Abstract
Objectives:
To describe the current state of non-intensive care unit (ICU) high flow nasal cannula (HFNC) protocols at children’s hospitals and explore associations between HFNC protocol type and utilization outcomes.
Methods:
We performed a cross-sectional study of the Pediatric Health Information Systems (PHIS) database. First, we designed a survey with the purpose of classifying HFNC protocols used at hospitals currently contributing data to PHIS. Next, we categorized hospitals based on their current HFNC protocol (ICU only, age-based non-ICU, or weight-based non-ICU). Finally, using the PHIS database, we compared hospital characteristics and patient-level bronchiolitis outcomes by HFNC protocol group.
Results:
We received survey responses from 36/44 (82%) hospitals contributing data to PHIS in 2021. During the time period studied, there was a steady increase in adoption of non-ICU HFNC protocols, with 71% of responding children’s hospitals reporting non-ICU HFNC protocols in 2021 compared to 11% prior to 2010. No differences in hospital characteristics were observed between ICU-only hospitals, age-based hospitals, or weight-based hospitals. Age-based hospitals had the highest proportion of bronchiolitis patients treated in the ICU (36.1%), while weight-based hospitals had the lowest proportion of patients treated in the ICU (21.0%, p<.001). Length of stay was longer at age-based hospitals (2.9 days) as compared to weight-based and ICU-only hospitals (1.9 days, p<.001).
Conclusion:
Most children’s hospitals have adopted non-ICU HFNC protocols for patients with bronchiolitis, the majority of which are now utilizing weight-based maximum flow rates. Weight-based HFNC protocols were associated with decreased ICU utilization as compared to age-based HFNC protocols.
Introduction:
The use of high flow nasal cannula (HFNC) as a respiratory support modality in children with bronchiolitis has risen dramatically.1–4 Though the full extent of its use has not been characterized, two single-institution studies found year-over-year increases in HFNC use in patients with bronchiolitis since HFNC adoption, with one site using it in up to 70% of hospitalized patients with bronchiolitis in 2017.3,5–8 Furthermore, the protocolized use of HFNC in non-intensive care unit (ICU) settings has become common, with one survey conducted in 2017 finding that 37 of 77 (48%) responding sites used HFNC on the pediatric wards.2
Early protocols for non-ICU use of HFNC utilized age-based (e.g. 8 liters of flow for children under 12 months of age) flow rates and have been associated with increased ICU utilization.1,9 More recently, there has been a shift toward the use of weight-based (e.g. 2 liters of flow per kilogram of body weight) flow rates, which have been studied in several recent randomized trials. However, these trials have not demonstrated that weight-based HFNC decreases ICU utilization or hospital length of stay (LOS).11,12
The extent to which weight-based flow rates have since been adopted in non-ICU HFNC protocols and whether they offer clinical benefits over age-based protocols has not been fully characterized. Our primary objective was to describe the current state of non-ICU HFNC protocols at children’s hospitals. Our secondary objective was to explore associations between HFNC protocol type and utilization outcomes.
Methods:
Design
We conducted a cross-sectional database study. We augmented our database study with a brief survey to obtain key details not otherwise available in the database.
Survey
We designed a survey with the purpose of classifying HFNC protocol use at all hospitals contributing to the Pediatric Health Information Systems (PHIS) database in 2021. We piloted the survey by e-mailing contacts at 4 hospitals and made changes to the survey based on feedback. Contacts at each hospital were identified from 2 sources: 1) our prior national HFNC survey and 2) membership in the American Academy of Pediatrics sponsored HFNC improvement collaborative, “High Flow Interventions to Facilitate Less Overuse”.9,13 The survey was administered electronically and included branching logic to first determine if their hospital allowed HFNC initiation outside the ICU and then to determine key dates of protocol initiation and transition. After this, survey questions on specific protocol features such as inclusion and exclusion criteria, maximum flow rates and fraction of inspired oxygen allowed, targeted the hospital’s current protocol.
Hospitals were categorized into three groups based on their current protocol: ICU only, age-based, and weight-based. ICU-only hospitals were defined as hospitals for which any HFNC use for bronchiolitis required at least initial care in the ICU. Age-based hospitals were defined as hospitals that allowed initial HFNC use on the non-ICU ward and targeted flow rates based on patient age. Weight-based hospitals were defined as hospitals that allowed initial HFNC use on the non-ICU ward and targeted flow rates based on patient weight.
Hospital Characteristics and Outcomes
We obtained hospital characteristics and patient outcomes from the PHIS database. The PHIS database includes patient-level data from children’s hospitals in the United States, including patient demographic characteristics, procedure codes, and discharge diagnosis codes.
Patients were included if they had an ICD-10 code consistent with bronchiolitis in any diagnostic position.14,15 We included four hospital-level utilization outcome variables including the proportion of patients treated in the ICU, total hospital LOS, the proportion treated with non-invasive positive pressure ventilation (NIPPV), which included continuous positive airway pressure and bi-level positive airway pressure, and the proportion treated with invasive mechanical ventilation (IMV). Patients were classified as having received NIPPV if they had an ICD-10 procedure code for NIPPV and having received IMV if they had a procedure or supply code for mechanical ventilation and a pharmacy charge code for a neuromuscular blocking agent based on previously published definitions.15–17 A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert previously published definitions that used ICD-9 codes to ICD-10 codes. A reliable measure of HFNC exposure is not available in the PHIS database.
Analysis
Survey responses were summarized using descriptive statistics. Hospital characteristics across the three groups (ICU only, age-based, and weight-based hospitals) were compared using Fisher’s exact test and ANOVA. Patient outcomes were compared across the three groups using multivariable regression analyses, adjusting for race, ethnicity, sex, age, presence of a complex chronic condition, census region, hospital type, and number of beds.18 Number of beds were grouped in 50 bed increments for the purpose of the analysis. For comparisons of patient outcomes, we limited data to 2021 in order to avoid misclassifying hospitals with recent changes in protocol. Analyses were performed using Stata version 15 (Stata-Corp, College Station, TX, USA).
Results:
Survey Results
We received survey responses from 36/44 (82%) hospitals (Figure 1). Eight hospitals were excluded from the analysis for missing data, such as dates of protocol adoption or transition, leaving 28 hospitals in our analysis. During the time period studied, there was a steady increase in adoption of non-ICU HFNC protocols, with 71% of responding children’s hospitals reporting non-ICU HFNC protocols in 2021 compared to 11% prior to 2010. Weight-based protocols were quite uncommon during the early study period (10% of non-ICU HFNC protocols in 2014), but as of 2021, 75% of non-ICU HFNC protocols are weight-based. The median maximum flow rate for weight-based protocols was 20 liters per minute (lpm) compared to 12 lpm for age-based protocols (p=.048). Exclusion criteria were specified in 40% of age-based protocols compared to 27% of weight-based protocols (p=0.35). Common exclusion criteria are chronic disease, comorbid acute disease, and prematurity.
Figure 1.
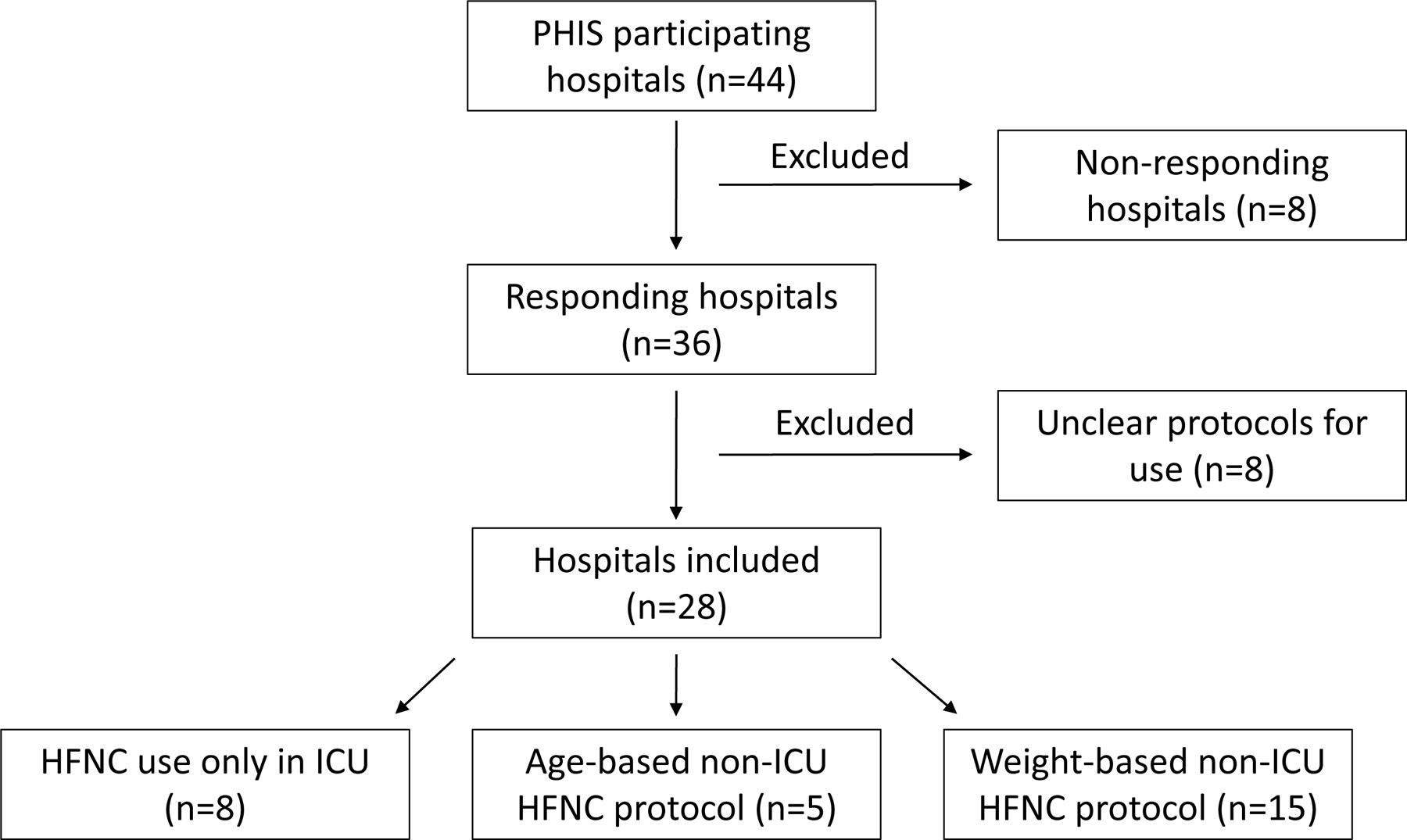
Flow diagram showing the number of included and excluded hospitals.
Hospital Characteristics
Average daily census was highest at hospitals utilizing age-based HFNC protocols. No differences in hospital type (freestanding vs non-freestanding), mean hospital bed size, nor census region were observed between ICU-only hospitals, age-based hospitals, or weight-based hospitals (Table 1).
Table 1.
Unadjusted hospital characteristics and adjusted outcomes in 2021 comparing hospitals with HFNC use in the ICU only, those with age-based non-ICU HFNC protocols, and those with weight-based non-ICU HFNC protocols.
| ICU only protocols (n=8) | Age-based protocols (n=5) | Weight-based protocols (n=15) | p-value | |
|---|---|---|---|---|
| Hospital Characteristics | ||||
| Freestanding | 5 (62.5) | 4 (80.0) | 12 (80.0) | .84 |
| Hospital size (mean # beds) | 369.8 | 406.8 | 410.3 | .1 |
| <200 | 0 | 0 | 1 (6.7) | |
| 200 to <300 | 0 | 0 | 3 (20.0) | |
| 300 to <400 | 3 (37.5) | 2 (40.0) | 3 (20.0) | |
| 400 to <500 | 2 (25.0) | 2 (40.0) | 1 (6.7) | |
| 500 to <600 | 0 | 0 | 2 (13.3) | |
| 600 to <700 | 0 | 0 | 2 (13.3) | |
| Missing | 3 (37.5) | 1 (20.0) | 3 (20.0) | |
| Avg daily census (mean #) | 241.0 | 316.3 | 289.5 | .01 |
| Geographic region | .96 | |||
| Midwest | 4 (50.0) | 1 (20) | 5 (33.3) | |
| Northeast | 1 (12.5) | 1 (20) | 3 (20.0) | |
| South | 1 (12.5) | 2 (40) | 4 (26.7) | |
| West | 1 (12.5) | 1 (20) | 3 (20.0) | |
| Patient Outcomes by protocol type | ||||
| n= 4,934 | n= 6,584 | n= 11,734 | ||
| ICU Utilization | 1,475 (29.9) | 2,397 (36.4) | 2,488 (21.2) | <.001 |
| LOS; median (IQR) | 1.9 (1.2–3.3 ) | 2.9 (1.2–4.4) | 1.9 (1.2–3.4) | <.001 |
| NIPPV | 168 (3.4) | 573 (8.7) | 810 (6.9) | <.001 |
| Mech vent (strict) | 64 (1.3) | 46 (0.7) | 59 (0.5) | .01 |
Outcomes
The proportion of bronchiolitis patients treated in the ICU was highest for age-based hospitals and lowest for weight-based hospitals (36.1% vs 21.0%, respectively, p<.001; Table 1). Additionally, the proportion of bronchiolitis patients treated with NIPPV was highest for age-based hospitals with 11.6% of patients at age-based hospitals receiving NIPPV compared to 6.4% and 3.5% at weight-based and ICU-only hospitals, respectively (p<.001). Length of stay was also significantly longer at age-based hospitals (2.9 days compared to 1.9 days at weight-based and ICU-only hospitals, p<.001). IMV use was most common in ICU-only hospitals with 1.4% of bronchiolitis patients receiving IMV at ICU-only hospitals compared to 0.4% at age-based hospitals and 0.6% at weight-based hospitals (p=.01).
Discussion:
We observed increased adoption of non-ICU based HFNC protocols, especially weight-based protocols. We did not observe differences in most hospital level characteristics between groups. We found decreased use of NIPPV and shorter LOS in weight-based hospitals compared to age-based hospitals. However, NIPPV use was lowest at hospitals using ICU-only HFNC protocols.
We observed a trend toward increasing use of weight-based protocols (75% of non-ICU HFNC protocols in our survey compared to 27% in the 2017 survey by Kalburgi et al).2 The transition to weight-based protocols was also associated with a trend toward allowance of higher maximum flow rates, consistent with flow rates used in recent published RCTs.10–12
We identified an association between the use of weight-based protocols and lower ICU utilization, similar to a single center study examining outcomes before and after transition to a weight-based HFNC protocol.5 It is possible that higher flow rates allowed by weight-based HFNC flow protocols, compared to age-based protocols, improve work of breathing and/or provide reassurance to providers considering an escalation of care.19 However, the use of weight-based protocols was associated with increased use of NIPPV compared to ICU-only protocols. One hypothesis for this finding is that once a respiratory escalation has occurred, subsequent respiratory escalations are more likely to occur. The differences in outcomes we observed between age-based protocols and weight-based protocols could also be related to the make-up of protocols such as restriction of use to bronchiolitis, inclusion of ICU transfer criteria, or protocol exclusion criteria.
Our study has several important limitations. The generalizability of our findings beyond the children’s hospitals that make up PHIS is unclear. Although, our response rate was very good (over 80%) there may be differences in hospitals that responded compared to those that did not. Additionally, only 78% of responding hospitals were included in the final analysis due to missing data. This left a smaller number in the age-based protocol group which means that a single hospital could have greater influence in this group. There may also be hospital-level differences in application of each type of protocol which we could not account for as well as differences created by the atypical timing of the 2021 respiratory surge. While we adjusted for hospital and patient-level covariates in comparing outcomes, other confounders may exist.
Conclusion:
Most children’s hospitals have adopted non-ICU HFNC protocols, the majority of which are now utilizing weight-based flow rates. Weight-based protocols were associated with decreased ICU utilization as compared to age-based protocols.
Figure 2.
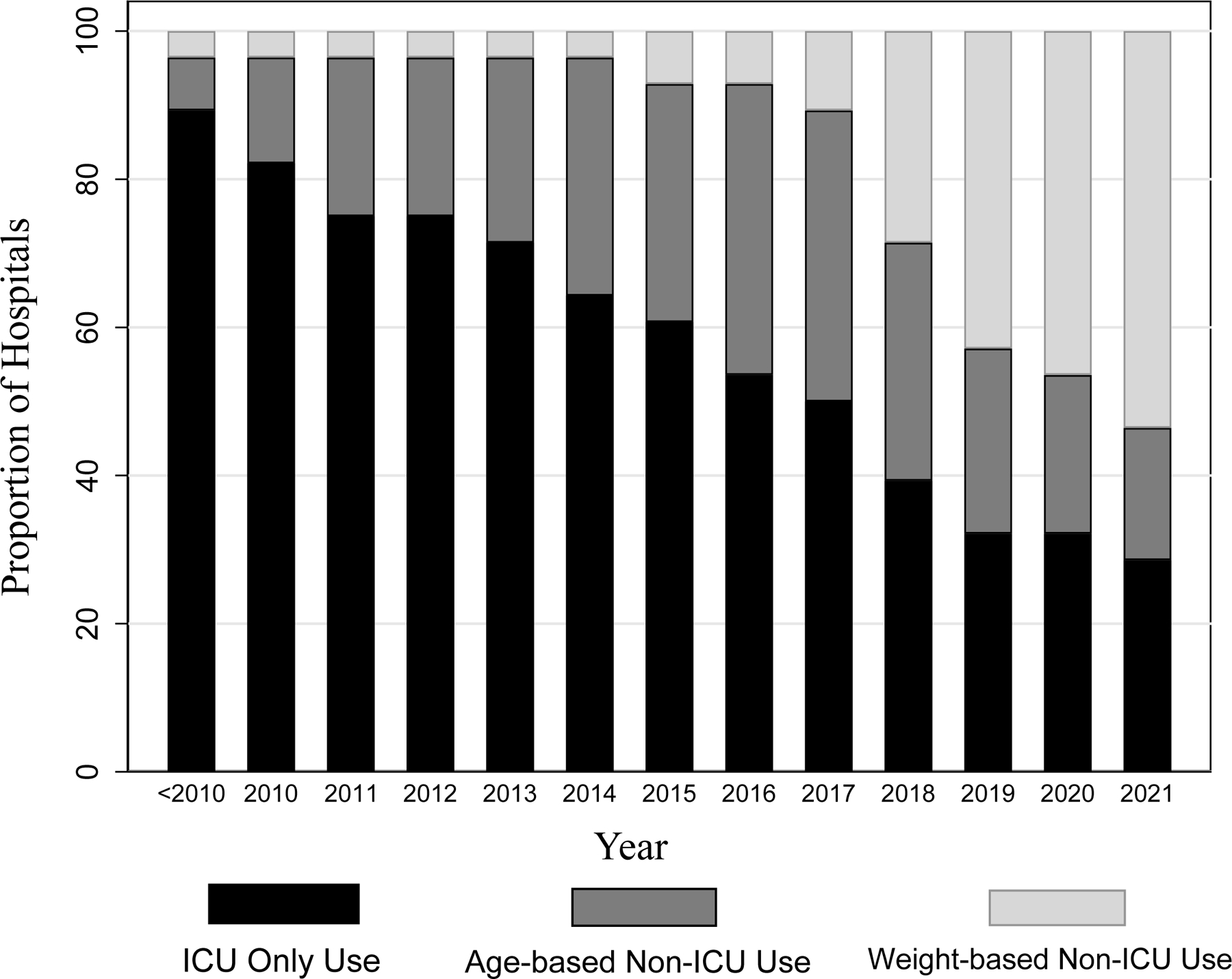
Bar chart displaying the proportions of hospitals who use ICU only, age-based non-ICU and weight-based non-ICU high-flow nasal cannula protocols, by year. “<2010”: survey respondents indicated that HFNC protocols started prior to 2010.
Funding Source:
No funding was secured for this study. Dr. Coon is the recipient of an Intermountain-Stanford Collaboration Grant (NCT03354325). Dr. Tyler is the recipient of a K08 Grant (HS026512) through AHRQ.
Role of Funders:
None of the above funders participated in this work.
Financial Disclosure:
The authors have no financial relationships relevant to this article to disclose.
Abbreviations:
- HFNC
high-flow nasal cannula
- NIV
non-invasive ventilation
- ICU
intensive care unit
- CCC
complex chronic condition
- ICD
international classification of diseases
- LOS
length of stay
- IMV
invasive mechanical ventilation
- PHIS
Pediatric Health Information Systems
Footnotes
Conflicts of Interest Disclosures: The authors have no conflicts of interest to disclose.
References:
- 1.Kline J, Kalburgi S, Halley T. High Flow Nasal Cannula Therapy for Bronchiolitis Across the Emergency Department and Acute Care Floor. Clin Pediatr Emerg Med 2018;19(1):40–45. [Google Scholar]
- 2.Kalburgi S, Halley T. High-Flow Nasal Cannula Use Outside of the ICU Setting. Pediatrics. 2020;146(5). doi: 10.1542/peds.2019-4083 [DOI] [PubMed] [Google Scholar]
- 3.Lo H, Moore RH, Rodkey T, et al. High-flow nasal cannula utilization rates and outcomes in bronchiolitis patients. Pediatrics. 2019;144(2 MeetingAbstract). [Google Scholar]
- 4.Slain KN, Shein SL, Rotta AT. The use of high-flow nasal cannula in the pediatric emergency department. J Pediatr (Rio J). 2017;93:36–45. doi: 10.1016/j.jped.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Willer RJ, Johnson MD, Cipriano FA, et al. Implementation of a Weight-Based High-Flow Nasal Cannula Protocol for Children With Bronchiolitis. Hosp Pediatr 2021;11(8):891–895. doi: 10.1542/hpeds.2021-005814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charvat C, Jain S, Orenstein EW, Miller L, Edmond M, Sanders R. Quality Initiative to Reduce High-Flow Nasal Cannula Duration and Length of Stay in Bronchiolitis. Hosp Pediatr 2021;11(4):309–318. doi: 10.1542/hpeds.2020-005306 [DOI] [PubMed] [Google Scholar]
- 7.Lipshaw MJ, Vukovic AA, Dean P, et al. High-Flow Nasal Cannula in Bronchiolitis at a Pediatric Emergency Department: Trends and Outcomes. Hosp Pediatr 2021;11(2):119–125. doi: 10.1542/hpeds.2020-002774 [DOI] [PubMed] [Google Scholar]
- 8.Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical Outcomes of Bronchiolitis After Implementation of a General Ward High Flow Nasal Cannula Guideline. Hosp Pediatr 2017;7(4):197–203. doi: 10.1542/hpeds.2016-0195 [DOI] [PubMed] [Google Scholar]
- 9.Coon ER, Stoddard G, Brady PW. Intensive Care Unit Utilization After Adoption of a Ward-Based High-Flow Nasal Cannula Protocol. J Hosp Med 2020;15(6):325–330. doi: 10.12788/jhm.3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin D, Babl FE, Schlapbach LJ, et al. A Randomized Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis. N Engl J Med 2018. doi: 10.1056/NEJMoa1714855 [DOI] [PubMed] [Google Scholar]
- 11.Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017. doi: 10.1016/S0140-6736(17)30061-2 [DOI] [PubMed] [Google Scholar]
- 12.Durand P, Guiddir T, Kyheng C, et al. A Randomised Trial of High-Flow Nasal Cannula in Infants with Moderate Bronchiolitis. Eur Respir J 2020:1901926. doi: 10.1183/13993003.01926-2019 [DOI] [PubMed] [Google Scholar]
- 13.VIP network. Accessed 9/30/2022. http://quiin.aap.org.
- 14.Willer RJ, Coon ER, Harrison WN, Ralston SL. Trends in Hospital Costs and Levels of Services Provided for Children with Bronchiolitis Treated in Children’s Hospitals. JAMA Netw Open. 2021;4(10):1–11. doi: 10.1001/jamanetworkopen.2021.29920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6). doi: 10.1542/peds.2019-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good RJ, Leroue MK, Czaja AS. Accuracy of Administrative Codes for Distinguishing Positive Pressure Ventilation From High-Flow Nasal Cannula. Hosp Pediatr 2018;8(7):426–429. doi: 10.1542/hpeds.2017-0230 [DOI] [PubMed] [Google Scholar]
- 17.Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal Changes in Prescription of Neuropharmacologic Drugs and Utilization of Resources Related to Neurologic Morbidity in Mechanically Ventilated Children with Bronchiolitis. Pediatr Crit Care Med 2017;18(12):e606–e614. doi: 10.1097/PCC.0000000000001351 [DOI] [PubMed] [Google Scholar]
- 18.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14(1). doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiler T, Kamerkar A, Hotz J, Ross PA, L Newth CJ, Khemani RG. The Relationship between High Flow Nasal Cannula Flow Rate and Effort of Breathing in Children. Vol 189.; 2017. www.jpeds.com. [DOI] [PubMed] [Google Scholar]


