Abstract
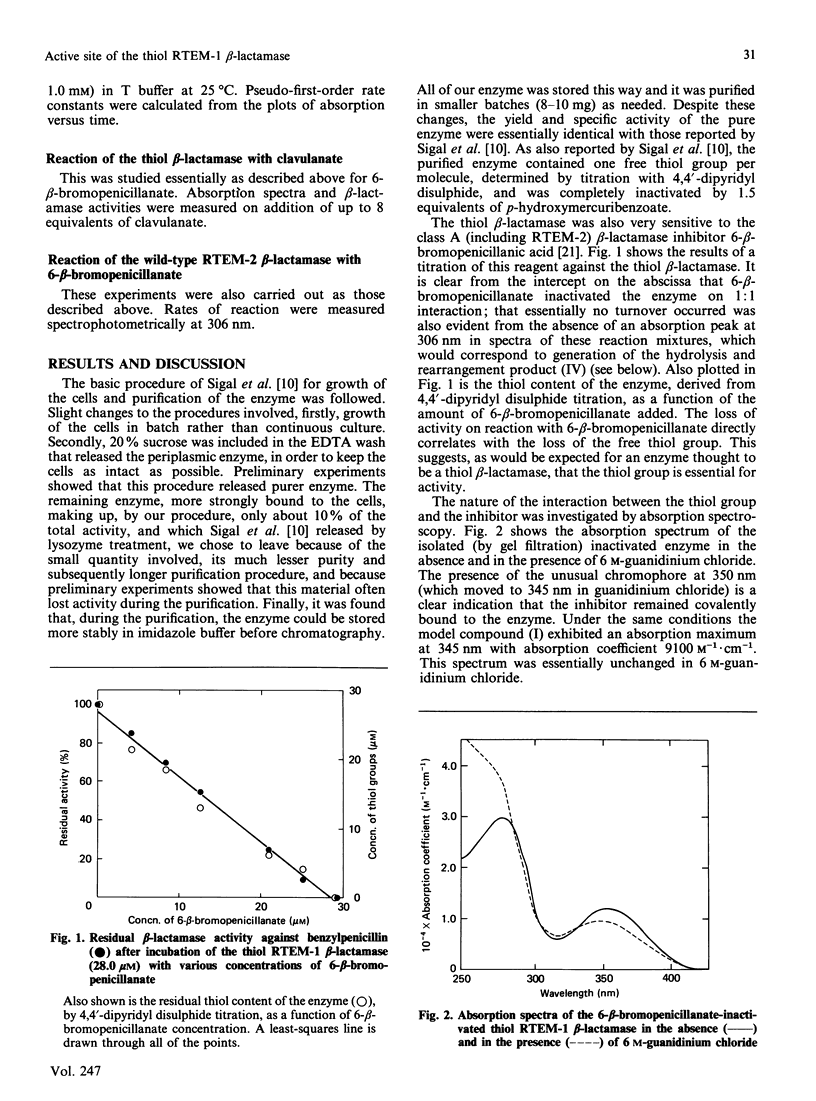
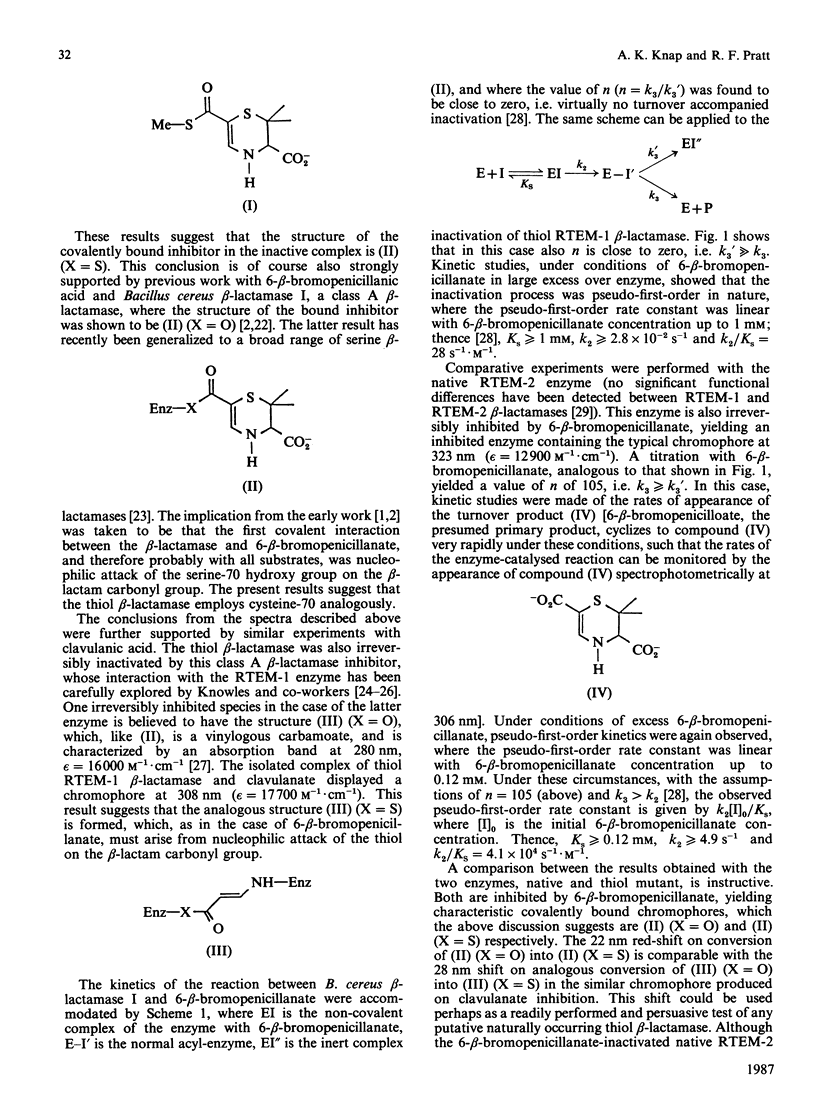
The thiol RTEM-1 beta-lactamase [Sigal, Harwood & Arentzen (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 7157-7160] is inactivated by 6-beta-bromopenicillanic acid with formation of a characteristic chromophore, absorbing maximally at 350 nm, which is covalently bound to the enzyme. Model studies suggest that the chromophore is that of a 6-carboxylate thiol ester of 2,3-dihydro-2,2-dimethyl-1,4-thiazine-3,6-dicarboxylate, which can arise by rearrangement of the thiol-penicilloate obtained by thiolysis of the beta-lactam of 6-beta-bromopenicillanate. Loss of activity of the enzyme is also concerted with disappearance of its single (cysteine) thiol group. These results indicate that the thiol group of this enzyme is indeed a nucleophilic catalyst in beta-lactam turnover. The thiol beta-lactamase is also inactivated by clavulanic acid with formation of a chromophore, presumably a 3-aminoacrylate thiol ester, at 308 nm. Both 6-beta-bromopenicillanate and clavulanate are hydrolysed more slowly by the thiol enzyme than by the native serine beta-lactamase, but, probably as a consequence of this, both compounds inactivate the former enzyme more efficiently. Cefoxitin, a poor substrate of the native enzyme, does not appear to interact covalently with the thiol beta-lactamase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: nature of irreversible inactivation of RTEM beta-lactamase from Escherichia coli. Biochemistry. 1984 Nov 20;23(24):5833–5839. doi: 10.1021/bi00319a024. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence that the lack of high catalytic activity of thiolsubtilisin towards specific substrates may be due to an inappropriately located proton-distribution system. Demonstration of highly nucleophilic character of the thiol group of thiolsubtilisin in the catalytically relevant ionization state of the active centre by use of a two-protonic-state reactivity probe. Biochem J. 1981 Mar 1;193(3):819–823. doi: 10.1042/bj1930819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnas R. L., Knowles J. R. Inactivation of RTEM beta-lactamase from Escherichia coli by clavulanic acid and 9-deoxyclavulanic acid. Biochemistry. 1981 May 26;20(11):3214–3219. doi: 10.1021/bi00514a035. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Pratt R. F. Inactivation of Bacillus cereus beta-lactamase I by 6 beta-bromopencillanic acid: mechanism. Biochemistry. 1980 Aug 19;19(17):3996–4003. doi: 10.1021/bi00558a017. [DOI] [PubMed] [Google Scholar]
- De Meester F., Frère J. M., Waley S. G., Cartwright S. J., Virden R., Lindberg F. 6-beta-Iodopenicillanate as a probe for the classification of beta-lactamases. Biochem J. 1986 Nov 1;239(3):575–580. doi: 10.1042/bj2390575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Hardy L. W., Kirsch J. F. Diffusion-limited component of reactions catalyzed by Bacillus cereus beta-lactamase I. Biochemistry. 1984 Mar;23(6):1275–1282. doi: 10.1021/bi00301a040. [DOI] [PubMed] [Google Scholar]
- Joris B., Dusart J., Frere J. M., van Beeumen J., Emanuel E. L., Petursson S., Gagnon J., Waley S. G. The active site of the P99 beta-lactamase from Enterobacter cloacae. Biochem J. 1984 Oct 1;223(1):271–274. doi: 10.1042/bj2230271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The acyl-enzyme mechanism of beta-lactamase action. The evidence for class C Beta-lactamases. Biochem J. 1982 Nov 1;207(2):315–322. doi: 10.1042/bj2070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Loosemore M. J., Cohen S. A., Pratt R. F. Inactivation of Bacillus cereus beta-lactamase I by 6 beta-bromopenicillanic acid: kinetics. Biochemistry. 1980 Aug 19;19(17):3990–3995. doi: 10.1021/bi00558a016. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Nanci A., Koshland D. E., Jr Properties of thiol-subtilisin. The consequences of converting the active serine residue to cysteine in a serine protease. J Biol Chem. 1968 Dec 25;243(24):6392–6401. [PubMed] [Google Scholar]
- Philipp M., Tsai I. H., Bender M. L. Comparison of the kinetic specificity of subtilisin and thiolsubtilisin toward n-alkyl p-nitrophenyl esters. Biochemistry. 1979 Aug 21;18(17):3769–3773. doi: 10.1021/bi00584a020. [DOI] [PubMed] [Google Scholar]
- Polgár L., Bender M. L. Simulated mutation at the active site of biologically active proteins. Adv Enzymol Relat Areas Mol Biol. 1970;33:381–400. doi: 10.1002/9780470122785.ch8. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Loosemore M. J. 6-beta-bromopenicillanic acid, a potent beta-lactamase inhibitor. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4145–4149. doi: 10.1073/pnas.75.9.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., DeGrado W. F., Thomas B. J., Petteway S. R., Jr Purification and properties of thiol beta-lactamase. A mutant of pBR322 beta-lactamase in which the active site serine has been replaced with cysteine. J Biol Chem. 1984 Apr 25;259(8):5327–5332. [PubMed] [Google Scholar]
- Sigal I. S., Harwood B. G., Arentzen R. Thiol-beta-lactamase: replacement of the active-site serine of RTEM beta-lactamase by a cysteine residue. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7157–7160. doi: 10.1073/pnas.79.23.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai I. H., Bender M. L. Conformation of the active site of thiolsubtilisin: reaction with specific chloromethyl ketones and arylacryloylimidazoles. Biochemistry. 1979 Aug 21;18(17):3764–3768. doi: 10.1021/bi00584a019. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Ojima S., Ishii S. Thioltrypsin. Chemical transformation of the active-site serine residue of Streptomyces griseus trypsin to a cysteine residue. J Biochem. 1977 Sep;82(3):869–876. doi: 10.1093/oxfordjournals.jbchem.a131763. [DOI] [PubMed] [Google Scholar]


