ABSTRACT
Background
Primary glomerular disease (PGD) is a major cause of end-stage kidney disease (ESKD) leading to kidney replacement therapy (KRT). We aimed to describe incidence (trends) in individuals starting KRT for ESKD due to PGD and to examine their survival and causes of death.
Methods
We used data from the European Renal Association (ERA) Registry on 69 854 patients who started KRT for ESKD due to PGD between 2000 and 2019. ERA primary renal disease codes were used to define six PGD subgroups. We examined age and sex standardized incidence, trend of the incidence and survival.
Results
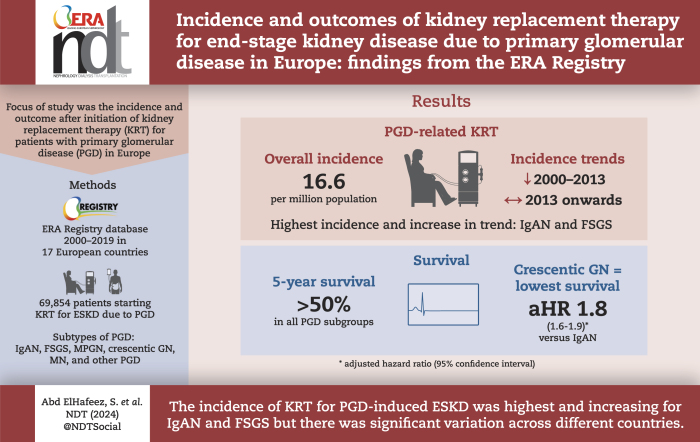
The standardized incidence of KRT for ESKD due to PGD was 16.6 per million population (pmp), ranging from 8.6 pmp in Serbia to 20.0 pmp in France. Immunoglobulin A nephropathy (IgAN) and focal segmental glomerulosclerosis (FSGS) had the highest incidences, of 4.6 pmp and 2.6 pmp, respectively. Histologically non-examined PGDs represented over 50% of cases in Serbia, Bosnia and Herzegovina, and Romania and were also common in Greece, Estonia, Belgium and Sweden. The incidence declined from 18.6 pmp in 2000 to 14.5 pmp in 2013, after which it stabilized. All PGD subgroups had 5-year survival probabilities above 50%, with crescentic glomerulonephritis having the highest risk of death [adjusted hazard ratio 1.8 (95% confidence interval 1.6–1.9)] compared with IgAN. Cardiovascular disease was the most common cause of death (33.9%).
Conclusion
The incidence of KRT for ESKD due to PGD showed large differences between countries and was highest and increasing for IgAN and FSGS. Lack of kidney biopsy facilities in some countries may have affected accurate assignment of the cause of ESKD. The recognition of the incidence and outcomes of KRT among different PGD subgroups may contribute to a more individualized patient care approach.
Keywords: dialysis, epidemiology, kidney replacement therapy, outcome, primary glomerular disease
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Primary glomerular disease (PGD) is a major cause of end-stage kidney disease (ESKD) in people initiating kidney replacement therapy (KRT).
So far, most studies on the epidemiology of people receiving KRT for PGD have combined the different PGD subgroups into one ‘overall’ PGD group, and only few epidemiological studies focused on different PGD subgroups.
We therefore aimed to examine the number of patients commencing KRT with PGD (overall and for different subgroups) as primary renal disease and their outcome after KRT.
This study adds:
We found significant variations in the standardized incidence of KRT for PGD across different countries, with the highest incidence observed for immunoglobulin A nephropathy (IgAN) and FSGS.
The percentage of histologically not examined PGD was high in several Eastern and Western European countries.
The mortality risk was highest for crescentic GN and lowest for IgAN.
Potential impact:
Understanding the burden and prognosis of individuals receiving KRT for specific PGD subgroups could help in facilitating the development of personalized and equitable patient care approaches.
INTRODUCTION
Primary glomerular disease (PGD) is among the leading causes of end-stage kidney disease (ESKD) in people initiating kidney replacement therapy (KRT). As shown in 2020 by large-scale renal registries, the incidence of KRT due to PGD per million population (pmp) was 19.0 pmp in Europe [1], 21.3 pmp in Canada [2] and 25.8 pmp in the USA [3].
PGD refers to several individual disorders, affecting and initially confined to glomeruli. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [4] classify PGD based on clinical, histopathological features on kidney biopsy, and immunological characteristics into immunoglobulin A nephropathy (IgAN), membranous nephropathy (MN), minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), immunoglobulin- and complement-mediated glomerular disease with a membranoproliferative glomerulonephritis (MPGN) pattern of injury, and anti-glomerular basement membrane (anti-GBM) antibody glomerulonephritis (GN), which typically manifests as rapidly progressive GN, characterized by a histological pattern of crescentic GN. Additionally, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis may present clinically and histologically as a pauci-immune crescentic GN.
Several studies have addressed the incidence, trends and outcomes among patients receiving KRT for ESKD secondary to the ‘overall’ PGD group [2, 3, 5–10]. However, few studies have investigated either the (trends in) incidence [11–13] or outcomes [14–18] of KRT among different PGD subgroups, and none of them compared the incidence and prognosis of KRT for ESKD across all PGD subgroups in multiple European countries. Studying the incidence and outcomes of KRT due to kidney failure for various PGD subgroups helps in understanding their prognosis, which could contribute to the development of a more individualized and equitable patient care approach.
The aim of this study was therefore to examine the (trends in) incidence of patients starting KRT for ESKD due to PGD between 2000 and 2019 and their survival and causes of death, for PGD groups combined and for the different PGD subgroups.
MATERIALS AND METHODS
Data collection
For this study, we used data from the following 32 renal registries providing individual patient data for 3 years or more between 2000 and 2019 on patients starting KRT to the ERA Registry: Austria, Dutch- and French-speaking Belgium, Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, the Netherlands, Norway, Romania, Serbia, Sweden, Switzerland, the Spanish regions of Andalusia, Aragon, Asturias, Basque country, Catalonia, Cantabria, Castile-La Mancha, Castile and León, Extremadura, Galicia, Madrid, Murcia, Navarre, Valencian region, the UK (England, Wales and Northern Ireland) and the UK (Scotland).
For the analysis on the trends in incidence over time, we included data from the national and regional renal registries that provided data for the entire study period from 2000 to 2019 with the general population coverage of 100% or almost 100%: Austria, Dutch- and French-speaking Belgium, Denmark, Finland, Greece, Iceland, the Netherlands, Norway, Serbia, Scotland, Sweden, the Spanish regions of Andalusia, Asturias, Basque country, Catalonia, Cantabria, Castile-La Mancha, Valencian region.
Definitions
Primary glomerular disease subgroups
Supplementary data, Table S1 presents the definition for the six PGD subgroups used in this study. For the main analysis, PGD was categorized into [old ERA primary renal disease (PRD) codes in brackets]: IgAN (‘12’), MPGN [type I (‘13’) and type II (‘14’)], MN (‘15’), FSGS [among adults (‘17’) and among children (‘11’)], crescentic GN (‘16’) and other PGD [histologically examined (‘19’) and histologically not examined (‘10’)] [19].
As a sensitivity analysis, we included three additional ERA PRD codes, referring to Henoch–Schönlein purpura (‘85’), anti-GBM disease formally known as Goodpasture syndrome (‘86’) and granulomatosis with polyangiitis formally known as Wegener's granulomatosis (‘74’). The kidney involvement in these diseases is indistinguishable from PGD and will be explained in more detail in the supplement (see footnotes to Supplementary data, Table S2).
We performed a second sensitivity analysis to calculate the incidence pmp based on the revised ERA PRD code from 2012 [20], which provides detailed information on the classification of histologically examined and histologically not examined subgroups compared with the old ERA PRD codes. This analysis was done for countries providing revised ERA PRD codes for the 2015–19 period (Austria, Bosnia and Herzegovina, Belgium, Denmark, Spain, Finland, Norway, Sweden and the UK (England, Wales, Northern Ireland and Scotland).
Causes of death
The causes of death were classified using the ERA coding system and grouped into the following categories: cardiovascular diseases (myocardial ischaemia and infarction, heart failure, cardiac arrest, other cause/unknown, cerebrovascular accident), infection, suicide/refusal of treatment, treatment withdrawal, cachexia, malignancies, miscellaneous and unknown/unavailable [19].
Statistical analysis
Results are expressed as median and interquartile range (IQR) for continuous variables and as percentage for categorical data. Comparisons between PGD subgroups were made using Mann–Whitney test (continuous variables) and chi-squared tests (categorical variables). Two tailed test with P < .05 was considered statistically significant.
Incidence
The crude incidence pmp was calculated by dividing the number of patients starting KRT (any form of dialysis or pre-emptive kidney transplantation) by the mid-year general population of the country or region, multiplied by 1 million. The standardized incidence was calculated using the age and sex distribution of the EU28 of 2015 as a reference [21]. In case of analysis by age group or sex, the number of patients commencing KRT was divided by the mid-year general population of that age or sex group. The incidence of KRT was studied for all participating countries combined with respect to total PGD and by PGD subgroup, and stratified by the age of patients at the onset of KRT, sex and country.
Time trends
Joinpoint regression analysis was used to determine the annual percentage change (APC) and 95% confidence interval (CI) of time trends in the age and sex standardized incidence of KRT for ESKD, both for total PGD and by PGD subgroup, with the observed incidence pmp for all participating countries combined as the outcome and the initiation year of KRT as the explanatory variable. The analysis was performed using Poisson regression as provided by the Joinpoint Regression Program (National Cancer Institute; version 4.2.0.2). Detailed information about this method can be found elsewhere [22].
Patient survival
Survival probabilities were analysed for 1, 2 and 5 years for people initiating KRT for PGD (total and by subgroup). To allow all patients to complete the follow-up period of 5 years, for the survival analysis only patients who started KRT between 2000 and 2014 were included. The first day of KRT was defined as the starting point and patient death was the event studied. Follow-up time was censored at recovery from KRT (defined as interruption of KRT for more than 30 days), loss to follow-up and after 5 years of follow-up. Switzerland, Serbia and Romania were excluded from the survival analyses as they did not provide data before 2014 to allow for the 5-year follow-up period.
Crude survival probabilities were calculated using the Kaplan–Meier method. Adjusted survival probabilities and hazard ratios (HRs) were computed using Cox regression analysis, adjusted for age, sex, time period and country. All crude and adjusted survival probabilities and HRs are reported with 95% CI.
Causes of death were examined for both total PGD and for the PGD subgroups. This analysis included all countries and regions except for France and the UK (England, Wales and Northern Ireland) as their registries reported 25% or more missing or unknown causes of death.
All national and regional registries contributing data to the ERA Registry followed national legislation regarding ethics committee approval. All analyses were performed with SAS version 9.4 [23].
RESULTS
Among 603 673 individuals who started KRT in 2000–19, 69 854 (11.6%) commenced this treatment because of PGD-induced kidney failure. Among those with PGD, 19 394 (27.8%) had a diagnosis of IgAN, 11 016 (15.8%) had FSGS, 5914 (8.5%) had MPGN, 4079 (5.8%) had crescentic GN and 3133 (4.5%) carried a diagnosis of MN. The remaining 26 318 (37.7%) patients were categorized as ‘other PGD’ (Table 1). The proportion of histologically non-examined PGD was the highest in Serbia (60%), Bosnia and Herzegovina (78%) and Romania (79%) (Supplementary data, Fig. S1). Overall, the median (IQR) age of PGD patients at the onset of KRT was 58.5 (44.5–70.6) years and 68.9% were men. Those with IgAN were the youngest (median age 51.9 years). Those with crescentic GN had the highest percentage of recovery of renal function within 3 months after KRT start (1.3%), but also the highest median age at KRT initiation (67.0 years) and the highest risk of death (4.9%) in the same timeframe (Table 1).
Table 1:
Baseline characteristics of patients starting KRT for ESKD due to PGD based on 2000–19 data.
| PGD subgroups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total PGD (N = 69 854) | IgAN (N = 19 394) (27.8%) |
FSGS (N = 11 016) (15.8%) |
MPGN (N = 5914) (8.5%) | Crescentic GN (N = 4079) (5.8%) | MN (N = 3133) (4.5%) |
Other PGD (N = 26 318) (37.7%) | P-value comparing PGD subgroups | |
| Age [median (Q1–Q3)] | 58.5 (44.5–70.6) |
51.9 (39.0–64.2) |
55.0 (40.8–67.3) |
65.2 (52.6–74.3) |
67.0 (54.6–76.0) |
57.9 (44.0–69.9) |
62.2 (48.8–73.3) |
<.001 |
| Age group at start of KRT (%) | <.001 | |||||||
| 0–19 years | 2.0 | 1.1 | 5.4 | 1.0 | 2.2 | 2.2 | 1.5 | |
| 20–44 years | 23.7 | 34.9 | 25.9 | 13.6 | 12.5 | 24.2 | 18.6 | |
| 45–64 years | 37.4 | 40.6 | 39.2 | 35.0 | 30.4 | 37.8 | 35.9 | |
| 65–74 years | 20.6 | 15.3 | 17.8 | 27.3 | 27.5 | 20.7 | 23.2 | |
| ≥75 years | 16.2 | 8.1 | 11.9 | 23.1 | 27.5 | 15.1 | 20.9 | |
| Male (%) | 68.9 | 77.3 | 65.4 | 70.6 | 57.7 | 62.1 | 66.6 | <.001 |
| Treatment modality at Day 1 (%) | <.001 | |||||||
| Haemodialysis | 72.8 | 63.2 | 72.7 | 76.2 | 89.2 | 73.9 | 76.5 | |
| Peritoneal dialysis | 20.4 | 25.5 | 20.8 | 18.3 | 9.4 | 19.9 | 18.7 | |
| Preemptive kidney transplantation | 6.8 | 11.3 | 6.5 | 5.5 | 1.5 | 6.3 | 4.8 | |
| Recovered kidney function ≤90 days after initiating KRT (%) | 0.6 | 0.4 | 0.4 | 0.6 | 1.3 | 0.7 | 0.7 | <.001 |
| Death ≤90 days after initiating KRT (%) | 2.6 | 1.4 | 1.9 | 3.1 | 4.9 | 2.8 | 3.2 | <.001 |
| Loss to follow-up ≤90 days after initiating KRT (%) | 0.3 | 0.2 | 0.3 | 0.2 | 0.6 | 0.5 | 0.3 | <.001 |
| Treatment modality at Day 90 (%) | <.001 | |||||||
| Haemodialysis | 69.1 | 58.5 | 69.6 | 73.7 | 84.6 | 69.5 | 73.3 | |
| Peritoneal dialysis | 22.0 | 26.8 | 22.0 | 19.4 | 13.2 | 22.1 | 20.4 | |
| Kidney transplantation | 8.9 | 14.7 | 8.4 | 6.9 | 2.2 | 8.4 | 6.3 | |
The percentages of the different PGD subgroups were column percentages.
Incidence
Overall and by country
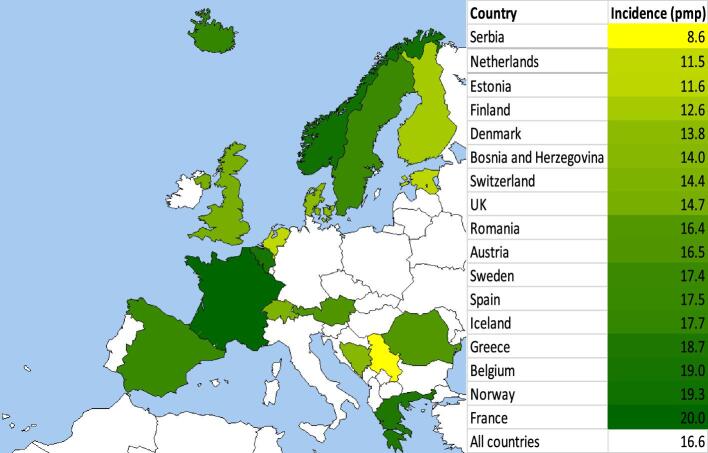
The standardized incidence of KRT for ESKD due to PGD for all countries combined was 16.6 pmp, ranging from 8.6 pmp in Serbia to 20.0 pmp in France (Fig. 1). No substantial differences were observed in the sensitivity analysis for which Henoch–Schönlein purpura, anti-GBM disease and granulomatosis with polyangiitis were added to the main definition of PGD (18.3 pmp in the sensitivity analysis versus 16.6 pmp in the main analysis) (Supplementary data, Table S2).
Figure 1:
Standardized incidence of kidney replacement therapy pmp for ESKD due to PGD by country based on 2000–19 data. For age and sex standardization, the EU28 population in 2015 was used. Dark green represents a higher standardized incidence pmp, while light green and yellow represents a lower standardized incidence pmp.
Among PGD subgroups, the standardized incidence of KRT in all countries combined ranged from 0.7 pmp for MN to 4.6 pmp for IgAN. There were large differences between countries in the standardized incidence of KRT for ESKD due to IgAN, which was lowest in Bosnia and Herzegovina (0.2 pmp) and highest in Sweden and France (both 6.2 pmp), and FSGS [lowest in Bosnia and Herzegovina (0.8 pmp) and highest in Iceland (6.2 pmp)]. International differences were less evident for MPGN, crescentic GN and MN (all ≤2.5 pmp). The standardized incidence of KRT for ESKD due to other PGD for all countries combined was 6.3 pmp, ranging from 2.0 pmp in Iceland to 14.0 pmp in Romania. Belgium (9.9 pmp), Greece (10.2 pmp), and Bosnia and Herzegovina (12.0 pmp) also had high standardized incidence of KRT for ESKD due to other PGD (Table 2 and Supplementary data, Fig. S2).
Table 2:
Incidence (crude and standardized) of KRT pmp for ESKD due to PGD, by country, age group and sex, based on 2000–19 data.
| PGD subgroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total PGD | IgAN | FSGS | MPGN | Crescentic GN | MN | Other PGD | ||||||||
| Crude | Standardized | Crude | Standardized | Crude | Standardized | Crude | Standardized | Crude | Standardized | Crude | Standardized | Crude | Standardized | |
| Countries | ||||||||||||||
| Austria | 16.1 | 16.5 | 3.8 | 3.8 | 3.3 | 3.3 | 1.0 | 1.1 | 1.0 | 1.0 | 0.6 | 0.7 | 6.4 | 6.6 |
| Belgium | 18.2 | 19.0 | 3.7 | 3.9 | 2.4 | 2.5 | 1.4 | 1.5 | 0.6 | 0.7 | 0.6 | 0.7 | 9.4 | 9.9 |
| Bosnia and Herzegovina | 13.4 | 14.0 | 0.2 | 0.2 | 0.8 | 0.8 | 0.5 | 0.5 | 0.1 | 0.1 | 0.4 | 0.4 | 11.5 | 12.0 |
| Denmark | 13.3 | 13.8 | 1.9 | 2.0 | 2.1 | 2.1 | 1.1 | 1.2 | 0.7 | 0.7 | 0.8 | 0.9 | 6.7 | 6.9 |
| Estonia | 11.4 | 11.6 | 5.0 | 5.1 | 1.0 | 1.0 | 0.0 | 0.0 | 0.6 | 0.5 | 0.2 | 0.2 | 4.6 | 4.7 |
| Finland | 12.4 | 12.6 | 5.1 | 5.1 | 2.1 | 2.2 | 0.7 | 0.7 | 0.4 | 0.4 | 0.6 | 0.6 | 3.4 | 3.5 |
| France | 18.9 | 20.0 | 5.9 | 6.2 | 3.8 | 4.0 | 1.6 | 1.7 | 1.3 | 1.4 | 0.7 | 0.8 | 5.6 | 5.9 |
| Greece | 18.8 | 18.7 | 2.7 | 2.7 | 2.9 | 2.9 | 1.6 | 1.6 | 1.0 | 0.9 | 0.4 | 0.4 | 10.3 | 10.2 |
| Iceland | 15.0 | 17.7 | 5.8 | 6.0 | 5.0 | 6.2 | 0.0 | 0.0 | 1.8 | 2.5 | 0.8 | 0.9 | 1.6 | 2.0 |
| Netherlands | 10.9 | 11.5 | 3.4 | 3.5 | 1.2 | 1.3 | 1.1 | 1.2 | 0.7 | 0.7 | 0.7 | 0.7 | 3.9 | 4.1 |
| Norway | 17.9 | 19.3 | 5.6 | 5.9 | 2.0 | 2.1 | 0.9 | 1.0 | 1.0 | 1.1 | 0.9 | 0.9 | 7.5 | 8.3 |
| Romania | 16.3 | 16.4 | 0.7 | 0.7 | 1.0 | 1.0 | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 13.9 | 14.0 |
| Serbia | 8.9 | 8.6 | 0.5 | 0.4 | 1.1 | 1.1 | 0.3 | 0.3 | 0.3 | 0.3 | 0.7 | 0.7 | 6.0 | 5.7 |
| Spain | 17.0 | 17.5 | 3.6 | 3.7 | 2.5 | 2.5 | 1.4 | 1.5 | 1.4 | 1.4 | 1.0 | 1.0 | 7.1 | 7.4 |
| Sweden | 17.0 | 17.4 | 6.0 | 6.2 | 1.9 | 2.0 | 0.9 | 0.9 | 0.8 | 0.8 | 0.6 | 0.7 | 6.7 | 6.9 |
| Switzerland | 14.5 | 14.4 | 4.2 | 4.1 | 2.8 | 2.8 | 1.1 | 1.0 | 1.0 | 1.0 | 0.5 | 0.5 | 5.0 | 4.9 |
| UK | 13.9 | 14.7 | 4.8 | 5.0 | 2.0 | 2.1 | 1.5 | 1.6 | 0.6 | 0.7 | 0.7 | 0.7 | 4.2 | 4.5 |
| All countries | 15.9 | 16.6 | 4.4 | 4.6 | 2.5 | 2.6 | 1.4 | 1.4 | 0.9 | 1.0 | 0.7 | 0.7 | 6.0 | 6.3 |
| Age group (years) at start of KRT | ||||||||||||||
| 0–19 | 1.4 | 1.4 | 0.2 | 0.2 | 0.6 | 0.6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.4 |
| 20–44 | 11.2 | 11.2 | 4.6 | 4.6 | 1.9 | 1.9 | 0.5 | 0.5 | 0.3 | 0.3 | 0.5 | 0.5 | 3.3 | 3.3 |
| 45–64 | 23.1 | 23.1 | 7.0 | 6.9 | 3.8 | 3.8 | 1.8 | 1.8 | 1.1 | 1.1 | 1.0 | 1.0 | 8.3 | 8.3 |
| 65–74 | 35.9 | 35.7 | 7.4 | 7.4 | 4.9 | 4.9 | 4.0 | 4.0 | 2.8 | 2.8 | 1.6 | 1.6 | 15.2 | 15.1 |
| ≥75 | 31.2 | 31.2 | 4.3 | 4.3 | 3.6 | 3.6 | 3.8 | 3.8 | 3.1 | 3.1 | 1.3 | 1.3 | 15.1 | 15.1 |
| Sex | ||||||||||||||
| Female | 9.7 | 10.1 | 2.0 | 2.0 | 1.7 | 1.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.5 | 0.6 | 3.9 | 4.1 |
| Male | 22.4 | 23.5 | 7.0 | 7.2 | 3.4 | 3.5 | 1.9 | 2.1 | 1.1 | 1.2 | 0.9 | 0.9 | 8.2 | 8.6 |
Based on the revised ERA PRD codes in 2012, the standardized incidence of KRT for ESKD due to other PGD for all countries combined was 5.75 pmp, ranging from 4.08 pmp in Austria to 11.82 pmp in Bosnia and Herzegovina. This high incidence in Bosnia and Herzegovina was mainly driven by histologically non-examined PGD (10.65 pmp). Similarly, other countries as Spain (3.56 pmp), Belgium (3.76 pmp), Norway (3.87 pmp) and Sweden (4.19 pmp) also exhibited high standardized incidences of non-biopsied PGD (Supplementary data, Table S3).
By age group
The crude incidence of KRT for PGD-induced kidney failure was lowest in the age group 0–19 years (1.4 pmp) and highest in the age group 65–74 years (35.9 pmp) (Table 2 and Supplementary data, Fig. S3).
Among PGD subgroups, the crude incidence of KRT was lowest in the age group 0–19 years (<0.6 pmp for all individual PGDs). The crude incidence of KRT was highest in patients aged 65–74 years for IgAN (7.4 pmp), FSGS (4.9 pmp), MPGN (4.0 pmp), MN (1.6 pmp) and other PGD (15.2 pmp), while for crescentic GN it was the highest in older patients (≥75 years; 3.1 pmp). There was no substantial difference between the crude and standardized incidence of KRT for PGD-induced kidney failure (Table 2 and Supplementary data, Fig. S3).
By sex
The crude incidence of KRT for ESKD due to PGD was 22.4 pmp for males and 9.7 pmp for females (Table 2 and Supplementary data, Fig. S4).
Within all PGD subgroups, the crude incidence of KRT was higher among males and ranged from 0.9 pmp for MN to 7.0 pmp for IgAN, while among females it ranged from 0.5 pmp for MN to 2.0 pmp for IgAN. The largest difference between males and females was observed for IgAN (7.0 pmp and 2.0 pmp, respectively). For other PGD, the crude incidence of KRT was 8.2 pmp in males and 3.9 pmp for females. There was no substantial difference between the crude and standardized incidence of KRT for PGD-induced kidney failure (Table 2 and Supplementary data, Fig. S4).
Time trends
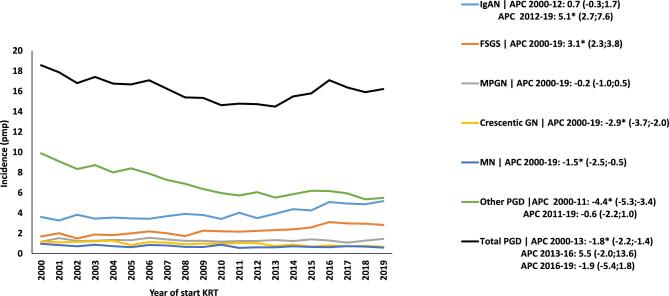
The standardized incidence of KRT for ESKD due to PGD showed a decline from 18.6 pmp in the year 2000 to 14.5 pmp in 2013 [APC –1.8 (95% CI –2.2; –1.4)]. Thereafter, it stabilized (Fig. 2).
Figure 2:
Trends in age- and sex-standardized incidence of KRT pmp for ESKD due to PGD, based on 2000–19 data. The APC and 95% CI are indicated in the righthand panel. *Significant change in APC.
Among PGD subgroups, the standardized incidence of KRT increased between 2012 and 2019 for IgAN [APC 5.1 (95% CI 2.7; 7.6)] and between 2000 and 2019 for FSGS [APC 3.1 (95% CI 2.3–3.8)]. Conversely, the standardized incidence of KRT for ESKD due to crescentic GN [APC –2.9 (95% CI –3.7; –2)] and MN [APC –1.5 (95% CI –2.5; –0.5)] declined during the whole study period. Also, the standardized incidence of KRT for ESKD for the other PGD decreased between 2000 and 2011 [APC –4.4 (95% CI –5.3; –3.4)] (Fig. 2).
Patient survival
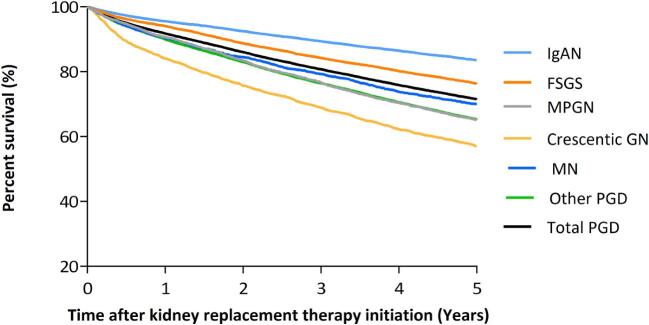
The crude and adjusted 5-year survival probabilities after the onset of KRT for PGD-induced kidney failure were 71.5% and 69.7%, respectively (Fig. 3 and Supplementary data, Table S4).
Figure 3:
Five-year survival for patients starting KRT for ESKD due to PGD based on 2000–14 data using Kaplan–Meier survival analyses. Adjustment for age, sex, time period and country.
Among PGD subgroups, the crude and adjusted 5-year survival probabilities after KRT initiation were lowest for crescentic GN (57.0% and 64.5%, respectively) and highest for IgAN (83.5% and 76.1%, respectively). Compared with IgAN, patients with crescentic GN had the highest risk of death [HR 1.8 (95% CI 1.6; 1.9); P < .001], followed by MN [HR 1.5 (95% CI 1.4; 1.7); P < .001] and other PGD [HR 1.5 (95% CI 1.4; 1.6); P < .001] (Fig. 3 and Supplementary data, Table S4).
Causes of death
The most common cause of death in patients receiving KRT for ESKD due to PGD was cardiovascular disease (33.9%), followed by infection (18.5%) (Table 3).
Table 3:
Causes of death among patients starting KRT for ESKD due to PGD based on 2000–14 data with 5 years of follow-up.
| Cause of death | Total PGD (N = 8928) | IgAN (N = 1146) | FSGS (N = 993) | MPGN (N = 848) | Crescentic GN (N = 763) | MN (N = 444) | Other PGD (N = 4734) |
|---|---|---|---|---|---|---|---|
| Cardiovascular diseases (%) | 33.9 | 31.8 | 36.2 | 30.9 | 26.5 | 30.6 | 35.9 |
| Infection (%) | 18.5 | 18.4 | 17.7 | 18.8 | 24.1 | 21.4 | 17.4 |
| Miscellaneous (%) | 16.3 | 18.5 | 16.6 | 13.9 | 18.2 | 14.9 | 15.9 |
| Malignancies (%) | 10.9 | 13.8 | 9.4 | 13.3 | 8.8 | 11.0 | 10.5 |
| Suicide/refusal treatment (%) | 3.4 | 3.5 | 3.5 | 5.9 | 3.5 | 1.8 | 3.2 |
| Cachexia (%) | 2.2 | 1.0 | 1.4 | 3.1 | 2.5 | 3.4 | 2.3 |
| Withdrawal (%) | 5.5 | 4.4 | 5.9 | 6.8 | 6.4 | 7.4 | 5.2 |
| Unknown/unavailable (%) | 9.3 | 8.6 | 9.3 | 7.3 | 10.0 | 9.5 | 9.6 |
Within PGD subgroups, the percentage of cardiovascular disease as cause of death varied from 26.5% for crescentic GN to 36.2% for FSGS. To the contrary, the percentage of death due to infection varied from 17.7% among FSGS patients to 24.1% among crescentic GN patients. Among patient deaths within the ‘other PGD’ subgroup, 35.9% were from cardiovascular disease and 17.4% due to infection (Table 3).
DISCUSSION
In this ERA Registry study using data from the past two decades, the standardized incidence of KRT for ESKD due to PGD varied significantly between countries, with an overall incidence rate of 16.6 pmp. IgAN and FSGS had the highest incidence rates, with a considerable variation across the European countries. Conversely, MPGN, crescentic GN and MN had lower incidence rates with minimal international variation. Over the study period, the standardized incidence of KRT for PGD-induced kidney failure decreased in the early 2000s, followed by stabilization after 2013. The ‘other PGD’ subgroup showed a decline in incidence until 2011. By contrast, there was a noticeable rise in KRT incidence from 2012 to 2019 for IgAN and throughout the study period for FSGS. These temporal trends were not explained by the demographic composition of the study population as analysis were standardized for the age and sex distribution of the EU28 population. Finally, the 5-year survival probabilities after initiating KRT were above 50% in each PGD subgroup, and cardiovascular disease was the most common cause of death, followed by infection.
Incidence
Our results demonstrate that 11.6% of incident KRT patients had PGD as PRD, which is higher than in the USA (7.1%) [21], but much lower than in Brazil (33.7%) [9]. Furthermore, similar to the USA [5], the standardized incidence of KRT for PGD-induced kidney failure decreased in the early years of this century.
Publications on the incidence of KRT for one or more PGD subgroups as PRD are scarce. IgAN was the most common PGD subgroup among patients who initiated KRT, which was similar to a small Spanish study examining incident peritoneal dialysis patients [12]. By contrast, FSGS was the most frequent PGD subgroup among incident KRT population in the USA, in both the white and Black individuals [3, 11]. In both the USA and Spain, MPGN was the least common PGD subgroup among patients who initiated KRT [11, 12], while our findings showed that in Europe MN was the least common PGD subgroup. Furthermore, hardly any previous studies have described the trends in the incidence of KRT for ESKD due to different PGD subgroups. The only exception is a Japanese study which reported stabilization in the age standardized incidence of KRT due to IgAN [13]. Data from the United States Renal Data System (USRDS) showed a decline in the percentage of incident KRT for all PGD subgroups between 2013 and 2020, except for proliferative GN [3].
The international differences in the incidence of people with PGD undergoing KRT may reflect variation in the burden of glomerular diseases worldwide [24, 25], ranging from 119.8 per 100 000 population in Central Europe to 379.3 per 100 000 population in Central Latin America [25]. However, because KRT can be prevented through earlier detection and/or treatment in a subset of PGDs, the variation may also reflect access to timely detection and care. There are also reporting issues, as most PGD subgroups are relatively rare and fall into the category of orphan diseases [26].
Considering the incidence of PGD subgroups, regional biopsy studies suggest that IgAN is more common in Europe [27–29] and Asia [30, 31], while FSGS is more common in the USA [32, 33], Canada [34, 35] and Latin America [36, 37]. Genetic susceptibility [38] and environmental factors [39, 40] may explain these differences, but such findings should be interpreted with caution as biopsy studies reflect access to care, and therefore may underestimate the true disease burden due to reluctance to refer patients with spontaneous remission or advanced kidney disease for biopsy [26]. Low biopsy rates were reported in Serbia (10.8 pmp/year) [41], Romania (11.3 pmp/year) [42] and Hungary (36.6 pmp/year) [43]. Higher biopsy rates were observed in Finland (176 pmp/year) [44], France (162 pmp/year) [45], Australia (215 pmp/year) [44] and the USA (175 pmp/year) [46]. Data from the current study showed a high incidence of histologically non-examined PGD causing ESKD in Serbia and Romania that was consistent with the low biopsy rate.
In addition to kidney biopsy, serological diagnostic techniques, such as testing for ANCA and anti-GBM autoantibodies, play a crucial role in differentiating between anti-GBM antibody GN or pauci-immune categories of crescentic GN. Also, testing for anti-phospholipase receptors A2 (anti-PLA2R) antibody could diagnose MN [4]. In the present study, patients with unspecified PGD diagnosis were grouped under other PGDs. Limited availability of diagnostic tools may lead to under diagnosis or misclassification of PGD subgroups, impacting accurate incidence rates and potentially negatively affecting treatment decisions [26].
Once people are diagnosed with PGD, the differences in the incidence of KRT for PGD-induced ESKD among European countries may be attributed to variable progression of PGD to KRT. Individuals with IgAN [47, 48] and MN [49] tend to have a lower rate of progression to ESKD, whereas those with FSGS [50, 51] and crescentic GN [52] experience a more rapid decline in kidney function. Despite the slower progression of IgAN, the incidence of KRT for ESKD was higher among IgAN compared with other PGD subgroups. Several factors, including traditional risk factors (proteinuria, hypertension, low glomerular filtration rate, histopathology) [53, 54], and demographic factors (age, sex, smoking, overweight/obesity) can influence the rate of progression to ESKD [51, 55]. Moreover, treatment may also impact outcomes [4]. Recent advances in the treatment of MN may have contributed to the progressive decrease in the incidence of KRT from MN [56] while KDIGO guidelines recommend enrolling IgAN patients in clinical trials, given the paucity of evidence [4]. FSGS is also problematic from the therapeutic point of view: being a non-specific histological term [4]. The present results are aligned with unmet needs regarding the treatment of IgAN and the appropriate diagnostic evaluation and treatment of FSGS.
When people with PGD have progressed to ESKD and need KRT, the variation in the access to KRT may be caused by the timing of KRT initiation, patient selection and availability of ESKD treatment options [57, 58]. Macroeconomic factors, such as healthcare expenditure, may also impact the access to KRT [57, 59].
Survival and causes of death
Patients starting KRT for ESKD due to PGD have a better 5-year survival rate (65.5%) than those with diabetes (46.4% %) and hypertension (55.9% %) as PRD, according to the ERA Registry annual report [10]. The current study revealed that among PGD subgroups, IgAN patients have the most favorable survival outcomes, while crescentic GN patients face the highest mortality risk. These findings align with data from studies in the USA [11] and Korea [49]. The substantially diverse mortality risks among PGD subgroups in this study were present also after adjustment for potential confounders although residual confounding may still exist. Cardiovascular disease and infection were the main causes of death among PGD patients undergoing KRT, which is consistent with previous research [6, 11, 15, 16, 18]. The differences in survival rates among various PGD subgroups can be attributed to several factors, including age, limited accessibility to KRT in certain countries [60], aggressive treatment strategies involving immunosuppression that may increase the risk of infections, and the potential occurrence of extrarenal complications [4]. The high cardiovascular mortality observed in PGD patients initiating KRT is linked to risk factors such as dyslipidemia, hypertension, proteinuria and endothelial dysfunction [61]. Patients with PGD are usually in the highest (A3) albuminuria category of cardiovascular risk [62, 63].
Strengths and limitations
This is the largest European study to date on the incidence and outcomes of patients initiating KRT with PGD as the cause of kidney failure, with (almost) 100% coverage in the participating countries. Another important strength of this study is the comparison of the different PGD subgroups regarding the incidence, trends and outcomes after KRT initiation. This study has limitations related to the registry-based nature of the data, including lack of information on potential confounders such as comorbidity, precluding their adjustment in the survival analysis. Finally, the histopathological classification, biopsy practices and management protocols could influence the PRD coding. In this study, we included both histologically and non-histologically confirmed diagnosis of PGD, despite the fact that using non-histologically confirmed diagnosis of PGD could lead to potential misclassification of PGD. However, including only histologically confirmed PGD would lead to an underestimation of the incidence of KRT due to PGD, particularly in countries with (mainly) non-histologically confirmed diagnosis of PGD.
CONCLUSION
Data from the ERA Registry reveals that the incidence of KRT for ESKD due to PGD was highest and increasing for IgAN and FSGS, with significant variation across the European countries. Notably, a substantial number of PGD cases lack histological examination, especially in Eastern and some Western European countries. This may lead to challenges in accurate diagnosis and appropriate treatment. The recognition of the incidence and prognosis of KRT among different PGD subgroups enables a more personalized approach to patient care. Future studies are warranted to assess the impact of novel therapies on disease progression and the initiation of KRT in PGD patients.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry (OEDTR) (F. Engler, G. Mayer and the Austrian Society of Nephrology); Dutch-speaking Belgian Society of Nephrology (NBVN]) (M. Couttenye, F. Schroven and J. De Meester); French-speaking Belgian Society of Nephrology (GNFB) (J.M. des Grottes and F. Collart); Estonian Society of Nephrology (Ü. Pechter and K. Lilienthal); Finnish Registry for Kidney Diseases (J. Helve and H. Niemelä); France: The Epidemiology and Information Network in Nephrology (REIN) (M. Lassalle and C. Couchoud); Hellenic Renal Registry (G. Moustakas); Norwegian Renal Registry (A.V. Reisæter); Romanian Renal Registry (RRR) (G. Mircescu, L. Garneata, and E. Podgoreanu); Renal Registry in Serbia (M. Lausevic, all of the Serbian renal units, and the Serbian Society of Nephrology); Spain Renal Registry (B. Mahillo Durán and M.O. Valentín Muñoz); Swedish Renal Registry (SRR) (K.G. Prütz, M. Stendahl, M. Evans, S. Schön, T. Lundgren and H. Rydell); Swiss Dialysis Registry (P. Ambühl and R. Guidotti); Dutch Renal Registry (RENINE) (L. Heuveling, S. Vogelaar and M. ten Dam); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (SRR) (all of the Scottish renal units); and the regional registries of Andalusia (SICATA) [P. Castro de la Nuez (on behalf of all users of SICATA)], Aragon (F. Arribas Monzón), Asturias (P. Beltrán, M. Rodríguez, J.R. Quirós and RERCA Working Group), Basque country (UNIPAR) (Á. Magaz, J. Aranzabal, M. Rodrigo and I. Moina), Cantabria (J.C. Ruiz San Millán), Castile and León (M.A. Palencia García and P. Ucio Mingo), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia (RMRC) (J. Comas and J. Tort), Community of Madrid (M.I. Aparicio de Madre and F. Tornero Molina), Extremadura [all the renal units (Nephrology and Dialysis)], Galicia (E. Bouzas-Caamaño), Murcia (I. Marín Sánchez and C. Santiuste de Pablos), Navarre (M.F. Slon Roblero, J. Manrique Escola and J. Arteaga Coloma) and Valencian region (O.L. Rodríguez-Arévalo and O. Zurriaga); and the other ERA Registry committee members not mentioned above for their advice in the analysis and the drafting of this paper: C. Wanner, P. Ambühl, P.M. Ferraro, J. Harambat, J. Helve, J.E. Sánchez-Alvarez, R. Boenink and B.A. Boerstra, and M.E. Astley in the AMC Registry office for data collection and management. The ERA Registry is funded by the European Renal Association (ERA).
Contributor Information
Samar Abd ElHafeez, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Epidemiology Department, High Institute of Public Health, Alexandria University, Alexandria, Egypt.
Anneke Kramer, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Mustafa Arici, Department of Nephrology, Faculty of Medicine, Hacettepe University, Ankara, Turkey.
Miha Arnol, Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia; Medical Faculty, University of Ljubljana, Ljubljana, Slovenia.
Anders Åsberg, The Norwegian Renal Registry, Department of Transplantation Medicine, Oslo University Hospital – Rikshospitalet, Oslo, Norway.
Samira Bell, Scottish Renal Registry, Meridian Court, Glasgow, UK; Division of Population Health and Genomics, University of Dundee, Dundee, UK.
Julie Belliere, Department of Nephrology and Organ Transplantation, Referral Centre for Rare Kidney Diseases, University Hospital of Toulouse, Toulouse, France.
Carmen Díaz Corte, Department of Nephrology, Hospital Universitario Central de Asturias, Oviedo University, Oviedo, Spain.
Gema Fernández Fresnedo, Hospital Universitario Marques de Valdecilla, Servicio de Nefrología, Cantabria, Spain.
Marc Hemmelder, Division of Nephrology, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, University of Maastricht, Maastricht, The Netherlands.
Line Heylen, Dutch-speaking Belgian Renal Registry (NBVN), Sint-Niklaas, Belgium; Dienst Nefrologie, Ziekenhuis Oost-Limburg, Genk, Belgium; University Hasselt, Hasselt, Belgium.
Kristine Hommel, Nephrology Department, Holbaek Hospital, Holbaek, Denmark.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV – Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria.
Radomir Naumović, Medical School, University of Belgrade, Serbia.
Dorothea Nitsch, London School of Hygiene and Tropical Medicine, London, UK; UK Renal Registry, Bristol, UK.
Rafael Santamaria, Andalusian Autonomous Transplant Coordination Information System, Seville, Spain; Nephrology Service, Reina Sofia University Hospital, Cordoba, Spain.
Patrik Finne, Helsinki University Central Hospital, Division of Nephrology, Helsinki, Finland.
Runolfur Palsson, Division of Nephrology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland; Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland.
Maria Pippias, University of Bristol, Department of Health Care Evaluation, Population Health Sciences, Bristol, UK; Bright Renal Unit, North Bristol NHS Trust, Bristol, UK.
Halima Resic, Renal Registry of Society of Nephrology, Dialysis and Transplantation of Bosnia and Herzegovina, Clinic for Hemodialysis Sarajevo, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina.
Mai Rosenberg, Competence Centre for Rare Diseases, Tartu University Hospital, Tartu, Estonia.
Carmen Santiuste de Pablos, Murcia Renal Registry, Department of Epidemiology, Murcia Regional Health Council, IMIB-Arrixaca, Murcia, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Madrid, Spain.
Mårten Segelmark, Division of Nephrology, Department of Clinical Sciences, Lund University, Lund, Sweden; Department of Endocrinology, Nephrology and Rheumatology, Skane University Hospital, Lund, Sweden.
Søren Schwartz Sørensen, Department of Nephrology Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Maria Jose Soler, Department of Nephrology, Vall d'Hebron University Hospital, Barcelona, Spain.
Enrico Vidal, Department of Medicine (DAME), University of Udine, Udine, Italy; Pediatric Nephrology Unit, University-Hospital of Padova, Padova, Italy.
Kitty J Jager, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain.
Vianda S Stel, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
FUNDING
K.J. and V.S. report grants from the European Renal Association. A.O.’s research is supported by Comunidad de Madrid en Biomedicina P2022/BMD-7223, CIFRA_COR-CM. Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001, RD21/0005/0016) funded by European Union—NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR) and SPACKDc PMP21/00 109, FEDER funds, COST Action PERMEDIK CA21165, supported by COST (European Cooperation in Science and Technology) and PREVENTCKD Consortium Project ID: 101 101 220 Programme: EU4H DG/Agency: HADEA.
AUTHORS’ CONTRIBUTIONS
V.S. and A.K. designed the study protocol and supervised the study. S.A. and A.K. analyzed the data. S.A. drafted the tables and figures. S.A., V.S., A.K. and K.J. interpreted the data and drafted the manuscript. All authors were involved in data collection for the ERA Registry and reviewed and approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Astley ME, Boenink R, Abd ElHafeez Set al. The ERA Registry Annual Report 2020: a summary. Clin Kidney J 2023;16:1330–54. 10.1093/ckj/sfad087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information. Treatment of end-stage organ failure in Canada, Canadian Organ Replacement Register, 2011 to2020: end-stage kidney disease and kidney transplants—Data tables. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.cihi.ca%2Fsites%2Fdefault%2Ffiles%2Fdocument%2Fend-stage-kidney-disease-transplant-2011-2020-data-table-en.xlsx&wdOrigin=BROWSELINK
- 3.United States Renal Data System . 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2022. [Google Scholar]
- 4.KDIGO . 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–S276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 5.Stel VS, Awadhpersad R, Pippias Met al. International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrology 2019;24:1064–76. 10.1111/nep.13531 [DOI] [PubMed] [Google Scholar]
- 6.Helve J, Haapio M, Groop PHet al. Primary kidney disease modifies the effect of comorbidities on kidney replacement therapy patients' survival. PLoS One 2021;16:e0256522. 10.1371/journal.pone.0256522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton AJ, Casula A, Ben-Shlomo Yet al. The clinical epidemiology of young adults starting renal replacement therapy in the UK: presentation, management and survival using 15 years of UK Renal Registry data. Nephrol Dial Transplant 2018;33:356–64. 10.1093/ndt/gfw444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra R, Chiu YW, Kalantar-Zadeh Ket al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011;171:110–8. 10.1001/archinternmed.2010.352 [DOI] [PubMed] [Google Scholar]
- 9.Batista PB, Lopes AA, Costa FA.. Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail 2005;27:651–6. 10.1080/08860220500234832 [DOI] [PubMed] [Google Scholar]
- 10.ERA Registry. ERA Registry Annual Report 2020 . Amsterdam UMC, Location AMC, Department of Medical Informatics, Amsterdam, the Netherlands, 2022. [Google Scholar]
- 11.O'Shaughnessy MM, Montez-Rath ME, Lafayette RAet al. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol 2015;10:1170. 10.2215/CJN.11261114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz Cuevas M, Limón Ramírez R, Pérez Contreras FJet al. Peritoneal dialysis in incident patients with primary glomerulonephritis. Results of a 20-year multicenter registry study. Nefrología (English Edition) 2021;41:53–61. [DOI] [PubMed] [Google Scholar]
- 13.Wakasugi M, Narita I.. Trends in the incidence of renal replacement therapy by type of primary kidney disease in Japan, 2006-2020. Nephrology 2023;28:119–29. 10.1111/nep.14134 [DOI] [PubMed] [Google Scholar]
- 14.Yang WL, Bose B, Zhang Let al. Long-term outcomes of patients with end-stage kidney disease due to membranous nephropathy: a cohort study using the Australia and New Zealand Dialysis and Transplant Registry. PLoS One 2019;14:e0221531. 10.1371/journal.pone.0221531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Liu X, Pascoe EMet al. Long-term outcomes of end-stage kidney disease for patients with IgA nephropathy: a multi-centre registry study. Nephrology 2016;21:387–96. 10.1111/nep.12629 [DOI] [PubMed] [Google Scholar]
- 16.Wilson GJ, Cho Y, Teixiera-Pinto Aet al. Long-term outcomes of patients with end-stage kidney disease due to membranoproliferative glomerulonephritis: an ANZDATA registry study. BMC Nephrol 2019;20:417. 10.1186/s12882-019-1605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert T, Jantzen R, Cambier Aet al. Spatiotemporal trends and prognosis of end-stage renal disease patients with biopsy-proven immunoglobulin A nephropathy in France from 2010 to 2014. Clin Kidney J 2021;14:898–908. 10.1093/ckj/sfaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo E, Mingozzi S, Mella Aet al. Clinical outcomes and temporal trends of immunological and non-immunological rare diseases in adult kidney transplant. BMC Nephrol 2021;22:386. 10.1186/s12882-021-02571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2019 . Amsterdam, The Netherlands: Amsterdam UMC, Location AMC, Department of Medical Informatics; 2021, 2019. [Google Scholar]
- 20.Venkat-Raman G, Tomson CRV, Gao Yet al. New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 2012;27:4414–9. 10.1093/ndt/gfs461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United Nations Statistics Division. Standard country or area codes for statistical use (M49). 1999; updated 2020. Available from: https://unstats.un.org/unsd/methodology/m49/ (17 October 2022, date last accessed)..
- 22.Joinpoint Regression Program, version 4.2.0.2. Statistical Research and Applications Branch, National Cancer Institute. 2015; https://surveillance.cancer.gov/joinpoint/
- 23.SAS/IML software : usage and reference, version 6: First edition. Cary, NC : SAS Institute, [1990] ©1990; 1990.
- 24.McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Ke R, Teixeira Wet al. Global, Regional, and national burden of CKD due to glomerulonephritis from 1990 to 2019: a systematic analysis from the Global Burden of Disease Study 2019. Clin J Am Soc Nephrol 2023;18:60–71. 10.2215/CJN.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floege J, Amann K.. Primary glomerulonephritides. Lancet North Am Ed 2016;387:2036–48. 10.1016/S0140-6736(16)00272-5 [DOI] [PubMed] [Google Scholar]
- 27.Horvatic I, Tisljar M, Bulimbasic Set al. Epidemiologic data of adult native biopsy-proven renal diseases in Croatia. Int Urol Nephrol 2013;45:1577–87. 10.1007/s11255-013-0397-z [DOI] [PubMed] [Google Scholar]
- 28.Zaza G, Bernich P, Lupo A.. Incidence of primary glomerulonephritis in a large North-Eastern Italian area: a 13-year renal biopsy study. Nephrol Dial Transplant 2013;28:367–72. 10.1093/ndt/gfs437 [DOI] [PubMed] [Google Scholar]
- 29.Nanchen G, Schutzbach K, Rotman Set al. Incidence of glomerulonephritis in the western part of Switzerland over the last decade. Swiss Med Wkly 2020;150:w20353. 10.4414/smw.2020.20353 [DOI] [PubMed] [Google Scholar]
- 30.Woo KT, Chan CM, Mooi CYet al. The changing pattern of primary glomerulonephritis in Singapore and other countries over the past 3 decades. Clin Nephrol 2010;74:372–83. 10.5414/CNP74372 [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Zhang Z, Zhuo Let al. The spectrum of biopsy-proven glomerular disease in China: a systematic review. Chin Med J (Engl) 2018;131:731–5. 10.4103/0366-6999.226906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Shaughnessy MM, Hogan SL, Poulton CJet al. Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol 2017;12:614–23. 10.2215/CJN.10871016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim JJ, Batech M, Hever Aet al. Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis 2016;68:533–44. 10.1053/j.ajkd.2016.03.416 [DOI] [PubMed] [Google Scholar]
- 34.Barbour S, Beaulieu M, Gill Jet al. An overview of the British Columbia Glomerulonephritis network and registry: integrating knowledge generation and translation within a single framework. BMC Nephrol 2013;14:236. 10.1186/1471-2369-14-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham A, Benediktsson H, Muruve DAet al. Trends in biopsy-based diagnosis of kidney disease: a population study. Can J Kidney Health Dis 2018;5:205435811879969. 10.1177/2054358118799690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DMdN C, Valente LM, Gouveia PACet al. Comparative analysis of primary and secondary glomerulopathies in the northeast of Brazil: data from the Pernambuco Registry of Glomerulopathies-REPEG. Brazilian Journal of Nephrology 2017;39:29–35. [DOI] [PubMed] [Google Scholar]
- 37.Garau M, Cabrera J, Ottati Get al. Temporal trends in biopsy proven glomerular disease in Uruguay, 1990-2014. PLoS One 2018;13:e0206637. 10.1371/journal.pone.0206637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Wang L, Shi DCet al. Genome-wide meta-analysis identifies three novel susceptibility loci and reveals ethnic heterogeneity of genetic susceptibility for IgA nephropathy. JASN 2020;31:2949–63. 10.1681/ASN.2019080799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couser WG, Johnson RJ.. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int 2014;86:905–14. 10.1038/ki.2014.49 [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Cui Z, Zhao M.. The genetic and environmental factors of primary membranous nephropathy: an overview from China. Kidney Dis 2018;4:65–73. 10.1159/000487136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naumovic R, Pavlovic S, Stojkovic Det al. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant 2009;24:877–85. 10.1093/ndt/gfn564 [DOI] [PubMed] [Google Scholar]
- 42.Covic A, Schiller A, Volovat Cet al. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 2006;21:419–24. 10.1093/ndt/gfi207 [DOI] [PubMed] [Google Scholar]
- 43.Molnár A, Thomas MJ, Fintha Aet al. Kidney biopsy-based epidemiologic analysis shows growing biopsy rate among the elderly. Sci Rep 2021;11:24479. 10.1038/s41598-021-04274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirta O, Mustonen J, Helin Het al. Incidence of biopsy-proven glomerulonephritis. Nephrol Dial Transplant 2008;23:193–200. 10.1093/ndt/gfm564 [DOI] [PubMed] [Google Scholar]
- 45.Fiorentino M, Bolignano D, Tesar Vet al. Renal biopsy in 2015–from epidemiology to evidence-based indications. Am J Nephrol 2016;43:1–19. 10.1159/000444026 [DOI] [PubMed] [Google Scholar]
- 46.Swaminathan S, Leung N, Lager DJet al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol 2006;1:483–7. 10.2215/CJN.00710805 [DOI] [PubMed] [Google Scholar]
- 47.Ebbestad R, Sanaei Nurmi M, Lundberg S. Long-term outcomes of patients with IgA nephropathy categorized by the International IgAN risk Prediction Tool and by the degree of hematuria at diagnosis. Nephron 2022;146:573–83. 10.1159/000525001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 2004;24:179–96. 10.1016/j.semnephrol.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 49.Lee H, Kim DK, Oh KHet al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol 2013;37:74–83. 10.1159/000345960 [DOI] [PubMed] [Google Scholar]
- 50.Cameron J, Turner D, Ogg Cet al. The long-term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol 1978;10:213–8. [PubMed] [Google Scholar]
- 51.Moranne O, Watier L, Rossert Jet al. Primary glomerulonephritis: an update on renal survival and determinants of progression. QJM 2008;101:215–24. 10.1093/qjmed/hcm142 [DOI] [PubMed] [Google Scholar]
- 52.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003;63:1164–77. 10.1046/j.1523-1755.2003.00843.x [DOI] [PubMed] [Google Scholar]
- 53.Eriksen BO, Ingebretsen OC.. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006;69:375–82. 10.1038/sj.ki.5000058 [DOI] [PubMed] [Google Scholar]
- 54.Jafar TH, Stark PC, Schmid CHet al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003;139:244–52. 10.7326/0003-4819-139-4-200308190-00006 [DOI] [PubMed] [Google Scholar]
- 55.Bonnet F, Deprele C, Sassolas Aet al. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis 2001;37:720–7. 10.1016/S0272-6386(01)80120-7 [DOI] [PubMed] [Google Scholar]
- 56.Rojas-Rivera J, Fervenza FC, Ortiz A. Recent clinical trials insights into the treatment of primary membranous nephropathy. Drugs 2022;82:109–32. 10.1007/s40265-021-01656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caskey FJ, Kramer A, Elliott RFet al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011;26:2604–10. 10.1093/ndt/gfq781 [DOI] [PubMed] [Google Scholar]
- 58.Jager KJ, Stel VS, Branger Pet al. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant 2018;33:560–2. 10.1093/ndt/gfx082 [DOI] [PubMed] [Google Scholar]
- 59.Liyanage T, Ninomiya T, Jha Vet al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet North Am Ed 2015;385:1975–82. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 60.Caskey FJ, Kramer A, Elliott RFet al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011;26:2604–10. 10.1093/ndt/gfq781 [DOI] [PubMed] [Google Scholar]
- 61.Hutton HL, Levin A, Gill Jet al. Cardiovascular risk is similar in patients with glomerulonephritis compared to other types of chronic kidney disease: a matched cohort study. BMC Nephrol 2017;18:95. 10.1186/s12882-017-0511-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rojas-Rivera JE, Bakkaloglu SA, Bolignano Det al. Chronic kidney disease: the missing concept in the 2019 EULAR/ERA-EDTA recommendations for lupus nephritis. Nephrol Dial Transplant 2023;39:151–8. 10.1093/ndt/gfad154 [DOI] [PubMed] [Google Scholar]
- 63.Ortiz A, Wanner C, Gansevoort R.. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: a position statement by the Council of the European Renal Association. Nephrol Dial Transplant 2023;38:527–31. 10.1093/ndt/gfac257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.