Abstract
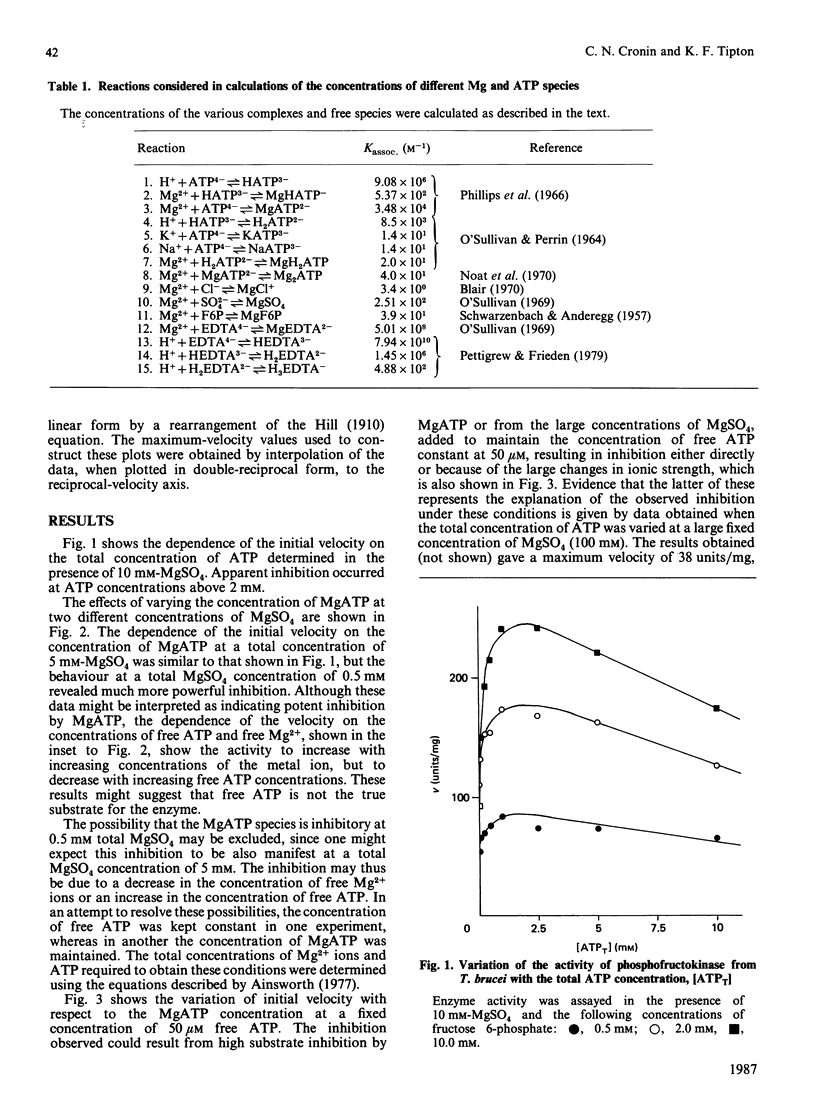
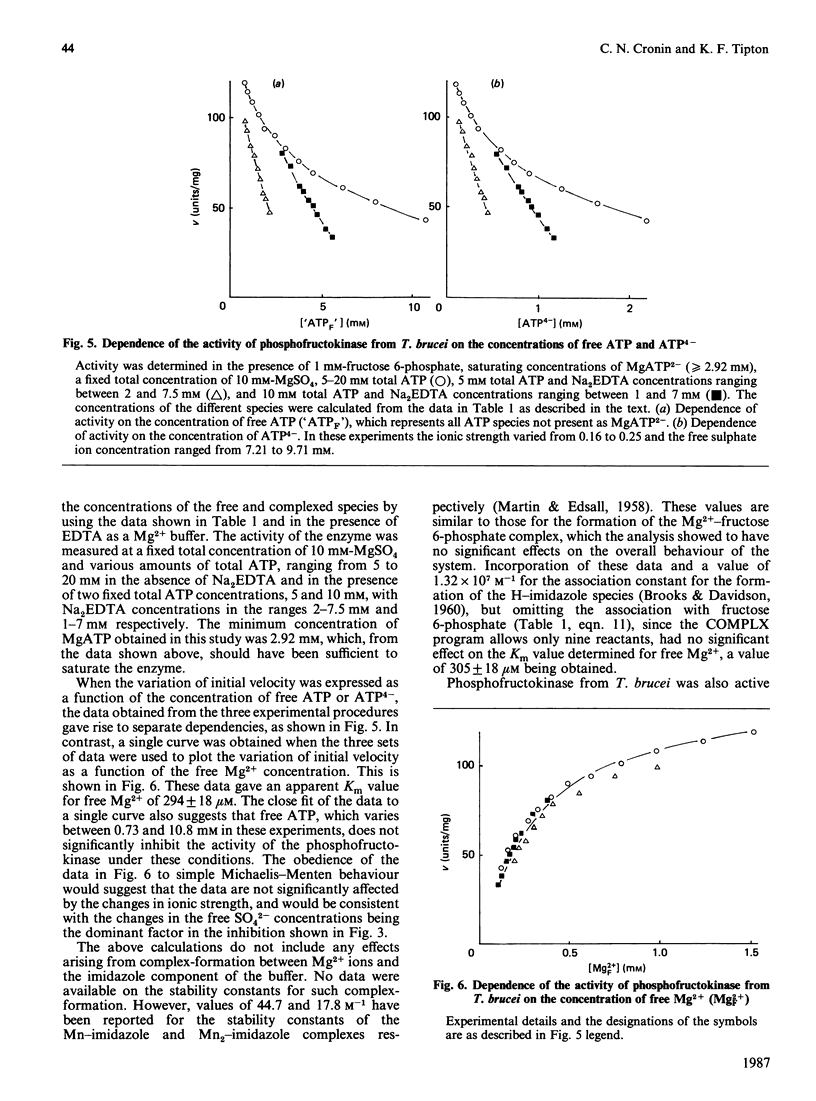
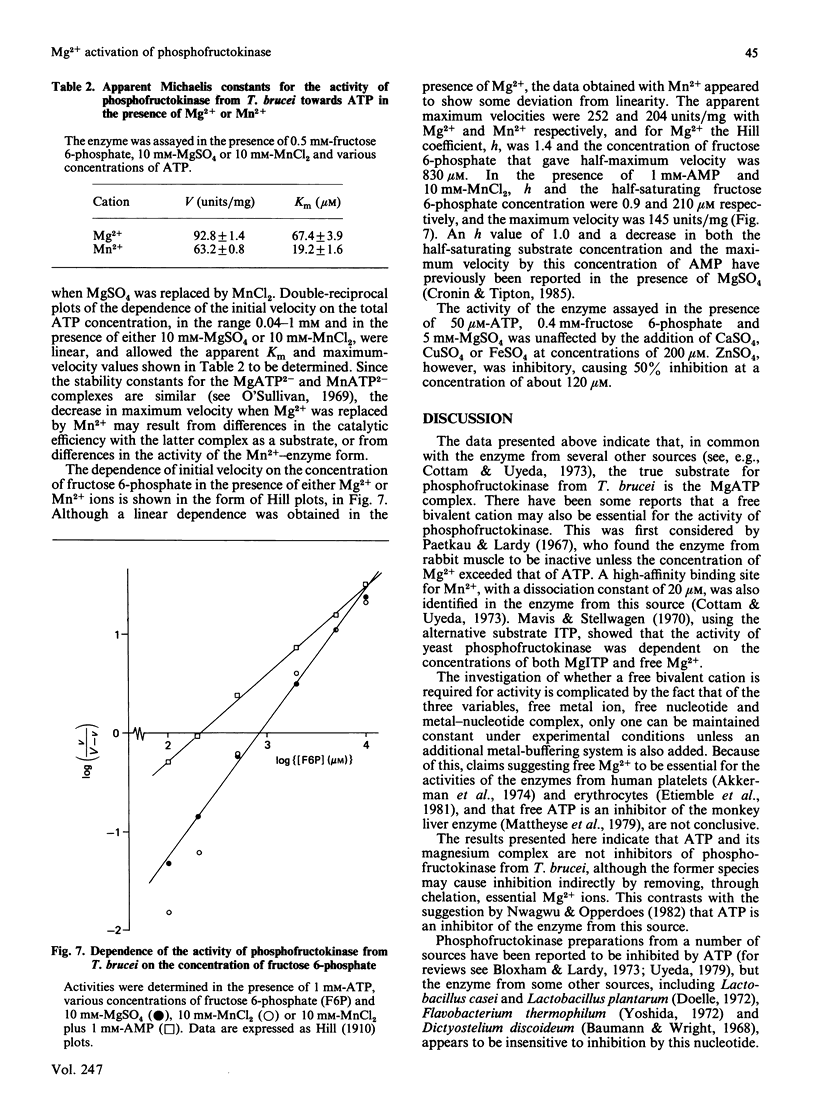
The involvement of Mg2+ ions in the reaction catalysed by phosphofructokinase from Trypanosoma brucei was studied. The true substrate for the enzyme was shown to be the MgATP2-complex, and free Mg2+ ions are also required for enzyme activity. At concentrations of MgATP2- of 2.92 mM and greater, and a fructose 6-phosphate concentration of 1 mM and in the presence of EDTA as a Mg2+ buffer, the Km value for Mg2+ was determined to be 294 +/- 18 microM. Neither MgATP nor free ATP is an inhibitor of the enzyme, although apparent inhibition by the latter can be observed as a consequence of the decrease in free Mg2+ by chelation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkerman J. W., Gorter G., Sixma J. J., Staal G. E. Influence of Mg2. Biochim Biophys Acta. 1974 Nov 25;370(1):113–119. doi: 10.1016/0005-2744(74)90037-0. [DOI] [PubMed] [Google Scholar]
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Wright B. E. The phosphofructokinase of Dictyostelium discoideum. Biochemistry. 1968 Oct;7(10):3653–3661. doi: 10.1021/bi00850a044. [DOI] [PubMed] [Google Scholar]
- Blair J. M. Magnesium, potassium, and the adenylate kinase equilibrium. Magnesium as a feedback signal from the adenine nucleotide pool. Eur J Biochem. 1970 Apr;13(2):384–390. doi: 10.1111/j.1432-1033.1970.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brohn F. H., Clarkson A. B., Jr Quantitative effects of salycylhydroxamic acid and glycerol on Trypanosoma brucei glycolysis in vitro and in vivo. Acta Trop. 1978 Mar;35(1):23–33. [PubMed] [Google Scholar]
- Brohn F. H., Clarkson A. B., Jr Trypanosoma brucei brucei: patterns of glycolysis at 37 degrees C in vitro. Mol Biochem Parasitol. 1980 Sep;1(5):291–305. doi: 10.1016/0166-6851(80)90062-6. [DOI] [PubMed] [Google Scholar]
- COHN M. MAGNETIC RESONANCE STUDIES OF METAL ACTIVATION OF ENZYMIC REACTIONS OF NUCLEOTIDES AND OTHER PHOSPHATE SUBSTRATES. Biochemistry. 1963 Jul-Aug;2:623–629. doi: 10.1021/bi00904a001. [DOI] [PubMed] [Google Scholar]
- Cottam G. L., Uyeda K. Manganese substrate complexes of phosphofructokinase studies by pulsed magnetic resonance. Arch Biochem Biophys. 1973 Feb;154(2):683–690. doi: 10.1016/0003-9861(73)90023-4. [DOI] [PubMed] [Google Scholar]
- Cronin C. N., Tipton K. F. Kinetic studies on the reaction catalysed by phosphofructokinase from Trypanosoma brucei. Biochem J. 1987 Jul 1;245(1):13–18. doi: 10.1042/bj2450013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin C. N., Tipton K. F. Purification and regulatory properties of phosphofructokinase from Trypanosoma (Trypanozoon) brucei brucei. Biochem J. 1985 Apr 1;227(1):113–124. doi: 10.1042/bj2270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelle H. W. Kinetic characteristics of phosphofructokinase from Lactobacillus casei var. Rhamnosus ATCC 7469 and Lactobacillus plantarum ATCC 14917. Biochim Biophys Acta. 1972 Feb 28;258(2):404–410. doi: 10.1016/0005-2744(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Etiemble J., Simeon J., Picat C., Boivin P. Influence of free Mg2+ on the kinetics of human erythrocyte phosphofructokinase. Biochimie. 1981 Jan;63(1):61–65. doi: 10.1016/s0300-9084(81)80147-2. [DOI] [PubMed] [Google Scholar]
- Hickman P. E., Weidemann M. J. The purification and properties of pig spleen phosphofructokinase. Biochem J. 1975 Nov;151(2):327–336. doi: 10.1042/bj1510327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey M. M., Chatterjee T., Cohen A. J., Stein A. L., Kemp R. G., Marcus F. Increased ATP inhibition of liver phosphofructokinase from genetically diabetic mice. Proc Natl Acad Sci U S A. 1980 May;77(5):2497–2499. doi: 10.1073/pnas.77.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheyse M. E., Cayanis E., Balinsky D. Purification and properties of monkey liver phosphofructokinase. Int J Biochem. 1979;10(4):295–302. doi: 10.1016/0020-711x(79)90093-4. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Stellwagen E. The role of cations in yeast phosphofructokinase catalysis. J Biol Chem. 1970 Feb 25;245(4):674–680. [PubMed] [Google Scholar]
- Meienhofer M. C., Cottreau D., Dreyfus J. C., Kahn A. Kinetic properties of human F4 phosphofructokinase: a poor regulatory enzyme. FEBS Lett. 1980 Feb 11;110(2):219–222. doi: 10.1016/0014-5793(80)80077-9. [DOI] [PubMed] [Google Scholar]
- Noat G., Ricard J., Borel M., Got C. Kinetic study of yeast hexokinase. Inhibition of the reaction by magnesium and ATP. Eur J Biochem. 1970 Apr;13(2):347–363. doi: 10.1111/j.1432-1033.1970.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Nwagwu M., Opperdoes F. R. Regulation of glycolysis in Trypanosoma brucei: hexokinase and phosphofructokinase activity. Acta Trop. 1982 Mar;39(1):61–72. [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Oduro K. K., Bowman I. B., Flynn I. W. Trypanosoma brucei: preparation and some properties of a multienzyme complex catalysing part of the glycolytic pathway. Exp Parasitol. 1980 Oct;50(2):240–250. doi: 10.1016/0014-4894(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Oduro K. K., Flynn I. W., Bowman I. B. Trypanosoma brucei: activities and subcellular distribution of glycolytic enzymes from differently disrupted cells. Exp Parasitol. 1980 Aug;50(1):123–135. doi: 10.1016/0014-4894(80)90014-4. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., Fonck K. The potential use of inhibitors of glycerol-3-phosphate oxidase for chemotherapy of African trypanosomiasis. FEBS Lett. 1976 Feb 15;62(2):169–172. doi: 10.1016/0014-5793(76)80045-2. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977 Aug 15;80(2):360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- Paetkau V., Lardy H. A. Phosphofructokinase. Correlation of physical and enzymatic properties. J Biol Chem. 1967 May 10;242(9):2035–2042. [PubMed] [Google Scholar]
- Pettigrew D. W., Frieden C. Binding of regulatory ligands to rabbit muscle phosphofructokinase. A model for nucleotide binding as a function of temperature and pH. J Biol Chem. 1979 Mar 25;254(6):1887–1895. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Visser N., Opperdoes F. R. Glycolysis in Trypanosoma brucei. Eur J Biochem. 1980 Feb;103(3):623–632. doi: 10.1111/j.1432-1033.1980.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Voorheis H. P., Gale J. S., Owen M. J., Edwards W. The isolation and partial characterization of the plasma membrane from Trypanosoma brucei. Biochem J. 1979 Apr 15;180(1):11–24. doi: 10.1042/bj1800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Allosteric nature of thermostable phosphofructokinase from an extreme thermophilic bacterium. Biochemistry. 1972 Mar 14;11(6):1087–1093. doi: 10.1021/bi00756a022. [DOI] [PubMed] [Google Scholar]


