Abstract
Esophageal dysphagia is most commonly caused by motility disorders and intrinsic mechanical obstruction. However, extrinsic obstruction, such as pericardial effusion, is rare causes of dysphagia. We present an 89-year-old male with history of Waldenstrom macroglobulinemia, Charcot-Marie-Tooth syndrome, and basal cell carcinoma presenting with generalized weakness, productive cough, shortness of breath, and dysphagia to both solids and liquids. A chest X-ray obtained showed cardiomegaly with suggested central vascular congestion and pulmonary edema. Further imaging with computed tomography (CT) abdomen and pelvis showed a moderate-to-large pericardial effusion. Patient later developed signs and symptoms of cardiac tamponade, requiring urgent pericardiocentesis with removal of 1 L of sanguineous fluid. Up to today, only 6 cases of dysphagia due to pericardial effusion have been described. This case displays another rare case and highlights the importance of recognizing dysphagia as a critical symptom as well as non-gastrointestinal (GI) causes of dysphagia.
Keywords: pericardial effusion, dysphagia, cardiac tamponade, echocardiography, case report
Introduction
Pericardial effusion is a serious cardiac condition that involves the accumulation of fluid in the pericardial sac that surmounts the normal amount of 15 to 50 mL of fluid. 1 The most common causes include acute pericarditis, autoimmune disease, trauma, postmyocardial infarction, or cardiac surgery, malignancy, radiation, and uremia.1-3 The accumulation of fluid may be rapid, resulting in an acute presentation, or more gradual, resulting in a subacute or chronic presentation.1,2 Signs and symptoms are non-specific. However, symptoms may include dyspnea, hoarseness, coughing, chest pain, and gastrointestinal symptoms via stimulation of the Vagus nerve and diaphragm.1,4 Once intrapericardial pressure rises to a level that is severe enough to impair chamber filling, cardiac tamponade ensues, with signs and symptoms including jugular vein distention (JVD), sinus tachycardia, hypotension, pulsus paradoxus, low voltage QRS on electrocardiogram (EKG), and muffled heart sounds.1,4,5 In this report, we present a case of subacute pericardial effusion and highlight dysphagia as an acute critical finding.
Case Presentation
An 89-year-old male with history of Waldenstrom macroglobulinemia, Charcot-Marie-Tooth syndrome, basal cell carcinoma, and hypertension (HTN) presented to the emergency department (ED) with complaints of general weakness and shortness of breath with productive cough of green phlegm that started about 3 weeks ago. Patient also reported orthopnea, dyspnea on exertion, and paroxysmal nocturnal dyspnea. His granddaughter stated that his clinical symptoms improved after 2 weeks, but started to worsen again 1 week later. Patient visited his oncologist, who prescribed Levofloxacin for upper respiratory infection. Since then, patient has not been able to eat or drink, reporting intermittent coughing with liquids and emesis with any oral intake. Denied any fever, chills, chest pain, headache, nausea, or abdominal pain.
On presentation to the ED, patient had a temperature of 98.1°F, heart rate 90 beats/minute, respiratory rate 18 breaths/minute, blood pressure (BP) of 140/65 mm Hg, and oxygen saturation of 99% on room air. Physical examination was remarkable for 2+ pitting edema of the bilateral lower extremities, swelling over the bilateral flanks, and ecchymosis of the upper extremities. Cardiovascular examination revealed an irregular rhythm but no murmurs, rubs, distant heart sounds, JVD, or pulsus paradoxus.
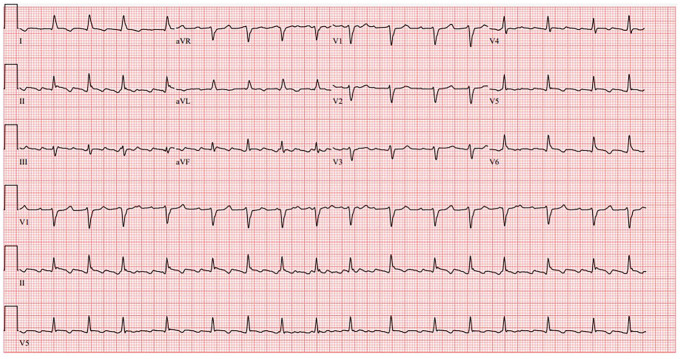
Initial laboratory values were remarkable for a white blood cell count of 7.9 × 103/µL (baseline 3-4 × 103/µL), hemoglobin 9.7 g/dL, platelets 130 × 103/µL, K 5.9 mmol/L, blood urea nitrogen (BUN) 60 mg/dL, Cr 3.62 mg/dL (baseline 2.0 mg/dL), HCO3 18 mmol/L, lactic acid 3.2 mmol/L, and albumin 2.4 g/dL. The EKG obtained (Figure 1) was significant for atrial flutter with variable AV block but not low-voltage QRS or electrical alternans. Chest X-ray (Figure 2) showed cardiomegaly with suggested central venous congestion, pulmonary edema, and possible small effusions. Computed tomography of the abdomen and pelvis without contrast (Figure 3) showed moderate-to-large pericardial effusion, small-to-moderate bilateral pleural effusions, mild diffuse increased soft tissue density throughout the mesentery, and prominent lymph nodes. While in the ED, patient was given calcium gluconate 1 g and insulin 5 units for his hyperkalemia, as well as bicarbonate 50 mEq push and 50 mL D50W for his acute kidney injury. Cardiology was consulted for further work-up and possible intervention.
Figure 1.
Electrocardiogram showing atrial flutter with variable AV block. Does not meet criteria for low voltage QRS. No electrical alternans.
Figure 2.
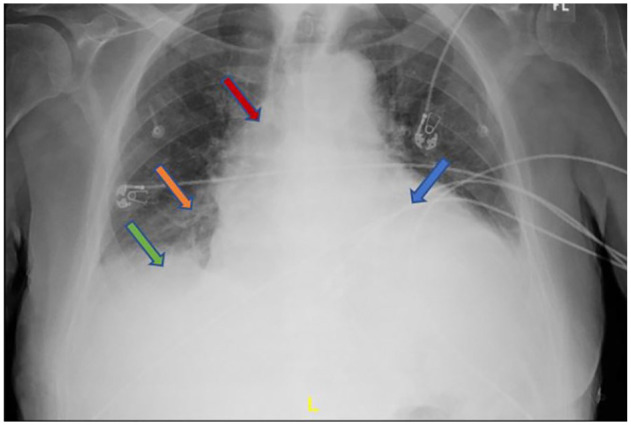
Chest X-ray showing cardiomegaly (blue arrow) with central venous congestion (red arrow), pulmonary edema (orange arrow), and pleural effusion (green arrow).
Figure 3.
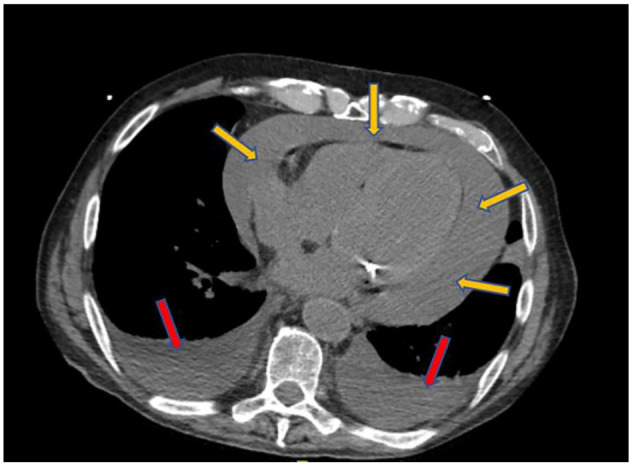
Computed tomography of the abdomen and pelvis without contrast showing a moderate-to-large pericardial effusion (yellow arrows) and small-to-moderate bilateral pleural effusions (red arrows).
A transthoracic echocardiogram obtained showed a normal ejection fraction at 60% and confirmed a large pericardial effusion without tamponade along with pleural effusion. The day after admission, the patient became hypotensive with mean arterial pressures (MAPs) in the high 50s. Physical examination also revealed distant heart sounds and decreased breath sounds with crackles, with concern for tamponade. Urgent pericardiocentesis was performed with drainage of 1 L of hemorrhagic fluid and sent for cytology. Patient’s hypotension immediately resolved. His dyspnea and weakness improved the following day. Patient no longer complained of dysphagia and was able to tolerate oral feeds. Cytology was negative for any neoplastic cells. A repeat echocardiogram performed several days after pericardiocentesis showed mild residual pericardial effusion. Patient was discharged in stable condition and on appropriate medications.
Discussion
Pericardial effusions can be seen in all ages and populations. The most common causes are infection, causing 16% to 27%, malignancy, causing 13% to 23%, and iatrogenic, causing 13% to 18%. 2 Within the developed world, viral pericarditis is the leading cause of effusion, whereas in developing areas, it is Mycobacterium tuberculosis. 3 Of malignancies, lymphoma and breast are the leading causes. 2 In the acute setting, only 100 to 150 mL of fluid is needed to cause tamponade. On the contrary, up to 1 to 2 L of fluid can accumulate in the chronic setting before tamponade ensues as the heart has adequate time to accommodate the increase in volume. In both cases, once the fluid level reaches a point that causes compression of the heart and impairs filling, tamponade develops with a progressive decline in cardiac output and blood pressure.1,3,4 Symptoms of pericardial effusion are non-specific, but include dyspnea (due to lung compression and atelectasis), hoarseness (due to compression of the recurrent laryngeal nerve), coughing, chest pain, and dysphagia (from esophageal compression).4,5 Of those with cardiac tamponade, only a small amount present with the classic Beck’s triad. 3 Other signs and symptoms of tamponade include low-voltage QRS complexes, electrical alternans, and pulsus paradoxus. 2
In our case, the patient presented with a subacute pericardial effusion of about 1 L with several non-specific symptoms including dyspnea, cough, and dysphagia that eventually led to tamponade associated with distant heart sounds, decreased breath sounds, and persistent hypotension. It is most likely that our patient’s effusion developed over the span of several weeks considering his complaints of dyspnea, weakness that began 3 weeks prior. Owing to the gradual progression of the effusion, the patient’s heart was able to accommodate the slow accumulation of fluid without compromise until the maximum level of fluid resulted in compression and decompensation.
The study of choice for pericardial effusion is an echocardiogram, either transthoracic or transesophageal, as it establishes the diagnosis and guides management.3,4 It allows for evaluation for the size of the effusion and whether tamponade is present.1,3,4 It appears as an anechoic area surrounding the heart.3,4 The size of the fluid in diastole is used to grade the effusion. 3 In our case, the echocardiogram was able to identify a moderate-to-large effusion.
The treatment for pericardial effusion depends on its size and etiology. It may include watching for small effusions without hemodynamic compromise with sequential echocardiograms to diagnostic and therapeutic pericardiocentesis for moderate-to-large effusions or symptomatic patients. Emergent treatment is necessary for patients presenting with hemodynamic instability.1,3 In our case, our patient presented with symptoms and a moderate-to-large effusion. However, due to development of instability, urgent pericardiocentesis was performed, with removal of about 1 L of hemorrhagic fluid. Cytology was negative for any malignant cells.
Although pericardial effusion is a known cause esophageal dysphagia, it is rare.6,7 Prior reports of dysphagia due to left atrium enlargement and mediastinal masses have been documented; however, only a few due to pericardial effusion have thus far been.4,7 Among individuals greater than 50 years of age, the estimated prevalence of dysphagia is 16% to 22%. 7 Causes of esophageal dysphagia can be broken down into 2 categories, which include motility dysfunction or mechanical obstruction. Of the causes of mechanical obstruction, intrinsic lesions such as strictures, masses, rings, or webs are the most common caused.7,8 Our patient presented with a week of dysphagia associated with intermittent coughing with liquids and emesis with any oral intake.
In the case that we present, the acute onset of dysphagia highlights the importance of recognizing dysphagia as a critical symptom of pericardial effusion. Although a non-specific symptom, recognizing its presence may aid identifying the size of the effusion prior to echocardiogram, as well as guide management of the effusion. However, we cannot definitively say this as only a handful of cases has been reported. Furthermore, it emphasizes that even though a small percentage of dysphagia are non–GI-related, they are crucial causes that should not be missed. For this reason, one must obtain a detailed history and characterize the symptoms to identify the cause.
Conclusion
Pericardial effusion is a rare but serious cause of acute dysphagia. In our patient, dysphagia developed 1 week prior to presentation, with the eventual development of tamponade and resolution of symptoms after pericardiocentesis. Given this presentation, the presence of dysphagia in those with pericardial effusion may warn of a large effusion and the potential for development of cardiac tamponade.
Acknowledgments
The views expressed in this publication represent those of the authors and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Prior Presentation of Abstract Statement: Presented at 2024 Western Medical Research Conference on January 19, 2024.
ORCID iD: Ariel Ahl  https://orcid.org/0009-0005-3247-5574
https://orcid.org/0009-0005-3247-5574
References
- 1. Hoit BD. Diagnosis and Treatment of Pericardial Effusion. UpToDate; 2022. Accessed August 23, 2023. https://www.uptodate.com/contents/diagnosis-and-treatment-of-pericardial-effusion?search=pericardial%20effusion&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H4463173 [Google Scholar]
- 2. Som R, Li A, McIntosh RP, et al. Acute dyspnoea, dysphagia, and non-specific chest pain in a smoker. BMJ. 2008;337: a2678. [DOI] [PubMed] [Google Scholar]
- 3. Willner DA. Pericardial effusion. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482365/ [Google Scholar]
- 4. Chauhan A, Khaja MS, Chauhan VK, et al. An unusual cause of dysphagia: pericardial effusion after implantable cardioverter-defibrillator placement. J Emerg Med. 2012;43(6): e405-e408. [DOI] [PubMed] [Google Scholar]
- 5. Hoit B. Etiology of Pericardial Disease. UpToDate; 2022. Accessed August 24, 2023. https://www.uptodate.com/contents/etiology-of-pericardial-disease?search=pericardial%20effusion&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2#H2 [Google Scholar]
- 6. Kadaba VB, Lattanzio N, Rahmany Z, et al. Dysphagia: the primary indicator of esophageal compression by a pericardial effusion. Am J Gastroenterol. 2018;113(suppl):S1037. [Google Scholar]
- 7. Vats HS. Dysphagia from extrinsic compression of esophagus by pericardial effusion. Clin Med Res. 2008;6(2):78-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fass R. Approach to the Evaluation of Dysphagia in Adults. UpToDate; 2022. Accessed August 23, 2023. https://www.uptodate.com/contents/approach-to-the-evaluation-of-dysphagia-in-adults?search=dysphagia%20&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H802823703 [Google Scholar]