay University, Garches, France); Eric FARFOUR, Pauline TOUCHE, Aurélie CHAN HEW WAI (Foch Hospital, Suresnes); Benoît PILMIS (Hôpital Saint-Joseph & Marie-Lannelongue, Paris); Sylvain DIAMANTIS (Hôpital de Melun, Melun, France); Rui BATISTA, Etienne CANOUÏ, Antoine CITERNE (Cochin Hospital, AP-HP, Centre—Université Paris Cité, Paris, France); Laurène DECONINCK, Chloé TESMOINGT (Bichat Hospital, AP-HP, Nord—Université Paris Cité, Paris, France); Laura BOUABDALLAH PERRIN, Matthieu LAFAURIE, Sophie TOURATIER (Saint-Louis Hospital, AP-HP, Nord—Université Paris Cité, Paris, France); Victoire de LASTOURS, Antoine HAMON (Beaujon hospital, AP-HP, Nord—Université Paris Cité, Clichy, France); Marie ANTIGNAC, Céline LEPLAY, Jean-Luc MEYNARD (Saint-Antoine Hospital, AP-HP, Sorbonne Université, Paris, France); Anne CATALDI, Clément OURGHANLIAN, Raphaël LEPEULE (Henri Mondor Hospital, AP-HP, HU Henri Mondor, Créteil, France); Marc-Antoine BILDAN, Marie-Caroline LOUSTALOT (Hôpital Européen Georges-Pompidou, AP-HP, Centre—Université Paris Cité, Paris, France); Ruxandra CALIN, Cédric MWAMBA, Jean Baptiste PAIN (Tenon Hospital, AP-HP, Sorbonne Université, Paris, France); Lelia ESCAUT, Benjamin WYPLOSZ (Bicêtre Hospital, AP-HP, Paris Saclay Université, Kremlin-Bicêtre, France); Alexandre BLEIBTREU, Helga JUNOT (Pitié-Salpêtrière Hospital, AP-HP, Sorbonne Université, Paris, France).
Contributor Information
Christel Mamona Kilu, Infectious Disease Department, Raymond Poincaré Hospital, APHP, Garches, France.
Camille Menvielle, Infectious Disease Department, Pitié-Salpêtrière Hospital, APHP, Paris, France.
Anne Cataldi, Pharmacy, Henri Mondor Hospital, APHP, Créteil, France.
Antoine Hamon, Internal Medicine, Beaujon Hospital, APHP, Clichy, France.
Clara Duran, Infectious Disease Department, Raymond Poincaré Hospital, APHP, Garches, France.
Cedric Mwanba, Pharmacy, Tenon Hospital, APHP, Paris, France.
Chloé Tesmoingt, Pharmacy, Bichat Hospital, APHP, Paris, France.
Laura Bouabdallah-Perrin, Pharmacy, Saint-Louis Hospital, APHP, Paris, France.
Pauline Touche, Pharmacy, Foch Hospital, Suresnes, France.
Aurélie Chanh Hew Wai, Pharmacy, Foch Hospital, Suresnes, France.
Clément Ourghanlian, Pharmacy, Henri Mondor Hospital, APHP, Créteil, France.
Marie Antignac, Pharmacy, Saint-Antoine Hospital, APHP, Paris, France.
Marc-Antoine Bildan, Pharmacy, Européen Georges Pompidou Hospital, APHP, Paris, France.
Alexandre Bleibtreu, Infectious Disease Department, Pitié-Salpêtrière Hospital, APHP, Paris, France.
Hugues Michelon, Infectious Disease Department, Raymond Poincaré Hospital, APHP, Garches, France.
Sylvain Diamantis, Infectious Disease Department, Melun Hospital, Melun, France.
Benoit Pilmis, Infectious Disease Department, Saint-Joseph & Marie-Lannelongue Hospital, Paris, France.
Antoine Citerne, Pharmacy, Cochin Hospital, APHP, Paris, France.
Eric Farfour, Pharmacy, Foch Hospital, Suresnes, France.
Aurélien Dinh, Infectious Disease Department, Raymond Poincaré Hospital, APHP, Garches, France; IHU PROMETHEUS, Raymond Poincaré Hospital, APHP, Garches, France.
Paris Temocillin study group:
Aurélien Dinh, Christel Mamona, Clara Duran, Hugues Michelon, Frédérique Bouchand, Eric Farfour, Pauline Touche, Aurélie Chan Hew Wai, Benoît Pilmis, Sylvain Diamantis, Rui Batista, Etienne Canouï, Antoine Citerne, Laurène Deconinck, Chloé Tesmoingt, Laura Bouabdallah Perrin, Matthieu Lafaurie, Sophie Touratier, Victoire de Lastours, Antoine Hamon, Marie Antignac, Céline Leplay, Jean-Luc Meynard, Anne Cataldi, Clément Ourghanlian, Raphaël Lepeule, Marc-Antoine Bildan, Marie-Caroline Loustalot, Ruxandra Calin, Cédric Mwamba, Jean Baptiste Pain, Lelia Escaut, Benjamin Wyplosz, Alexandre Bleibtreu, and Helga Junot
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
References
- 1. Cassini A, Högberg LD, Plachouras D et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol 2010; 300: 371–9. 10.1016/j.ijmm.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 3. ECDC . Assessing the health burden of infections with antibiotic-resistant bacteria in the EU/EEA, 2016-2020. 2022. https://www.ecdc.europa.eu/en/publications-data/health-burden-infections-antibiotic-resistant-bacteria-2016-2020.
- 4. Van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 19: 460–9. 10.1080/21505594.2016.1222343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantón R, Novais A, Valverde A et al. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect Dis 2008; 14 Suppl 1: 144–53. 10.1111/j.1469-0691.2007.01850.x [DOI] [PubMed] [Google Scholar]
- 6. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis 2019; 69 Suppl 7: 521–8. 10.1093/cid/ciz824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birgand G, Moore LSP, Bourigault C et al. Measures to eradicate multidrug-resistant organism outbreaks: how much do they cost? Clin Microbiol Infect Dis 2016; 22: 162.e1–e9. 10.1016/j.cmi.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 8. Bolivar R, Weaver SS, Bodey GP. Comparative in vitro study of temocillin (BRL 17421), a new penicillin. Antimicrob Agents Chemother 1982; 21: 641–5. 10.1128/AAC.21.4.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slocombe B, Basker MJ, Bentley PH et al. BRL 17421, a novel beta-lactam antibiotic, highly resistant to beta-lactamases, giving high and prolonged serum levels in humans. Antimicrob Agents Chemother 1981; 20: 38–46. 10.1128/AAC.20.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jules K, Neu HC. Antibacterial activity and beta-lactamase stability of temocillin. Antimicrob Agents Chemother 1982; 22: 453–60. 10.1128/AAC.22.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Livermore DM, Warner M, Mushtaq S et al. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents 2011; 37: 415–9. 10.1016/j.ijantimicag.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 12. Spencer RC. Temocillin. J Antimicrob Chemother 1990; 26: 735–7. 10.1093/jac/26.6.735 [DOI] [PubMed] [Google Scholar]
- 13. Chenouard R, Mahieu R, Luque Paz D et al. Impact of ceftriaxone and temocillin on fecal abundance of extended-spectrum β-lactamase producing Escherichia coli in a mouse model. PLoS One 2021; 16: e0248177. 10.1371/journal.pone.0248177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edlund C, Ternhag A, Skoog Ståhlgren G et al. The clinical and microbiological efficacy of temocillin versus cefotaxime in adults with febrile urinary tract infection, and its effects on the intestinal microbiota: a randomised multicentre clinical trial in Sweden. Lancet Infect Dis 2022; 22: 390–400. 10.1016/S1473-3099(21)00407-2 [DOI] [PubMed] [Google Scholar]
- 15. Heard KL, Killington K, Mughal N et al. Clinical outcomes of temocillin use for invasive Enterobacterales infections: a single-centre retrospective analysis. JAC Antimicrob Resist 2021; 3: dlab005. 10.1093/jacamr/dlab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dinh A, Duran C, Singh S et al. Real-life temocillin use in Greater Paris area, effectiveness and risk factors for failure in infections caused by ESBL-producing Enterobacterales: a multicentre retrospective study. JAC Antimicrob Resist 2023; 5: dlac132. 10.1093/jacamr/dlac132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta ND, Smith RE, Balakrishnan I. Clinical efficacy of temocillin. J Antimicrob Chemother 2009; 64: 431–3. 10.1093/jac/dkp208 [DOI] [PubMed] [Google Scholar]
- 18. EUCAST . Clinical breakpoints and dosing of antibiotics. 2023. https://www.eucast.org/clinical_breakpoints.
- 19. Alexandre K, Fantin B. Pharmacokinetics and pharmacodynamics of temocillin. Clin Pharmacokinet 2018; 57: 287–96. 10.1007/s40262-017-0584-7 [DOI] [PubMed] [Google Scholar]
- 20. Alexandre K, Réveillon-Istin M, Fabre R et al. Temocillin against Enterobacteriaceae isolates from community-acquired urinary tract infections: low rate of resistance and good accuracy of routine susceptibility testing methods. J Antimicrob Chemother 2018; 73: 1848–53. 10.1093/jac/dky101 [DOI] [PubMed] [Google Scholar]
- 21. Livermore DM, Hope R, Fagan EJ et al. Activity of temocillin against prevalent ESBL- and AmpC-producing Enterobacteriaceae from south-east England. J Antimicrob Chemother 2006; 57: 1012–4. 10.1093/jac/dkl043 [DOI] [PubMed] [Google Scholar]
- 22. Delory T, Gravier S, Le Pluart D et al. Temocillin versus carbapenems for urinary tract infection due to ESBL-producing Enterobacteriaceae: a multicenter matched case-control study. Int J Antimicrob Agents 2021; 58: 106361. 10.1016/j.ijantimicag.2021.106361 [DOI] [PubMed] [Google Scholar]
- 23. BSAC . Susceptibility testing. 2024. https://bsac.org.uk/susceptibility/.
- 24. Alexandre K, Leysour de Rohello F, Dahyot S et al. Efficacy of temocillin against MDR Enterobacterales: a retrospective cohort study. J Antimicrob Chemother 2021; 76: 784–8. 10.1093/jac/dkaa486 [DOI] [PubMed] [Google Scholar]
- 25. Legge JS, Reid TM, Palmer JB. Clinical efficacy, tolerance and pharmacokinetics of temocillin in patients with respiratory tract infections. Drugs 1985; 29 Suppl 5: 118–21. 10.2165/00003495-198500295-00025 [DOI] [PubMed] [Google Scholar]
- 26. Gray JM, Leiper JM, Lawson DH et al. Temocillin in the treatment of chest infections. Drugs 1985; 29 Suppl 5: 197–200. 10.2165/00003495-198500295-00043 [DOI] [PubMed] [Google Scholar]
- 27. Verbist L, Verhaegen J. Effect of temocillin in combination with other beta-lactam antibiotics. Antimicrob Agents Chemother 1984; 25: 142–4. 10.1128/AAC.25.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lekkas A, Gyi KM, Hodson ME. Temocillin in the treatment of Burkholderia cepacia infection in cystic fibrosis. J Cyst Fibros 2006; 5: 121–4. 10.1016/j.jcf.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 29. Habayeb H, Sajin B, Patel K et al. Amoxicillin plus temocillin as an alternative empiric therapy for the treatment of severe hospital-acquired pneumonia: results from a retrospective audit. Eur J Clin Microbiol Infect Dis 2015; 34: 1693–9. 10.1007/s10096-015-2406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Jongh R, Hens R, Basma V et al. Continuous versus intermittent infusion of temocillin, a directed spectrum penicillin for intensive care patients with nosocomial pneumonia: stability, compatibility, population pharmacokinetic studies and breakpoint selection. J Antimicrob Chemother 2008; 61: 382–8. 10.1093/jac/dkm467 [DOI] [PubMed] [Google Scholar]
- 31. Layios N, Visée C, Mistretta V et al. Modelled target attainment after temocillin treatment in severe pneumonia: systemic and epithelial lining fluid pharmacokinetics of continuous versus intermittent infusions. Antimicrob Agents Chemother 2022; 66: e0205221. 10.1128/aac.02052-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poston GJ, Greengrass A, Moryson CJ. Biliary concentrations of temocillin. Drugs 1985; 29 Suppl 5: 140–5. 10.2165/00003495-198500295-00029 [DOI] [PubMed] [Google Scholar]
- 33. Pfeiffer M, Fock RR. Therapeutic experience with temocillin in peritonitis. Drugs 1985; 29 Suppl 5: 194–6. 10.2165/00003495-198500295-00042 [DOI] [PubMed] [Google Scholar]
- 34. Wise R, Donovan IA, Drumm J et al. The intraperitoneal penetration of temocillin. J Antimicrob Chemother 1983; 12: 93–6. 10.1093/jac/12.1.93 [DOI] [PubMed] [Google Scholar]
- 35. Wittke RR, Adam D, Klein HE. Therapeutic results and tissue concentrations of temocillin in surgical patients. Drugs 1985; 29 Suppl 5: 221–6. 10.2165/00003495-198500295-00049 [DOI] [PubMed] [Google Scholar]
- 36. Schulze B, Heilmann HD. Treatment of severe infections with temocillin clinical and bacteriological evaluation. Drugs 1985; 29 Suppl 5: 207–9. 10.2165/00003495-198500295-00046 [DOI] [PubMed] [Google Scholar]
- 37. Saylam K, Anaf V, Kirkpatrick C. Successful medical management of multifocal psoas abscess following cesarean section: report of a case and review of the literature. Eur J Obstet Gynecol Reprod Biol 2002; 102: 211–4. 10.1016/S0301-2115(01)00604-2 [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez M, Nelson M, Kelly JE et al. Successful use of temocillin as salvage therapy for cervical osteomyelitis secondary to multidrug-resistant Burkholderia cepacia. J Pediatr Infect Dis Soc 2014; 3: 77–80. 10.1093/jpids/pis110 [DOI] [PubMed] [Google Scholar]
- 39. Duerinckx JFH. Case report: subacute synovitis of the knee after a rose thorn injury: unusual clinical picture. Clin Orthop 2008; 466: 3138–42. 10.1007/s11999-008-0482-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barker S, Nichol T, Harrison PL et al. Temocillin: a new candidate antibiotic for local antimicrobial delivery in orthopaedic surgery? J Antimicrob Chemother 2015; 70: 780–3. 10.1093/jac/dku425 [DOI] [PubMed] [Google Scholar]