Abstract
Background
Although hypertension is a significant public health challenge globally, only a few studies have assessed the effectiveness of risk factor control and adherence to recommended lifestyle among United States hypertension patients.
Methods
In this study, a detailed, stratified analysis of the 1999–2018 National Health and Nutrition Examination Survey was conducted to assess the adequacy of risk factor control and conformity to recommended lifestyle among United States patients with hypertension. Logistic regression analysis was used to identify influencing factors associated with not acheving risk factors and lifestyle targets.
Results
A total of 21,770 participants (mean age, 62 ± 15 years) were enrolled in this study. About one in five (20%) participants achieved the recommended body mass index goal, 40% achieved the low-density lipoprotein cholesterol goal, and 30% achieved the recommended waist circumference. Most patients (80%) achieved the recommended smoking goal, 58% met the recommended alcohol consumption, and 19% achieved the recommended physical activity goal. Multivariate analysis demonstrated that age, gender, race, education, metabolic syndrome, and diabetes mellitus were independent predictors of not achieving risk factors and lifestyle targets.
Conclusions
Controlling risk factors and adherence to recommended lifestyles are not ideal for hypertension patients. Therefore, further research should assess how to improve the compliance rate and take targeted measures based on influencing factors for long-term prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20401-3.
Keywords: Risk factor, Lifestyle, Hypertension
Introduction
Hypertension (HTN) affects about a quarter of the adult population worldwide. HTN may affect about 29% by 2025 due to increased population ageing [1]. Besides, HTN is a major risk factor (RF), causing an estimated 9.4 million deaths yearly [2]. Almost half of the United States (U.S.) adults aged 20 and older have HTN based on data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2011 to 2014 [3]. Therefore, effective HTN prevention programs and timely control are needed to reduce the burden of HTN.
Many HTN patients have major RFs for cardiovascular disease (CVD), which significantly impact morbidity and mortality [4, 5]. Derek Weycker et al. showed that more than 50% of HTN patients also have diabetes mellitus (DM), hyperlipidemia, or high body mass index (BMI), additional RFs that significantly increase the risk of CVD [4]. Only 18% of HTN patients across 28 U.S. practices do not have any additional cardiometabolic RFs [6]. Therefore, the identification and management of CVD RFs is also an important aspect of HTN treatment. However, most previous studies [4, 7–9] only focused on HTN awareness, treatment, control, or RF clustering, disregarding the adequacy of RF control and how to strengthen RF management. Few studies investigated the RF control in HTN patients. Lifestyle intervention is crucial for non-drug treatment of HTN. ACC/AHA [10] and ESC/ESH [11] guidelines have shown that lifestyle interventions can prevent and treat HTN. Eighteen meta-analyses and systematic reviews involving 594,129 adult participants have shown that physical activity (PA) reduces blood pressure (BP) in individuals with prehypertension or HTN [12]. Weight loss (including energy-restricted diets) significantly reduces systolic blood pressure (SBP) and diastolic blood pressure (DBP) [13]. Niels Graudal et al. showed that sodium restriction decreases BP in a dose-dependent manner. Specifically, 100 mmol of sodium restriction decreases SBP and DBP by 7.7 mmHg and 3.0 mmHg, respectively [14]. Unfortunately, the majority of the aforementioned research only looked at the connection between a healthy lifestyle and lowering BP. Additionally, only a small number of studies examined HTN patients’ adherence to suggested lifestyle changes. Moreover, a number of earlier research [15–20] have examined how RFs and lifestyle are managed in various nations and areas. However, the majority of these studies have focused on coronary patients, with relatively few on HTN patients.
Therefore, with a large sample size and a long-time span, this study aimed to: (1) investigate the effectiveness of RF management by evaluating patients who successfully achieved their target RFs from NHANES 1999–2018; analyze recommended lifestyle adherence in non-institutionalized American adults with HTN; and (2) analyze the possible influencing factors of not achieving RFs targets and lifestyle targets.
Methods
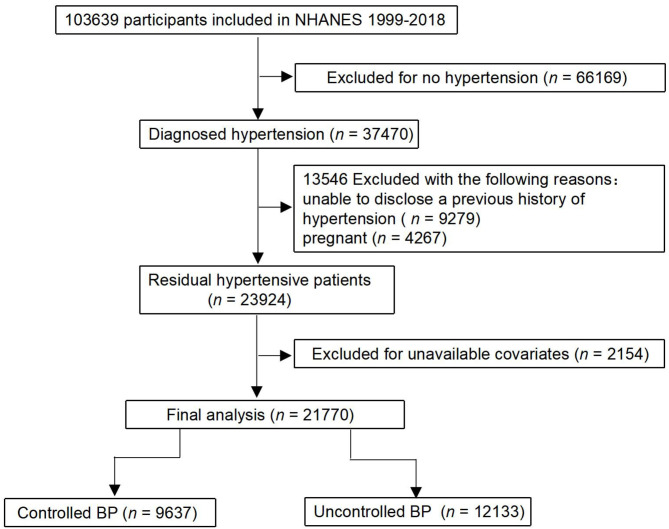
The NHANES is a cross-sectional health survey conducted by the U.S. Centers for Disease Control and Prevention (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Data was collected from NHANES survey cycles from 1999 to 2018. The U.S. adults aged 18 years and older who self-reported a history of HTN were included. HTN was defined as: (1) mean SBP ≥ 140 mmHg, mean DBP ≥ 90 mmHg (≥ 130/80 mmHg if DM) [21] or receiving treatment with antihypertensive drugs; (2) Diagnosed with doctors. Herein, 21,770 participants were included. The participants signed written informed consent. The process of participant selection is shown in Fig. 1.
Fig. 1.
Study flowchart showing the process of participant selection. This study selected 103,639 participants from the National Health and Nutrition Examination Survey (NHANES), and it remained 21,770 persons in the final analysis
Demographic information (age, gender, race), lifestyle information (PA, smoking, alcohol consumption, sodium intake, fiber intake), and socioeconomic information were collected from the participants using questionnaires. A standardized physical examination, including BP (SBP/DBP), BMI, waist circumference measurements, was conducted by a medical professional at a mobile examination center (MEC). An analysis of blood lipids and glycosylated hemoglobin (HbA1c) was analyzed.
In the univariate logistic regression analysis, age, gender, race, socioeconomic status, education status, current health insurance status, MS, CHD, stroke, HF, DM, CKD were modeled as independent variables, and significant variables were subjected to multiple logistic regression analysis.
Definition of comorbidities
Participants meeting three or more of the following criteria were diagnosed as metabolic syndrome (MS) [22]: (1) HTN: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg; (2) Triglycerides (TG) levels: TG > 150 mg/dL; (3) HDL-C levels: male HDL-C < 40 mg/dL, female HDL-C < 50 mg/dL; (4) Abdominal obesity: waist circumference > 102 cm for men or > 88 cm for women; (5) Fasting glucose levels ≥ 110 mg/dL. Participants with angina and myocardial infarction (“heart attack”) were diagnosed with coronary heart disease (CHD). Participants meeting the below criteria were diagnosed with stroke: (1) Imaging showing cerebral tissue ischemia, hypoxia, necrosis, or bleeding; (2) Those diagnosed with “stroke” before. Participants who had been previously diagnosed with heart failure (HF) by a health professional were regarded as HF patients. Participants meeting the following criteria were diagnosed with DM: (1) Fasting blood glucose level ≥ 126 mg/dL (7.0 mmol/L); (2) Two-hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during oral glucose tolerance test (OGTT); (3) HbA1c ≥ 6.5% (48 mmol/mol); (4) A random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) in a patient with classic symptoms [23]; (5) Participants receiving treatment with hypoglycemic drugs; (6) Those previously diagnosed with “DM” by a doctor. Participants with glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 were diagnosed with CKD [24]. GFR was estimated using the dietary modification equation for kidney disease, as follows: GFR = 186 ×serum creatinine-10,154 × age-0.2.3 × (1.212 if black) × (0.742 if female).
Definition of targets
Physiological targets were set as follows. BMI: 18.5–25 kg/m2, LDL-C < 131 mg/mL, waist circumference: < 89 cm for women and < 102 cm for men, HbA1c < 7% for HTN patients with DM. Lifestyle targets: smoking status: never smoked or quit smoking after HTN. Alcohol consumption: ≤ 2 drinks/day for men and ≤ 1 drink/day for women; PA: ≥ 5 days/week and ≥ 30 min/session; Sodium intake: < 1.5 g/d; Fiber intake: 20 gm/d-30 gm/d. These two targets were referenced from the guideline of AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update [25].
In this study, not achieving RFs targets was defined as ≥ 2 RFs not achieving the targets, not achieving lifestyle targets was defined as ≥ 2 lifestyles not achieving the targets [16, 26, 27].
Statistical analysis
Patients were grouped by age, gender, race, socioeconomic status, education status, current health insurance status and co-morbidities to evaluate the proportion of HTN patients with BP controlled, and the statistical differences was analyzed by Chi-square test. Age, BP (SBP and DBP), serum lipids (LDL-C, HDL-C, and TC), BMI, sodium intake, fiber intake, and waist circumference between BP controlled and uncontrolled patients were compared using Student’s t-test. HTN patients with controlled or uncontrolled BP with comorbidities (including MS, CHD, stroke, HF, DM, and CKD) were detected by Chi-square test. The participants were grouped by gender, age, race, socioeconomic, education, and health insurance status to determine the proportions of patients achieving recommended RFs targets, and compared by Chi-square test were measured. In addition, the potential differences in achieving RF targets between participants with or without each co-morbidity were compared with Chi-square test.
First, the not achieving RFs targets or not achieving lifestyle targets were modeled as dichotomous variables, and then independent variables (including age, gender, race, socioeconomic status, education status, current health insurance status, MS, CHD, stroke, HF, DM, CKD) were modeled for univariate logistic regression analysis. After that, the variables with statistical differences (p < 0.05) were selected for multiple logistic regression analysis. Multivariate logistic regression analysis was performed to analyze the influencing factors of not achieving RFs targets (the variables were including age, gender, race, education status, health insurance status, MS, CHD, stroke, DM, and CKD). Notably, in addition to socioeconomic status and HF being different, multivariate logistic regression analysis was also performed to analyze the influencing factors of not achieving lifestyle targets. For all analyses, statistical significance was set at p < 0.05, SAS statistical software (version 9.3; SAS Institute, Cary, NC, USA) was used for data management and analysis.
Results
A total of 21,770 participants were enrolled in this study. The basic characteristics of the participants are shown in Table 1. The mean age of the respondents was 62 ± 15. Most participants were females (51%). Most respondents were non-Hispanic white (NHW) (49%), 46% were low-income earners (< $35,000), 43% were associate degree (AA) holders or had high school education, and 34% were public-medicare personnel. Compared with the BP controlled group, the proportion of SBP, DBP, LDL-C, HDL-C, TC, smoking, and DM was higher in the uncontrolled group. However, salt intake, fiber intake, and CKD were not significantly different between the two groups.
Table 1.
Characteristics of the final analytic sample of participants (n = 21770)
| Characteristics | Overall (n = 21770) |
Controlled BP (n = 9637) |
Uncontrolled BP (n = 12133) |
P Value |
|---|---|---|---|---|
| Age (yrs), mean ± SD | 61.61 ± 15.07 | 61.35 ± 14.62 | 61.83 ± 15.41 | 0.02 |
| Gender, n(%) | < 0.0001 | |||
| Male | 10,634 (48.85) | 4473 (46.41) | 6161 (50.78) | |
| Female | 11,136 (51.15) | 5164 (53.59) | 5972 (49.22) | |
| Race, n(%) | < 0.0001 | |||
| Non-Hispanic White | 9760 (48.46) | 4667 (52.20) | 5093 (45.48) | |
| Hispanic | 4672 (23.20) | 1816 (20.31) | 2856 (25.50) | |
| Non-Hispanic Black | 5707 (28.34) | 2457 (27.48) | 3250 (29.02) | |
| Socioeconomic Status, n(%) | < 0.0001 | |||
| Low | 6089 (45.71) | 2765 (43.87) | 3324 (47.37) | |
| Middle | 4466 (33.53) | 2126 (33.73) | 2340 (33.35) | |
| High | 2765 (20.76) | 1412 (22.40) | 1353 (19.28) | |
| Education Status, n(%) | < 0.0001 | |||
| < high school | 6905 (32.08) | 2765 (28.94) | 4140 (34.58) | |
| High school diploma | 5281 (24.53) | 2368 (24.78) | 2913 (24.33) | |
| AA or high | 9341 (43.39) | 4422 (46.28) | 4919 (41.09) | |
| Current Health Insurance Status, n(%) | <0.0001 | |||
| Uninsured | 2027 (12.74) | 744 (10.00) | 1283 (15.15) | |
| Private | 4067 (25.56) | 1967 (26.43) | 2100 (24.80) | |
| Public-medicare | 5368 (33.74) | 2529 (33.98) | 2839 (33.52) | |
| Public others | 4450 (27.97) | 2203 (29.60) | 2247 (26.53) | |
| BP | ||||
| SBP (mmHg), mean ± SD | 139.29 ± 21.01 | 121.36 ± 11.00 | 151.22 ± 17.28 | < 0.0001 |
| DBP (mmHg), mean ± SD | 72.67 ± 16.51 | 67.64 ± 12.77 | 76.01 ± 17.82 | < 0.0001 |
| Risk Factors | ||||
| LDL-C (mg/dL), mean ± SD | 111.99 ± 36.65 | 108.77 ± 35.00 | 114.93 ± 37.87 | < 0.0001 |
| HDL-C (mg/dL), mean ± SD | 52.46 ± 16.69 | 51.92 ± 16.12 | 52.91 ± 17.13 | 0.0004 |
| TC (mg/dL), mean ± SD | 193.62 ± 44.21 | 188.49 ± 42.30 | 197.88 ± 45.30 | < 0.0001 |
| Current smoking, n (%) | 10,618 (48.77) | 4588 (47.61) | 6030 (49.70) | 0.005 |
| Current drinker, n (%) | 5532 (57.90) | 2451 (59.68) | 3081 (56.56) | 0.002 |
| BMI | 30.60 ± 7.24 | 31.26 ± 7.25 | 30.13 ± 7.20 | < 0.0001 |
| Sodium intake (g/d) | 3.15 ± 1.46 | 3.17 ± 1.42 | 3.13 ± 1.48 | 0.078 |
| Fiber intake (gm/d) | 15.81 ± 8.56 | 15.93 ± 8.55 | 15.72 ± 8.56 | 0.124 |
| Waist circumference | 104.14 ± 15.84 | 105.59 ± 15.56 | 103.11 ± 15.96 | < 0.0001 |
| Co-morbidities, n (%) | ||||
| MS | 3984 (66.03) | 1917 (68.54) | 2067 (63.86) | 0.0001 |
| CHD | 3225 (14.94) | 1650 (17.22) | 1575 (13.12) | < 0.0001 |
| Stroke | 1716 (7.95) | 832 (8.68) | 884 (7.36) | 0.0004 |
| HF | 1539 (7.13) | 828 (8.64) | 711 (5.92) | < 0.0001 |
| DM | 5710 (26.23) | 2011 (20.87) | 3699 (30.49) | < 0.0001 |
| CKD | 2488 (18.99) | 1177 (19.67) | 1311 (18.42) | 0.07 |
Data are presented as n (%) or mean ± standard deviation (SD)
Uncontrolled blood pressure (BP): BP ≥ 140/90mmHg, or BP ≥ 130/80 mm Hg in those hypertensive patients with diabetes mellitus (DM) or chronic kidney disease (CKD)
Socioeconomic status: low, <$35,000; middle, $35,000e$75,000; high, >$75,000
AA: associate degree; SBP: systolic blood pressure; DBP: diastolic blood pressure; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; BMI: body mass index; MS: metabolic syndrome; CHD: coronary heart disease; HF: heart failure
P value indicates comparison of means or proportions between controlled and uncontrolled groups
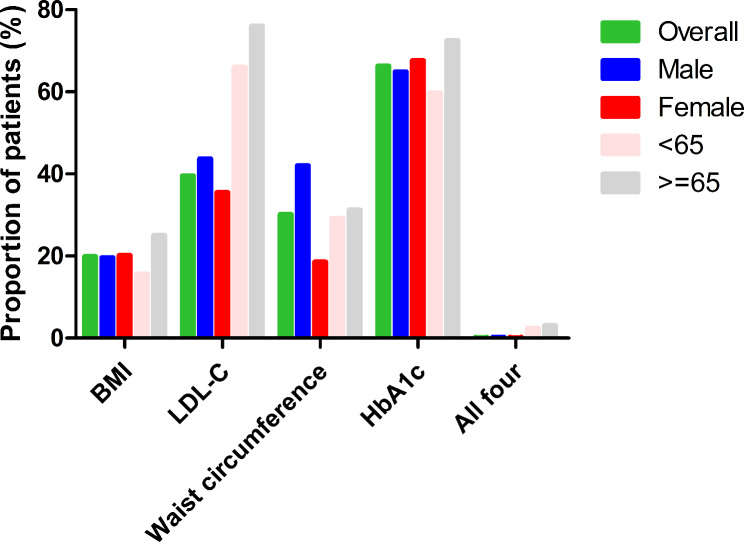
The percentage of patients who met the recommended physiological goals is shown in Table S1 and Fig. 2. About one in five (20%) participants reached the recommended BMI, with the elderly, NHW, low-income, less than high school education, and public-medicare populations more likely to achieve this goal. Approximately 40% of respondents achieved the LDL-C goal, mostly male, elderly, NHW, less than high school education, and public-medicare groups. About 30% achieved the recommended waist circumference, with male, elderly, non-Hispanic black, high-income, less than high school education, and uninsured population more likely to achieve this goal. About 66% met the recommended HBA1c target, mostly for female, elderly, NHW, and public-medicare populations. However, only 32 (0.2%) respondents completed the all four goals.
Fig. 2.
Proportion (%) of US adults with hypertension at recommended cardiovascular risk factor goals by gender and age group (years). BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin
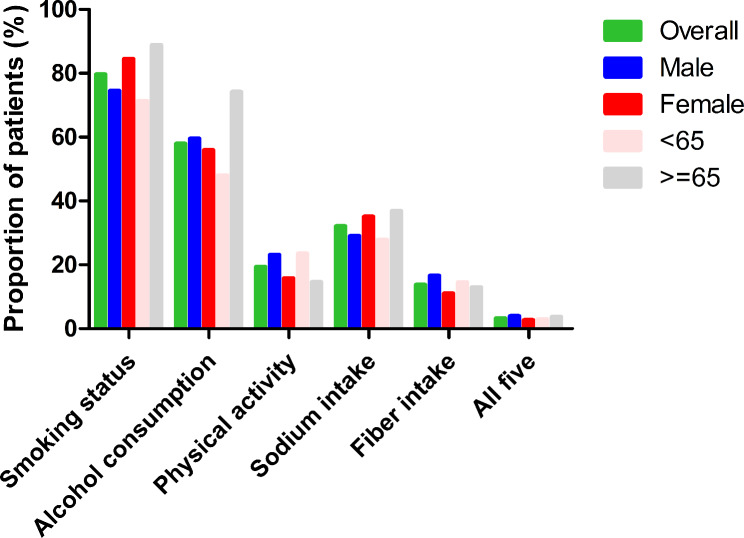
The proportion of participants who completed the recommended lifestyle modifications is shown in Table S2 and Fig. 3. Most patients (80%) achieved the recommended smoking status, with female, elderly, Hispanic, high-income, AA or high, and public-medicare populations doing better than other groups. About 58% met the recommended alcohol consumption, with male, elderly, NHW, high-income, AA or high, and public-medicare populations more likely to accomplish the goal. Only 19% achieved the recommended PA goal, with females, elderly, NHW, less than high school education, and public medicare groups performing worse. Only 32% met the recommended salt intake, where males < 65 years old, NHW, high-income, and AA or high education groups were doing worse. Only 14% met the recommended fiber intake, and 3% met all five goals.
Fig. 3.
Proportion (%) of US adults with hypertension at recommended lifestyle modification goals by gender and age group (years)
The proportion of HTN subjects achieving recommended RF targets according to presence of comorbidities is shown in Table S3. About 53% of HTN patients with MS achieved the recommended HbA1c target, with only 5% reaching the recommended BMI, 5% achieving the waist circumference target, and no patients achieving all four goals (0 (0), p < 0.01). Furthermore, 61% of patients with CHD achieved the LDL-C target, 69% achieved the recommended HbA1c, and only 0.3% achieved all four goals. About 22% of patients with stroke achieved the recommended BMI, 78% achieved the LDL-C target, 73% achieved the HbA1c target, and only 0.4% achieved all four goals. Only 17% of patients with HF achieved the recommended BMI, 56% achieved the recommended LDL-C goal, 24% achieved the waist circumference, 72% achieved the HbA1c target, and 0.4% achieved all four goals. About 13% of patients with DM achieved the BMI target, 79% achieved the recommended LDL-C, and 20% achieved the waist circumference target. About 20%, 49%, 25%, and 57% of patients with CKD achieved the BMI goal, LDL-C, recommended waist circumference, and glycolysis targets, respectively.
The proportion of HTN subjects achieving recommended lifestyle targets according to presence of comorbidities is shown in Table S4. Furthermore, 79%,23%, and 1% of patients with MS achieved the recommended smoking status, PA, and all five goals, respectively. About 70% of patients with CHD met the recommended alcohol consumption, only 15% met the PA goal, 35% met the recommended sodium intake, and 12% met the fiber intake goal. Also, 65% of patients with stroke achieved the recommended alcohol consumption, 12% achieved PA goal, 37% achieved sodium intake, and 10% achieved fiber intake. Similarly, 68% of patients with HF achieved the recommended alcohol consumption, 12% achieved the recommended PA goal, 37% met sodium intake, and 11% met fiber intake target. Most DM patients (82%) achieved smoking status, and only 17% achieved recommended PA. Similarly, most patients with CKD (83%) achieved the smoking goal, 69% achieved recommended alcohol consumption, 17% achieved recommended PA, 16% achieved sodium intake, 14% achieved fiber intake, and only 6% completed the five goals.
The potential factors that determine patient’s experience of not achieving RFs targets and lifestyle targets are shown in Tables 2 and 3, respectively. Univariate analysis revealed that the age, gender, race, education status, health insurance status, MS, CHD, stroke, DM, and CKD did not achieve RFs targets (Table 4). Also, age, gender, race, socioeconomic status, education status, MS, CHD, stroke, HF, and CKD did not achieve lifestyle targets (Table 4). Furthermore, multivariate analysis demonstrated that age, gender, race, education status, MS, and DM were the independent predictors of not achieving RFs targets (Table 2). Also, age, gender, race, socioeconomic status, MS, and CKD were the independent predictors of not achieving lifestyle targets (Table 3).
Table 2.
Odds ratios by multivariate logistic regression for not achieving risk factors targets
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (yrs) (vs. < 65) | |||
| ≥ 65 | 0.67 | 0.51–0.86 | 0.002 |
| Gender (vs. Male) | |||
| Female | 1.67 | 1.41–1.97 | < 0.0001 |
| Race (vs. Non-Hispanic White) | |||
| Hispanic | 1.13 | 0.90–1.42 | 0.29 |
| Non-Hispanic Black | 1.23 | 1.00-1.50 | 0.05 |
| Education Status (vs. < high school) | |||
| High school diploma | 1.08 | 0.86–1.36 | 0.50 |
| High | 1.28 | 1.04–1.58 | 0.02 |
| Health Insurance Status (vs. Uninsured) | |||
| Private | 1.23 | 0.93–1.62 | 0.14 |
| Public-medicare | 1.26 | 0.89–1.77 | 0.19 |
| Public others | 1.11 | 0.82–1.50 | 0.49 |
| Co-morbidities(No vs. Yes) | |||
| MS | 0.03 | 0.02–0.03 | < 0.0001 |
| CHD | 1.19 | 0.93–1.53 | 0.17 |
| Stroke | 0.92 | 0.65–1.27 | 0.59 |
| DM | 0.58 | 0.47–0.73 | < 0.0001 |
| CKD | 1.12 | 0.89–1.42 | 0.32 |
Not achieving risk factors targets: ≥2 risk factors (including LDL-C ≥ 131 mg/dL, body mass index ≥ 25 kg/m2, HbA1c ≥ 7% for those hypertensive patients with diabetes mellitus, waist circumference [≥ 89 cm for women and ≥ 102 cm for men]) not achieving targets
Socioeconomic status: low, <$35,000; middle, $35,000-$75,000; high, >$75,000
MS: metabolic syndrome; DM: diabetes mellitus; CHD: coronary heart disease; CKD: chronic kidney disease; CI: confidence interval; OR: odds ratio
Table 3.
Odds ratios by multivariate logistic regression for not achieving lifestyle targets
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (yrs) (vs. < 65) | |||
| ≥ 65 | 0.59 | 0.45–0.78 | 0.0001 |
| Gender (vs. Male) | |||
| Female | 1.44 | 1.11–1.87 | 0.007 |
| Race (vs. Non-Hispanic White) | |||
| Hispanic | 0.89 | 0.65–1.23 | 0.49 |
| Non-Hispanic Black | 1.64 | 1.13–2.40 | 0.01 |
| Socioeconomic Status (vs. Low) | |||
| Middle | 0.80 | 0.59–1.07 | 0.13 |
| High | 0.64 | 0.45–0.91 | 0.01 |
| Education Status (vs. < high school) | |||
| High school diploma | 1.14 | 0.78–1.67 | 0.51 |
| High | 0.80 | 0.58–1.12 | 0.19 |
| Co-morbidities(No vs. Yes) | |||
| MS | 0.64 | 0.49–0.83 | 0.0008 |
| CHD | 1.20 | 0.83–1.73 | 0.34 |
| Stroke | 0.91 | 0.53–1.56 | 0.73 |
| HF | 0.85 | 0.48–1.52 | 0.59 |
| CKD | 0.59 | 0.39–0.89 | 0.01 |
Not achieving lifestyle targets: ≥2 lifestyle (including current smoking, current drinker [> 2 drinks/day for men and > 1 drink/day for women], sodium intake ≥ 1.5 g/d, fiber intake < 20 gm/d, physical activity not reached at least 5 days a week and no less than 30 min per session) not achieving targets
Socioeconomic status: low, <$35,000; middle, $35,000-$75,000; high, >$75,000
MS: metabolic syndrome; CHD: coronary heart disease; CKD: chronic kidney disease; HF: heart failure; CI: confidence interval; OR: odds ratio
Table 4.
Univariate logistic analysis for not achieving risk factors targets and not achieving lifestyle targets
| Group | Not achieving risk factors targets | Not achieving lifestyle targets | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | ||||||
| < 65 | 1 | reference | 1 | reference | ||
| ≥ 65 | 0.63 | 0.60–0.67 | < 0.0001 | 0.75 | 0.68–0.83 | < 0.0001 |
| Gender | ||||||
| Male | 1 | reference | 1 | reference | ||
| Female | 0.87 | 0.83–0.92 | < 0.0001 | 1.18 | 1.07–1.31 | 0.0014 |
| Race | ||||||
| Non-Hispanic White | 1 | reference | 1 | reference | ||
| Hispanic | 1.28 | 1.19–1.37 | < 0.0001 | 0.65 | 0.57–0.74 | < 0.0001 |
| Non-Hispanic Black | 1.54 | 1.44–1.65 | < 0.0001 | 1.13 | 0.98–1.29 | 0.09 |
| Socioeconomic Status | ||||||
| Low | 1 | reference | 1 | reference | ||
| Middle | 1.08 | 0.99–1.17 | 0.07 | 0.76 | 0.67–0.90 | 0.001 |
| High | 1.03 | 0.94–1.14 | 0.48 | 0.63 | 0.53–0.74 | < 0.0001 |
| Education Status | ||||||
| < high school | 1 | reference | 1 | reference | ||
| High school diploma | 1.21 | 1.12–1.30 | < 0.0001 | 1.39 | 1.20–1.61 | < 0.0001 |
| AA or high | 1.22 | 1.14–1.30 | < 0.0001 | 1.07 | 0.95–1.20 | 0.26 |
| Current Health Insurance Status | ||||||
| Uninsured | 1 | reference | 1 | reference | ||
| Private | 0.63 | 0.56–0.71 | < 0.0001 | 0.97 | 0.78–1.19 | 0.75 |
| Public-medicare | 0.39 | 0.35–0.43 | < 0.0001 | 1.00 | 0.82–1.23 | 0.99 |
| Public others | 0.55 | 0.49–0.62 | < 0.0001 | 1.11 | 0.89–1.37 | 0.36 |
| Co-morbidities | ||||||
| MS | 0.03 | 0.02–0.03 | < 0.0001 | 0.73 | 0.58–0.92 | 0.0068 |
| CHD | 1.10 | 1.02–1.19 | 0.01 | 0.83 | 0.71–0.96 | 0.02 |
| Stroke | 1.35 | 1.22–1.49 | < 0.0001 | 0.61 | 0.49–0.77 | < 0.0001 |
| HF | 1.10 | 0.99–1.23 | 0.07 | 0.63 | 0.49–0.80 | 0.0002 |
| DM | 0.56 | 0.52–0.59 | < 0.0001 | 0.92 | 0.82–1.03 | 0.16 |
| CKD | 1.17 | 1.06–1.28 | 0.0013 | 0.71 | 0.58–0.88 | 0.0014 |
Not achieving risk factors targets: ≥2 risk factors (including LDL-C ≥ 131 mg/dL, body mass index ≥ 25 kg/m2, HbA1c ≥ 7% for those hypertensive patients with diabetes mellitus, waist circumference [≥ 89 cm for women and ≥ 102 cm for men]) not achieving targets
Not achieving lifestyle targets: ≥2 lifestyle (including current smoking, current drinker [> 2 drinks/day for men and > 1 drink/day for women], sodium intake ≥ 1.5 g/d, fiber intake < 20 gm/d, physical activity not reached at least 5 days a week and no less than 30 min per session) not achieving targets
Socioeconomic status: low, <$35,000; middle, $35,000-$75,000; high, >$75,000
MS: metabolic syndrome; CHD: coronary heart disease; HF: heart failure; DM: diabetes mellitus; CKD: chronic kidney disease; CI: confidence interval; OR: odds ratio
Discussion
These findings suggest that controlling RFs and adherence to recommended lifestyles are not ideal for U.S. HTN patients. Meanwhile, age, gender, race, education, MS, and DM were determined to be associated with not achieving RF targets. Moreover, age, gender, race, socioeconomic status, MS, and CKD were determined to be associated with not achieving lifestyle targets.
A study published by GP Vyssoulis et al. [28] investigated 21,280 Greek patients with HTN. The study found that over half (53.1%) of the patients had one type of RF, 32.9% had two, and only 10.2% had no accompanying RFs. Another study [4] also found more than half (56%) of patients with HTN at a large managed care facility with at least one CVD RF: DM (15%), hyperlipidemia (24%), and high BMI (37%). Herein, about 26% of HTN patients had DM. These different results may be due to differences in the definition of the study population. Compared to participants without DM, those with DM have a two- to threefold increased risk of developing CVD [29]. Our findings, however, suggest that blood glucose control among hypertensive patients with DM is suboptimal. Only three-fifths (60%) of this group achieved the recommended HbA1c level. This aligns with previous studies demonstrating less than ideal fasting blood glucose control in hypertensive patients with DM, with reported ranges between 158.4 and 170.2 mg/dL [4]. Although the specific indicator differs from ours, the conclusions align. Additionally, our study identified subgroups with poorer glycemic control, including males, individuals younger than 65 years old, Hispanics, and the uninsured. These findings highlight the need for increased focus on improving blood sugar control in these specific populations.
Derek Weycker [4] found that the LDL-C ranges for HTN patients with hyperlipidemia and those without hyperlipidemia are 129.6-145.1 mg/dL and 109.9-118.2 mg/dL, respectively. Although the National Cholesterol Education Program (NCEP) guidelines have been widely publicized, published U.S. data show that hypercholesterolemia is still poorly treated and controlled [30]. Similar to our findings on blood glucose control, the present study revealed that blood lipid control was also suboptimal. Only two-thirds (66.7%) of patients achieved the recommended LDL-C target of < 131 mg/dL. Subgroups with lower achievement rates included females younger than 65 years old, Hispanics, individuals with high school education or less, and the uninsured population. These findings highlight the need for further research to identify strategies for improving blood lipid management in these specific patient groups.
One risk estimate health study [31] showed that obesity is the major cause of HTN, accounting for 40%. Epidemiological studies have shown that BMI is directly associated with BP [32]. An Italian study [33] showed that a BMI 27 ± 4 kg/m2 and 52.6% may indicate overweight or obesity. This study found that compared with HbA1c and LDL-C control rates, a lower proportion of HTN patients achieved recommended BMI and waist circumference goals. This aligns with findings from another NHANES study [16], suggesting that weight management remains a significant challenge for HTN patients in the U.S.
Earlier research indicated that physical activity serves as a crucial lifestyle intervention for preventing and treating HTN [34, 35]. Regular PA can lower BP in HTN patients [36]. Poor BP control is associated with a lack of PA (OR 1.195; 95% CI 1.175–3.387; p = 0.011) [37]. Another study found that obese men who engage in no physical activity have a relative risk of 1.50 (95% CI: 1.27–1.77), while obese men with high activity levels have a relative risk of 1.16 (95% CI 0.79–1.70) [38]. A survey of 1000 people with HTN in Jordan showed that most respondents received advice on PA (n = 690, 69%) [39]. However, our study also revealed a concerningly low percentage of hypertensive patients (only 19%) meeting recommended PA targets. This disparity was further accentuated in females, elderly individuals, those with lower educational attainment, and low-income groups. These findings highlight the need for increased public awareness and educational initiatives on the importance of physical activity for this population. Moreover, considering the diverse exercise capabilities and economic limitations within these subgroups, further research is crucial to develop tailored PA prescription strategies that are both feasible and effective.
Smoking is a powerful cardiovascular RF that increases the risk of CVD through multiple mechanisms [40]. Therefore, quitting smoking cessation is one of the effective lifestyle measures to prevent many CVD [41]. ESC/ESH guidelines recommend quitting smoking to prevent and manage HTN [11]. Similarly, the Jordanian survey showed that less than half (n = 430, 43%) of patients received advice to quit smoking [39]. In a study conducted in Greece (21,280 people), 33% of patients with HTN smoked [28]. In this study, 80% of people achieved the goal of quitting smoking, which is higher than that reported in previous studies.
Numerous observational epidemiological studies have indicated that heavy alcohol consumption is a RF for elevated BP [42]. Both ACC/AHA [10] and ESC/ESH [11] guidelines recommend reducing alcohol intake to manage HTN. In this study, more than half patients met the recommended alcohol consumption target, with more male and older adults reaching the target. However, future studies should assess the long-term effectiveness of limiting alcohol consumption in reducing BP [43].
Sodium restriction is the most popular recommendation for preventing HTN and lowering BP. Besides, the current dietary guidelines recommend reducing sodium (or salt) intake [10, 11]. A study [44] estimated that the global sodium intake in 2010 was 3,950 mg per day, significantly higher than the recommended level in all published guidelines (2,300 mg per day). In this study, only 32% of patients met the recommended salt intake level. An increase in dietary fiber intake may reduce BP in HTN patients [45]. Herein, only 14%of patients reached the target, and very few completed all five goals.
Furthermore, age, gender, and race were independent predictors of achieving RFs targets and lifestyle targets. Notably, patients aged 65 and older were more likely to achieve the targets. Compared with males, females were less likely to achieve the targets, consistent with another NHANES study [26]. Besides, non-Hispanic black showed the worst results in achieving the lifestyle target. Socioeconomic status significantly affects cardiovascular and CHD burden, with occupation, income, and education being the most critical measures [46]. In this study, education significantly influenced the achievement of RFs. Notably, people with higher degree were less likely to achieve RFs targets. Our findings regarding the impact of education level on risk factor control differ from previous research. A study of 7,937 CHD patients [47] found that individuals with higher education had significantly better control of their risk factors. In addition, people with higher incomes were more likely to achieve lifestyle targets than those with lower incomes. This is similar to the results of previous study [48]. Landon BE et al. compared treatment patterns and outcomes for patients with acute myocardial infarction in six countries and observed that survival outcomes were significantly better in high-income populations than in low - and high-income populations. Therefore, more effective strategies are needed to target low-income populations. Furthermore, MS is significantly associated with DM and CVD in the general population [49]. Herein, patients with MS were less likely to achieve RFs targets and lifestyle targets. DM, as one of the major RFs for CVD, has reached epidemic proportions [50]. Herein, patients with DM were less likely to achieve RFs targets. Furthermore, CKD, as a public health threat affecting cardiovascular risk [51], also affected the achievement of lifestyle targets. However, this study was not designed to elucidate the specific influence of the aforementioned comorbidities on the achievement of RF targets and lifestyle goals. Further research is necessary to explore these relationships in greater detail.
This study used the large sample size of the NHANES 1999–2018 period and thus can project the findings as closely as possible to real-world HTN patients in the U.S. Furthermore, standardized methods and instruments were used to perform physical examinations and collect blood information, such as BP, waist circumference measurements, and laboratory analysis of lipids and blood sugar. However, this study has some limitations. This study was based on a cross-sectional descriptive analysis of large observational studies and clinical investigations and can only identify associations but cannot establish causation. Second, data including lifestyle and socioeconomic status were collected using self-reports which may vary from the actual situation. Therefore, there may be bias in the analysis of sample characteristics in the final participants. Thirdly, NHANES measurements are only captured at one moment in time, potentially leading to misclassification of some participants as having controlled or uncontrolled BP. Similarly, the characteristics of participants and our conclusions may be biased and fail to reflect the real situation. In addition, our analysis was based on secondary data from databases, which may have excluded some information depending on their research objectives and data collection methods. Therefore, the accuracy and completeness of the data may be relatively poor. Lastly, we did not incorporate medication data, and thus, we did not analyze and discuss patients’ drug use. Further studies are advocated to resolve these limitations and validate our findings.
Conclusion
In summary, controlling RFs and adherence to recommended lifestyles are not ideal in American patients with HTN. Therefore, further research should assess how to improve the compliance rate and take targeted measures according to influencing factors for improving long-term prognosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the staff of the NHANES and the reviewers who participated in the review.
Author contributions
Conceived and designed the experiments: Lijiang Tang and Xiaowei Liu. Performed the experiments: Zhi Zhang and Xiaowei Liu. Analyzed and interpreted the data: Changqing Du and Lijiang Tang. Contributed reagents, materials, analysis tools or data: Changqing Du and Xiaowei Liu. Wrote the paper: Lin Yang and Zhi Zhang.
Funding
We did not receive any form of financial support.
Data availability
Publicly available datasets were analyzed in this study. All the raw data used in this study are derived from the public NHANES data portal (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).
Declarations
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The data was obtained from the NHANES database, which is a public database approved by the ethics committee of NHANES, and thus this study does not need approval by ethics committee.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lin Yang and Zhi Zhang contributed equally to this work.
Contributor Information
Lijiang Tang, Email: zjyytang@126.com.
Xiaowei Liu, Email: liuxiaowei144138@163.com.
References
- 1.Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis Mar. 2010;55(3):590–8. 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Poulter NR, Prabhakaran D, Caulfield M, Hypertension. Lancet. Aug 22 2015;386(9995):801 – 12. 10.1016/s0140-6736(14)61468-9 [DOI] [PubMed]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH et al. /ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. Oct 23 2018;138(17):e426-e483. 10.1161/cir.0000000000000597 [DOI] [PubMed]
- 4.Weycker D, Nichols GA, O’Keeffe-Rosetti M, Edelsberg J, Khan ZM, Kaura S, Oster G. Risk-factor clustering and cardiovascular disease risk in hypertensive patients. Am J Hypertens Jun. 2007;20(6):599–607. 10.1016/j.amjhyper.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol Apr. 2020;16(4):223–37. 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belletti DA, Zacker C, Wogen J. Effect of cardiometabolic risk factors on hypertension management: a cross-sectional study among 28 physician practices in the United States. Cardiovasc Diabetol Feb 1. 2010;9:7. 10.1186/1475-2840-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J, Mi Y, Li Y, Xin B, Wang Y. Factors associated with awareness, treatment and control of hypertension among 3579 hypertensive adults in China: data from the China Health and Nutrition Survey. BMC Public Health Mar. 2021;1(1):423. 10.1186/s12889-021-10417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhauser HK, Adler C, Rosario AS, Diederichs C, Ellert U. Hypertension prevalence, awareness, treatment and control in Germany 1998 and 2008-11. J Hum Hypertens Apr. 2015;29(4):247–53. 10.1038/jhh.2014.82. [DOI] [PubMed] [Google Scholar]
- 9.Khdour MR, Hallak HO, Shaeen M, Jarab AS, Al-Shahed QN. Prevalence, awareness, treatment and control of hypertension in the Palestinian population. J Hum Hypertens Oct. 2013;27(10):623–8. 10.1038/jhh.2013.26. [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH, ASPC/NMA/PCNA Guideline for the Prevention. /, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. Jun 2018;71(6):e13-e115. 10.1161/hyp.0000000000000065 [DOI] [PubMed]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J Sep. 2018;1(33):3021–104. 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 12.Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. Jun 2019;51(6):1314–23. 10.1249/mss.0000000000001943. [DOI] [PubMed]
- 13.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertens Nov. 2003;42(5):878–84. 10.1161/01.Hyp.0000094221.86888.Ae. [DOI] [PubMed] [Google Scholar]
- 14.Graudal N, Hubeck-Graudal T, Jürgens G, Taylor RS. Dose-response relation between dietary sodium and blood pressure: a meta-regression analysis of 133 randomized controlled trials. Am J Clin Nutr May. 2019;1(5):1273–8. 10.1093/ajcn/nqy384. [DOI] [PubMed] [Google Scholar]
- 15.Kotseva K, Jennings CS, Turner EL, Mead A, Connolly S, Jones J, Bowker TJ, Wood DA. ASPIRE-2-PREVENT: a survey of lifestyle, risk factor management and cardioprotective medication in patients with coronary heart disease and people at high risk of developing cardiovascular disease in the UK. Heart Jun. 2012;98(11):865–71. 10.1136/heartjnl-2011-301603. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Patao C, Chuang J, Wong ND. Cardiovascular risk factor control and adherence to recommended lifestyle and medical therapies in persons with coronary heart disease (from the National Health and Nutrition Examination Survey 2007–2010). Am J Cardiol Oct. 2013;15(8):1126–32. 10.1016/j.amjcard.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 17.De Smedt D, De Sutter J, De Pauw M, Vandekerckhove H, Trouerbach J, De Backer G, Willems AM, Pardaens S, Vervaet P, Deweerdt N, et al. Lifestyle behaviour and risk factor control in coronary patients: Belgian results from the cross-sectional EUROASPIRE surveys. Acta Cardiol Feb. 2019;74(1):21–7. 10.1080/00015385.2018.1438092. [DOI] [PubMed] [Google Scholar]
- 18.Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, Maggioni A, Marques-Vidal P, Jennings C, Abreu A, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol May. 2019;26(8):824–35. 10.1177/2047487318825350. [DOI] [PubMed] [Google Scholar]
- 19.Kilic S, Sümerkan M, Emren V, Bekar L, Cersit S, Tunc E, Gök G, Altuntas E, Canpolat U, Sinan UY, et al. Secondary prevention of coronary heart disease in elderly population of Turkey: a subgroup analysis of ELDERTURK study. Cardiol J. 2019;26(1):13–9. 10.5603/CJ.a2017.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil Apr. 2009;16(2):121–37. 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]
- 21.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens Jun. 2020;38(6):982–1004. 10.1097/hjh.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 22.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama May. 2001;16(19):2486–97. 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care Jan. 2023;1(Suppl 1):S19–40. 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease’. Kidney Int Sep. 2013;84(3):622–3. 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 25.Smith SC Jr., Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation Nov. 2011;29(22):2458–73. 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Yan J, Tang L, Liu X. Risk factor control among heart failure patients in the United States: results from the NHANES 1999–2018. Int J Cardiol Cardiovasc Risk Prev Jun. 2022;13:200128. 10.1016/j.ijcrp.2022.200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Du C, Zhong X, Wang R, Tang L, Liu X. The secondary prevention of coronary heart disease in US adults 75 years and older in daily practice: results from the National Health and Nutrition Examination Survey 1999–2018 survey. Heliyon Apr. 2024;15(7):e28239. 10.1016/j.heliyon.2024.e28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyssoulis GP, Karpanou EA, Liakos CI, Kyvelou SM, Tzamou VE, Michaelides AP, Triantafyllou AI, Spanos PG, Stefanadis CI. Cardiovascular risk factor(s) prevalence in Greek hypertensives. Effect of gender and age. J Hum Hypertens Jul. 2012;26(7):443–51. 10.1038/jhh.2011.55. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg RB. Hyperlipidemia and cardiovascular risk factors in patients with type 2 diabetes. Am J Manag Care Aug. 2000;6(13 Suppl):S682–91. discussion S692-6. [PubMed] [Google Scholar]
- 30.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation May. 2003;6(17):2185–9. 10.1161/01.Cir.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 31.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health Oct. 2018;6(10):e1077–86. 10.1016/s2214-109x(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med Jan. 1998;15(2):81–8. 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Tocci G, Ferrucci A, Pontremoli R, Ferri C, Rosei EA, Morganti A, Trimarco B, Mancia G, Borghi C, Volpe M. Blood pressure levels and control in Italy: comprehensive analysis of clinical data from 2000–2005 and 2005–2011 hypertension surveys. J Hum Hypertens Nov. 2015;29(11):696–701. 10.1038/jhh.2015.4. [DOI] [PubMed] [Google Scholar]
- 34.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med Apr. 2002;2(7):493–503. 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Lesniak KT, Dubbert PM. Exercise and hypertension. Curr Opin Cardiol Nov. 2001;16(6):356–9. 10.1097/00001573-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Börjesson M, Onerup A, Lundqvist S, Dahlöf B. Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. Br J Sports Med. Mar 2016;50(6):356–61. 10.1136/bjsports-2015-095786. [DOI] [PubMed]
- 37.Yang MH, Kang SY, Lee JA, Kim YS, Sung EJ, Lee KY, Kim JS, Oh HJ, Kang HC, Lee SY. The Effect of Lifestyle changes on blood pressure control among hypertensive patients. Korean J Fam Med Jul. 2017;38(4):173–80. 10.4082/kjfm.2017.38.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenehjem JS, Hjerkind KV, Nilsen TIL. Adiposity, physical activity, and risk of hypertension: prospective data from the population-based HUNT Study, Norway. J Hum Hypertens Apr. 2018;32(4):278–86. 10.1038/s41371-018-0042-5. [DOI] [PubMed] [Google Scholar]
- 39.Alefan Q, Huwari D, Alshogran OY, Jarrah MI. Factors affecting hypertensive patients’ compliance with healthy lifestyle. Patient Prefer Adherence. 2019;13:577–85. 10.2147/ppa.S198446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conklin DJ, Schick S, Blaha MJ, Carll A, DeFilippis A, Ganz P, Hall ME, Hamburg N, O’Toole T, Reynolds L, et al. Cardiovascular injury induced by tobacco products: assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am J Physiol Heart Circ Physiol. Apr 2019;1(4):H801–27. 10.1152/ajpheart.00591.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518–25. 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: the atherosclerosis risk in communities Study. Hypertens May. 2001;37(5):1242–50. 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs FD, Fuchs SC. The Effect of Alcohol on blood pressure and hypertension. Curr Hypertens Rep Nov. 2021;11(10):42. 10.1007/s11906-021-01160-7. [DOI] [PubMed] [Google Scholar]
- 44.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open Dec. 2013;23(12):e003733. 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens Mar. 2005;23(3):475–81. 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 46.Beauchamp A, Peeters A, Wolfe R, Turrell G, Harriss LR, Giles GG, English DR, McNeil J, Magliano D, Harrap S, et al. Inequalities in cardiovascular disease mortality: the role of behavioural, physiological and social risk factors. J Epidemiol Community Health Jun. 2010;64(6):542–8. 10.1136/jech.2009.094516. [DOI] [PubMed] [Google Scholar]
- 47.Bruthans J, Mayer O Jr., De Bacquer D, De Smedt D, Reiner Z, Kotseva K, Cífková R. Educational level and risk profile and risk control in patients with coronary heart disease. Eur J Prev Cardiol May. 2016;23(8):881–90. 10.1177/2047487315601078. [DOI] [PubMed] [Google Scholar]
- 48.Landon BE, Hatfield LA, Bakx P, Banerjee A, Chen YC, Fu C, Gordon M, Heine R, Huang N, Ko DT, et al. Differences in Treatment Patterns and outcomes of Acute myocardial infarction for low- and high-income patients in 6 countries. Jama Apr. 2023;4(13):1088–97. 10.1001/jama.2023.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: an epidemiologic perspective. Epidemiol Rev. 1998;20(2):157–72. 10.1093/oxfordjournals.epirev.a017978. [DOI] [PubMed] [Google Scholar]
- 50.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their Cardiovascular complications. Circ Res May. 2016;27(11):1723–35. 10.1161/circresaha.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmena R, Ascaso JF, Redon J. Chronic kidney disease as a cardiovascular risk factor. J Hypertens Nov. 2020;38(11):2110–21. 10.1097/hjh.0000000000002506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. All the raw data used in this study are derived from the public NHANES data portal (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).