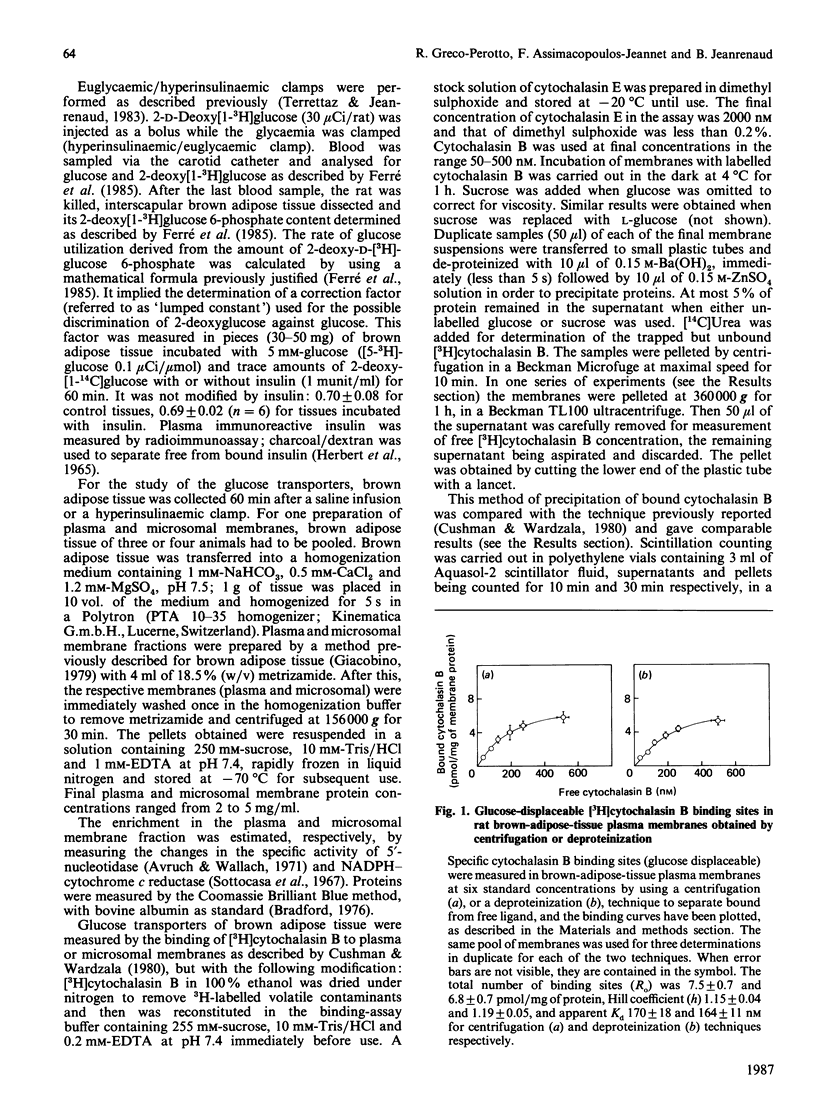
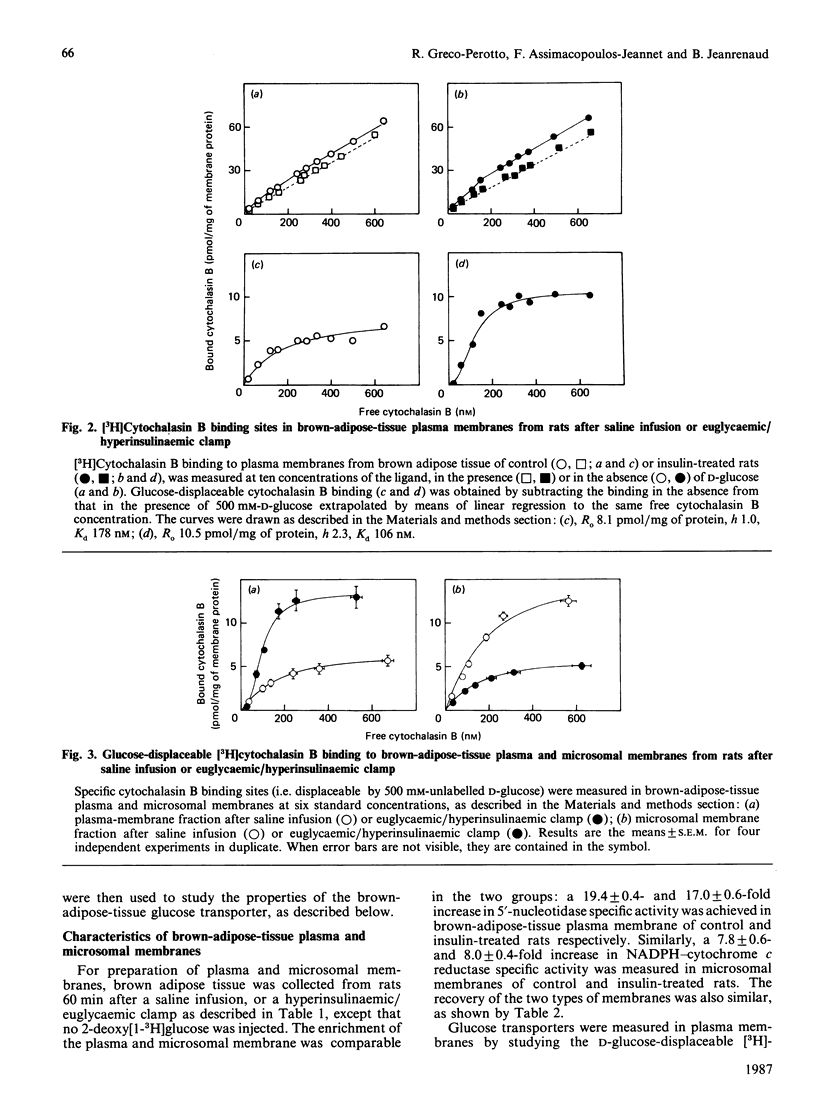
Abstract
The properties of glucose transporters associated with plasma and microsomal membranes have been studied in brown adipose tissue of rats after treatment by saline infusion or hyperinsulinaemic/euglycaemic clamp. In this tissue, insulin produces a 40-fold increase in glucose utilization as measured by the 2-deoxy-D-glucose technique, and therefore a 40-fold increase in the rate-limiting glucose transport. This increase, promoted by insulin, is associated with: (a) translocation of the transporters from a pool associated with the microsomal fraction to the plasma membrane without modification of the total number of transporters; (b) an increase in the Hill coefficient of the plasma-membrane glucose transporters for cytochalasin B from 1.1 to 2.5, indicating the presence of positive co-operativity; (c) a decrease in the Kd (apparent dissociation constant) of the transporters towards cytochalasin B from 148 to 82 nM; (d) no change in the Hill coefficient or Kd for the transporters associated with the microsomal membranes. These data indicate that, in addition to causing translocation of the glucose transporters, insulin modifies their properties and behaviour towards cytochalasin B. This may reflect modifications in their properties and behaviour towards glucose, and by this contribute to bringing about the marked effect of this hormone on glucose transport in brown adipose tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Newsholme E. A. The maximum capacity of glycolysis in brown adipose tissue and its relationship to control of the blood glucose concentration. FEBS Lett. 1982 Nov 8;148(2):198–200. doi: 10.1016/0014-5793(82)80807-7. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- Ezaki O., Kono T. Effects of temperature on basal and insulin-stimulated glucose transport activities in fat cells. Further support for the translocation hypothesis of insulin action. J Biol Chem. 1982 Dec 10;257(23):14306–14310. [PubMed] [Google Scholar]
- Ferré P., Burnol A. F., Leturque A., Terretaz J., Penicaud L., Jeanrenaud B., Girard J. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochem J. 1986 Jan 1;233(1):249–252. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobino J. P. Subcellular fractionation of brown adipose tissue. J Supramol Struct. 1979;11(4):445–449. doi: 10.1002/jss.400110403. [DOI] [PubMed] [Google Scholar]
- Greco-Perotto R., Zaninetti D., Assimacopoulos-Jeannet F., Bobbioni E., Jeanrenaud B. Stimulatory effect of cold adaptation on glucose utilization by brown adipose tissue. Relationship with changes in the glucose transporter system. J Biol Chem. 1987 Jun 5;262(16):7732–7736. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hill A. V. A new mathematical treatment of changes of ionic concentration in muscle and nerve under the action of electric currents, with a theory as to their mode of excitation. J Physiol. 1910 May 11;40(3):190–224. doi: 10.1113/jphysiol.1910.sp001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Cellular thermogenesis. Annu Rev Physiol. 1976;38:315–351. doi: 10.1146/annurev.ph.38.030176.001531. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Kuhn C. E., Sauerheber R. D. Insulin stimulation of glucose transport in isolated rat adipocytes. Functional evidence for insulin activation of intrinsic transporter activity within the plasma membrane. Biochem J. 1985 Nov 15;232(1):245–254. doi: 10.1042/bj2320245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab Rev. 1985;1(3):203–227. doi: 10.1002/dmr.5610010301. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. Biochem J. 1977 Sep 15;166(3):627–630. doi: 10.1042/bj1660627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. The regulation of fatty acid synthesis in brown adipose tissue by insulin. Prog Lipid Res. 1982;21(3):195–223. doi: 10.1016/0163-7827(82)90009-1. [DOI] [PubMed] [Google Scholar]
- Oka Y., Czech M. P. Photoaffinity labeling of insulin-sensitive hexose transporters in intact rat adipocytes. Direct evidence that latent transporters become exposed to the extracellular space in response to insulin. J Biol Chem. 1984 Jul 10;259(13):8125–8133. [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Marshall C. E., McCormack J. G. Brown-adipose-tissue lipogenesis in starvation: effects of insulin and (-) hydroxycitrate. Biosci Rep. 1982 May;2(5):289–297. doi: 10.1007/BF01115114. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrettaz J., Jeanrenaud B. In vivo hepatic and peripheral insulin resistance in genetically obese (fa/fa) rats. Endocrinology. 1983 Apr;112(4):1346–1351. doi: 10.1210/endo-112-4-1346. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Identification of the D-glucose-inhibitable cytochalasin B binding site as the glucose transporter in rat diaphragm plasma and microsomal membranes. Biochim Biophys Acta. 1983 Apr 21;730(1):49–56. doi: 10.1016/0005-2736(83)90315-2. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Young P., Cawthorne M. A., Levy A. L., Wilson K. Reduced maximum capacity of glycolysis in brown adipose tissue of genetically obese, diabetic (db/db) mice and its restoration following treatment with a thermogenic beta-adrenoceptor agonist. FEBS Lett. 1984 Oct 15;176(1):16–20. doi: 10.1016/0014-5793(84)80903-5. [DOI] [PubMed] [Google Scholar]


