Abstract
Background
In Australia, diabetes is the fastest growing chronic condition, with prevalence trebling over the past three decades. Despite reported sex differences in diabetes outcomes, disparities in management and health targets remain unclear. This population-based retrospective study used MedicineInsight primary healthcare data to investigate sex differences in diabetes prevalence, incidence, management, and achievement of health targets.
Methods
Adults (aged ≥ 18 years) attending 39 general practices in Western Australia were included. Diabetes incidence and prevalence were estimated by age category. Health targets assessed included body mass index (BMI), blood pressure, blood lipids, and glycated haemoglobin (HbA1c) levels. Medical management of diabetes-associated conditions was also investigated. Time-to-incident diabetes was modelled using a Weibull regression. A multilevel mixed-effects logistic regression model investigated risk-adjusted sex differences in achieving the HbA1c health target (HbA1c ≤ 7.0% (≤ 53 mmol/mol)).
Results
Records of 668,891 individuals (53.4% women) were analysed. Diabetes prevalence ranged from 1.3% (95% confidence interval (CI) 1.2%-1.3%) in those aged < 50 years to 7.2% (95% CI 7.1%-7.3%) in those aged ≥ 50 years and was overall higher in men. In patients younger than 30 years, incidence was higher in women, with this reversing after the age of 50. Among patients with diabetes, BMI ≥ 35 kg/m2 was more prevalent in women, whereas current and past smoking were more common in men. Women were less likely than men to achieve lipid health targets and less likely to receive prescriptions for lipid, blood pressure, or glucose-lowering agents. Men with incident diabetes were 21% less likely than women to meet the HbA1c target. Similarly, ever recorded retinopathy, nephropathy, neuropathy, hypertension, dyslipidaemia, coronary heart disease, heart failure, peripheral vascular disease and peripheral artery disease were higher in men than women.
Conclusions
This research underscores variations in diabetes epidemiology and management based on sex. Tailoring diabetes management should consider the patient's sex.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03698-0.
Keywords: Diabetes, Sex, Prevalence, Incidence, Health targets
Background
Diabetes is a leading cause of disability and mortality worldwide with increasing global prevalence and over 1.5 million deaths directly attributable to it each year [1]. In 2021, the International Diabetes Federation estimated that 537 million people lived with diabetes globally [2], with estimates projected to rise to 1.3 billion in 2050 [3]. Diabetes manifests primarily in two prevalent forms, namely type 1 and type 2. Among these, type 2 diabetes is the more widespread type, responsible for approximately 95% of disability-adjusted life years attributed to this chronic condition [3]. Diabetes is also a key risk factor for stroke and coronary heart disease [4, 5], which are the two global leading causes of disease burden [6].
Previous research has highlighted sex differences in diabetes prevalence, incidence, and outcomes. The global age-standardised diabetes prevalence is higher in men than in women, with a male-to-female ratio of 1.14 (6.5% versus 5.8% respectively) [3]. Compared to women, men are often diagnosed with type 2 diabetes at a younger age and with a lower body mass index (BMI) [7, 8]. In contrast, at the time of diagnosis, women, especially young women, often exhibit a greater burden of risk factors such as higher blood pressure and more obesity than men [9]. Sex differences in diabetes associated outcomes have also been reported with some being worse in women. A systematic review showed that relative risks of developing coronary heart disease and stroke due to diabetes were higher in women compared to men, and after adjusting for major vascular risk factors, diabetes was linked to a nearly 50% higher rate of occlusive vascular mortality among women compared to men [10, 11]. Increased cardiovascular risk factors in women with diabetes and disparities in diabetes treatment favouring men have been suggested as contributing factors [12, 13]. It has been reported that women with diabetes are less likely than their male counterparts to achieve glycaemic control and target levels of glycated haemoglobin (HbA1c) [14]. A study conducted in the US found poorer control of blood pressure and low-density lipoprotein (LDL) cholesterol in women compared to men, suggesting that such treatment disparities contributed to the observed sex differences in cardiovascular mortality, to the detriment of women [15].
In Australia, diabetes is the fastest growing chronic condition, increasing at a faster rate than other chronic diseases such as heart disease and cancer, with prevalence trebling over the past three decades [16, 17]. As of 2021, approximately 1 in every 20 Australians was living with diabetes. While there has been a decline in age-standardised diabetes-related mortality over the years, peaking in 2008 (62 per 100,000 population) and steadily declining to 54 per 100,000 population in 2020 [16], there has been an increase in the incidence of medical complications, particularly among men [18]. Despite the increasing prevalence and the sex disparities in diabetes outcomes, it is not known if there are disparities in management and in achievement of health targets in Australia.
To shed more light on this matter, this population-based study investigated sex differences in the prevalence, incidence, and management of diabetes, using a large sample of routinely collected primary healthcare data in Western Australia. This study aimed to provide a sex-stratified snapshot of glycaemic control and diabetes management over the last year of clinical interactions between patients and their general practitioners (GPs). Our approach of examining diabetes management as a snapshot is well-established and frequently employed [19, 20].
Methods
Data source and study sample
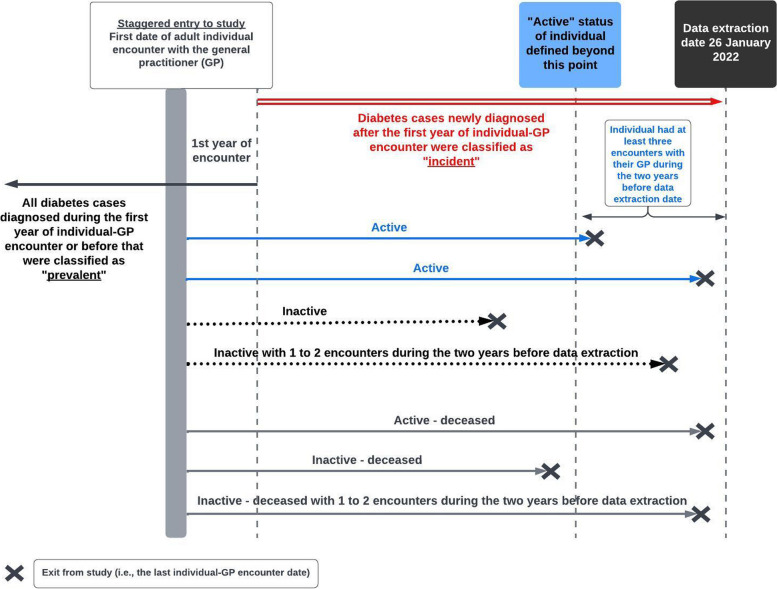
The study followed a retrospective cohort design with staggered entry. Adults (aged ≥ 18 years) who visited a GP for any reason at one of the 39 MedicineInsight participating general practices in Western Australia were included in this study. As of the data extraction date of January 26, 2022, patients attending these clinics were categorised as follows: "active," defined as having had at least three encounters with the GP in the two years preceding data extraction; "inactive," defined as having had fewer than three such encounters during that period; or "deceased" (Fig. 1).
Fig. 1.
Staggered entry sequence diagram
The de-identified electronic health records were extracted from the MedicineInsight database, an Australian national general practice data programme established by NPS MedicineWise, which included records from general practices that had consented to be part of the programme [21, 22]. The programme uses validated diagnostic algorithms to identify individuals with chronic diseases [23]. Approval for access to the data was given by the NPS MedicineWise Data Governance Committee (2020–003).
Information from MedicineInsight used in this study included demographics, diagnoses, reasons for consultations, laboratory and pathology investigation requests and their results, prescription data, patient screening, anthropometric measurements based on measured weight and height, smoking status and clinical measurements. Dates of diagnoses, tests, referrals, and medical treatments were also available. Socioeconomic disadvantage measure was based on the Socio-Economic Indexes for Areas – Index of Relative Socio-Economic Disadvantage (SEIFA-IRSD) [24], which is a residential postcode-based composite score that ranks geographic areas across Australia according to their relative socioeconomic advantage or disadvantage. All diagnoses were obtained from the “diagnosis”, “reason for encounter” and “reason for prescription” data fields using data extraction methods used by MedicineInsight, including standard clinical terminologies, misspellings, and abbreviations [21–23].
Ascertainment of diabetes mellitus (type 1, type 2, or unspecified type)
Diabetes mellitus (in this paper, referred to as “diabetes”) case identification was based on recorded diagnoses, prescription reasons, pathology results [25–27], and Medicare Benefits Schedule (MBS) item codes indicating presence of diabetes. MBS is an Australian government-funded list of medical services subsidised for Australian citizens, promoting accessible and affordable healthcare. To optimise the accuracy of diabetes detection, it was necessary to use two distinct records for the same individual to confirm the presence of diabetes [28]. To be defined as having diabetes, a patient needed to meet any of the following criteria:
1) Two separate diagnosis records indicating diabetes; 2) two separate HbA1c results ≥6.5% (48 mmol/mol); 3) two separate fasting plasma glucose tests ≥7.0 mmol/L; 4) two separate plasma glucose tests ≥11.1 mmol/L; 5) two separate recorded prescriptions of glucose lowering medications (Anatomical Therapeutic Chemical code: A10); and 6) two separate MBS item codes indicating management or diagnosis of diabetes (codes 66551, 66554, 66841, 73812, 73826, 73839, 73840, 81100, 81105, 81110, 81115, 81120, 81125, and historic codes 2517-2526, and 2620-2635).
The earliest recorded date of any of the above criteria was used as the diagnosis date.
Exclusion criteria
Without evidence of type 1, type 2, or unspecified type of diabetes, the following conditions were not counted as diabetes for the purposes of this study:
1) Gestational diabetes mellitus; 2) pre-diabetes managed with metformin; and 3) polycystic ovary syndrome managed with metformin.
Definitions
Type of diabetes
Individuals identified as having diabetes were categorised as having type 2 if they had a recorded diagnosis indicating type 2 diabetes, non-insulin-dependent diabetes, or adult-onset diabetes. Individuals were recorded as having type 1 if they had a recorded diagnosis indicating type 1 diabetes or insulin-dependent diabetes. Those with a recorded diagnosis of diabetes with an unknown type (for example, “diabetes mellitus”) were classified as having unspecified diabetes. The majority rule was applied in cases where multiple types of diabetes were documented for a patient, determining the patient's classification based on the most frequently documented type. If different types were equally documented, the patient was classified as having "unspecified diabetes".
Study entry and exit
Patients entered the study on the initial date of their adult clinical encounter with the GP and exited either upon the patients’ death or upon their last clinical encounter in any of the 39 participating general practices. (Fig. 1).
Prevalence versus incidence
Cases diagnosed with diabetes over a period spanning 395 days from the first date of adult clinical encounter or before that were classified as prevalent (Fig. 1). To account for delays in patients’ electronic health recordings, “395 days” instead of the yearly “365 days” was selected. Similarly, patients diagnosed with diabetes based on abnormal HbA1c levels within 12 weeks after 395 days from the first patient-GP encounter were regarded as prevalent cases as HbA1c levels reflect average plasma glucose over the previous 8–12 weeks from the time of the test [29].
Cases diagnosed after 395 days from the first patient-GP encounter (or after 479 days for HbA1c criterion) were classified as incident cases. All prevalent cases were excluded from the incidence estimation. Women who had a history of gestational diabetes but did not show evidence of type 1, type 2, or unspecified diabetes were included among those at risk of developing diabetes mellitus as such women were at high risk of developing type 2 diabetes [30, 31].
Cases with unknown diagnosis date were classified as unknown prevalent or incident diabetes.
Patient-GP consultations
Multiple consultations occurring on the same day for the same patient were considered as single consultations.
BMI measurements
We used the BMI estimate recorded in the MedicineInsight database. If this estimate was not available, we computed BMI using the measured weight and height of the participants.
Health targets and management
The clinical management goals assessed in this study align with guidelines from the Royal Australian College of General Practitioners (RACGP) [27]. Individual management goals encompass smoking cessation and BMI, while treatment management goals include HbA1c, lipid levels, urine albumin creatinine ratio, vaccination, and blood pressure. Screening for potential diabetes-related conditions (ever recorded in patient health records) and the pharmacological approaches to managing blood pressure, dyslipidaemia, and diabetes were also investigated.
Statistical analysis
Diabetes incidence (all types) (measured as cases per 1,000 person-years of follow-up) and prevalence rates (measured as cases diagnosed over a period spanning 395 days from the first date of adult clinical encounter or before, divided by the number of people in the sample) were estimated with their 95% confidence intervals (CI). Characteristics of the overall cohort as well as individuals with and without diabetes were summarised using standard measures of central tendency and dispersion. Pearson’s χ2 test compared the frequencies in categorical variables, while a Mann–Whitney test compared the mean ranks of continuous variables. Prevalence and incidence of diabetes were each compared by the sexes stratified by age groups. Comorbidities and medical conditions were compared by sex stratified by diabetes type and duration.
Clinical and screening measures and health targets were compared by sex and type of diabetes. To be included in this analysis, patients with prevalent diabetes needed at least three years of follow-up while those with incident diabetes required three years of follow-up after their diabetes diagnosis.
Multivariable analysis: time to incident diabetes
The proportional hazards assumption was violated, rendering Cox regression unsuitable for analysis. Instead, time-to-incident diagnosis of diabetes (all types) was modelled using an accelerated time Weibull regression which provided the best fit with the lowest Akaike Information Criteria (AIC) compared to other parametric survival distributions. Study participants without evidence of prevalent diabetes were followed up from the first adult clinical encounter until they were diagnosed with incident diabetes or died or were right censored at the last clinical encounter. The analysis adjusted for age at first clinical encounter, sex, SEIFA-IRSD, smoking status and BMI (both at the first adult clinical encounter), and Indigenous ethnicity, while also accounting for clustering effects within the 39 participating general practices. Risk adjusted probability of remaining free of diabetes over time was plotted by sex.
Multivariable analysis: achievement of glycaemic control in the last year of clinical encounter among patients with incident diabetes
Sex differences in achieving glycaemic control [HbA1c ≤ 7.0% (≤ 53 mmol/mol)] (yes/no) over a period of up to 395 days, ending at the last clinical encounter, in patients with incident type 2 or unspecified diabetes who had at least three years of follow-up from diagnosis, were modelled using a multilevel mixed effects logistic regression. The multivariable model adjusted for 1) demographics (sex, age at last clinical encounter, SEIFA-IRSD, and Indigenous ethnicity); 2) BMI and smoking status as recorded over a period spanning 395 days up to the last clinical encounter); 3) years of follow-up; 4) active status of the patient; 5) baseline adult first recorded HbA1c level; and 5) clinical conditions that could have resulted in falsely high or falsely low HbA1c levels including anaemia, chronic kidney disease, chronic liver disease, hypertriglyceridaemia, and pregnancy [32–35]. The model also adjusted for cluster effects within the 39 participating general practices.
Multivariable analysis: sex differences in diabetes management in prevalent and incident cases
Sex disparities in the absence of tests or clinical assessments, lack of screening for diabetes-related conditions, and non-treatment with medications for diabetes-associated conditions over a period spanning 395 days up to the last clinical encounter were each analysed using multilevel mixed-effects logistic regression models. These models were adjusted for age at the last clinical encounter, sex, SEIFA-IRSD, smoking status, BMI, Indigenous ethnicity, rurality, duration of follow-up, and type of diabetes. Additionally, the models accounted for clustering effects within the 39 participating general practices.
The analyses were conducted separately for prevalent and incident cases, each requiring at least three years of follow-up from their initial adult clinical encounter or from their diabetes incident diagnosis, respectively.
Sensitivity analyses
In sensitivity analyses, sex differences in achieving glycaemic control were separately modelled after excluding pregnant women (over the period of 395 days up to the last clinical encounter) and/or after limiting the analyses to type 2 diabetes.
All analyses were performed using Stata/MP 17.0 (StataCorp, College Station, TX, USA).
Results
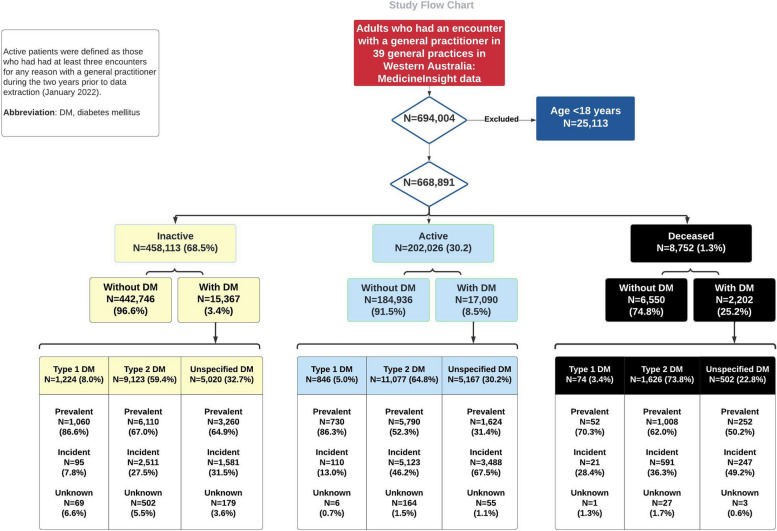
Records of 668,891 individuals (53.4% women, mean follow-up 3.7 ± 5.1 years) from 39 general practices in Western Australia were analysed. Of these, 202,026 (30.2%) were classified as “active”, 458,113 (68.5%) as “inactive”, and 8,752 (1.3%) as “deceased” at the time of data extraction. Within these categories, diabetes was identified in 8.5%, 3.4%, and 25.2%, respectively. Among the total 34,659 patients diagnosed with diabetes, 6.2% were classified as type 1 diabetes, 63.0% as type 2 diabetes, and 30.8% had unspecified diabetes (Fig. 2). Compared to those without diabetes, patients with diabetes were more likely to be older, male, overweight or obese, to come from disadvantaged socioeconomic backgrounds, and to have had more consultations over a period of 395 days up to the last year of clinical encounter with longer years of follow-up (7.3 ± 6.5 years in those with diabetes versus 3.5 ± 6.5 years in those without) (Additional file 1: Table S1).
Fig. 2.
Cohort selection – study flow chart
As expected, type 1 diabetes was predominantly (85.9%) prevalent in our adult cohort, whereas 37.7% of type 2 diabetes and 49.7% of unspecified type cases were incident diabetes (Fig. 2).
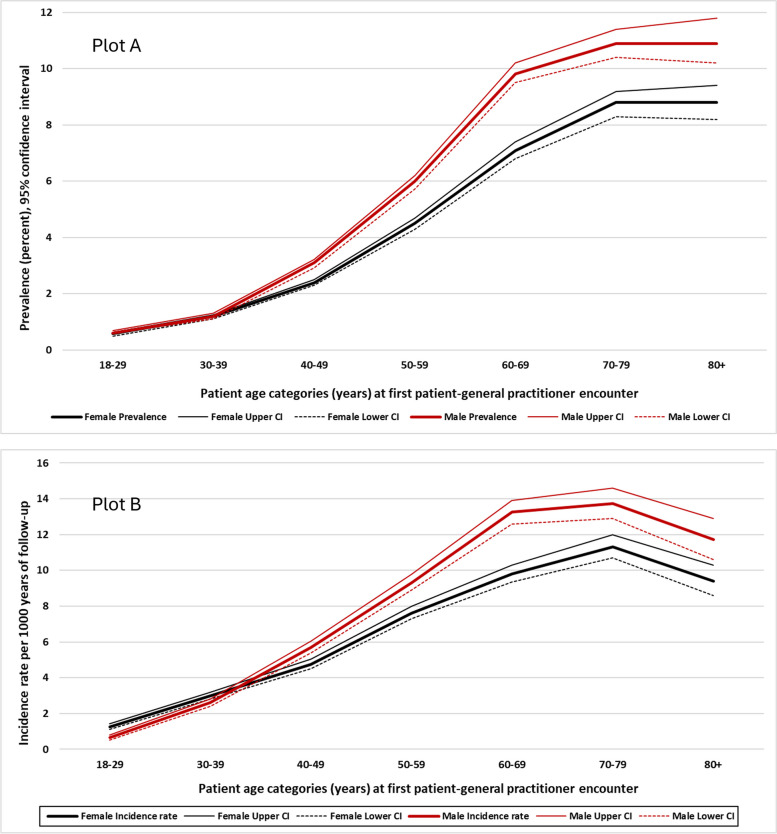
Prevalence and incidence estimates of diabetes (all types) classified by the “active” patient status, age and sex are presented in Table 1. Overall, the prevalence among those aged ≥ 50 years was 7.2% (95% CI 7.1%—7.3%), being significantly higher in men (8.3% (95% CI 8.1% – 8.5%)) than in women (6.3% (95% CI 6.2%—6.5%)), p < 0.001. The prevalence was also higher in men among individuals younger than 50 years old (Fig. 3, Plot A).
Table 1.
Prevalence and incidence rates of diabetes (all types) by sex, age category, and active statusa of the individual
| Women N = 356,910 |
Men N = 305,719 |
Allb N = 668,891 |
|||||
|---|---|---|---|---|---|---|---|
| Age <50 years | Age ≥50 years | Age <50 years | Age ≥50 years | Age <50 years | Age ≥50 years | ||
|
Prevalence Percent (95% CI) |
Active | 1.7 (1.6 – 1.7) | 7.6 (7.3 – 7.8) | 2.1 (2.0 – 2.3) | 10.1 (9.7 – 10.4) | 1.9 (1.8 – 1.9) | 8.7 (8.5 – 8.9) |
| Inactive | 0.9 (0.8 – 1.0) | 5.2 (5.1 – 5.4) | 1.1 (1.0 – 1.2) | 6.7 (6.4 – 6.9) | 1.0 (0.9 – 1.0) | 5.8 (5.7 – 6.0) | |
| Deceased | 8.9 (6.6 – 12.0) | 15.0 (13.8 – 16.2) | 6.5 (4.9 – 8.7) | 17.0 (15.9 – 18.2) | 7.5 (6.1 – 9.2) | 16.1 (15.2 – 16.9) | |
| All | 1.2 (1.1 – 1.2) | 6.3 (6.2 – 6.5) | 1.4 (1.3 – 1.5) | 8.3 (8.1 – 8.5) | 1.3 (1.2 – 1.3) | 7.2 (7.1 – 7.3) | |
| Incidence rate per 1000 years (95% CI) | Active | 5.7 (5.5 – 6.0) | 10.5 (10.0 – 11.0) | 5.9 (5.7 – 6.2) | 13.1 (12.6 – 13.7) | 5.8 (5.7 – 6.0) | 11.7 (11.3 – 12.1) |
| Inactive | 1.9 (1.8 – 2.0) | 7.5 (7.1 – 8.0) | 2.5 (2.4 – 2.7) | 9.8 (9.2 – 10.4) | 2.2 (2.1 – 2.3) | 8.5 (8.2 – 8.9) | |
| Deceased | 11.9 (8.8 – 16.1) | 13.7 (12.3 – 15.3) | 10.8 (8.2 – 14.1) | 16.4 (15.0 – 18.1) | 11.2 (9.2 – 13.7) | 15.1 (14.1 – 16.2) | |
| All | 3.8 (3.7 – 3.9) | 9.5 (9.2 – 9.8) | 4.2 (4.0 – 4.4) | 12.1 (11.7 – 12.5) | 4.0 (3.9 – 4.1) | 10.7 (10.4 – 10.9) | |
aActive status was defined as having at least three encounters for any reason with a general practitioner during the two years prior to data extraction date (January 2022)
bThe total sample also included 6,262 individuals whose sex was either different from male or female or was unknown
Fig. 3.
Prevalence and incidence of diabetes mellitus by sex and age category
The overall incidence rate of diabetes per 1000 years of follow-up was 5.9 (95% CI 5.8–6.0), ranging from 4.0 per 1000 years (95% CI 3.9–4.1) among those younger than 50 years to 10.7 per 1000 years (95% CI 10.4–10.9) in those aged ≥ 50 years (Table 1). In patients ≤ 30 years, diabetes incidence was higher in women compared to men; however, higher incident rates in men were consistently observed after the age of 50 years (Fig. 3, Plot B and Fig. 4).
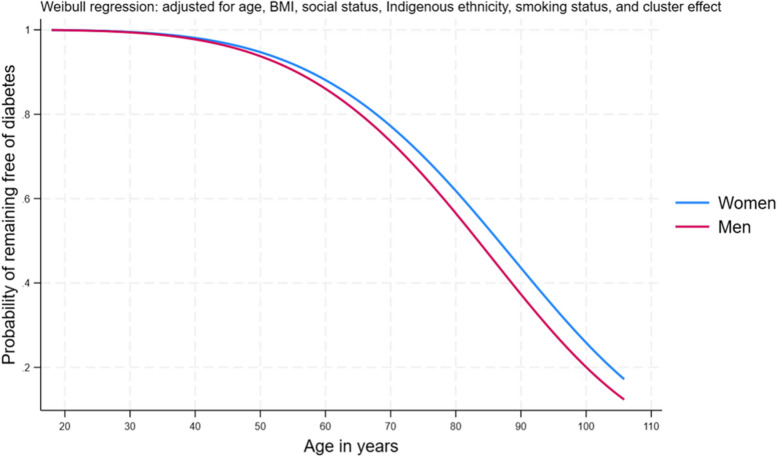
Fig. 4.
Risk-adjusted probability of remaining free of diabetes by sex
Adjusting for age, BMI and smoking status (all three as recorded at first adult clinical encounter), and Indigenous ethnicity, socioeconomic disadvantage, and cluster effect within the general practices, men were 19% more likely than women to be diagnosed with incident diabetes (adjusted hazard ratio 1.19, 95% CI 1.09–1.30, p < 0.001) (Additional file 1: Table S2). Over time, the increased risk was considerably higher in men than women as shown in Fig. 4.
At the last clinical encounter, burden of diseases varied by the sexes who had diabetes. Ever recorded coronary heart disease, heart failure, hypertension, peripheral vascular disease and peripheral artery disease were significantly higher in men compared to women (Table 2). In type 1 diabetes, diabetes associated metabolic conditions, specifically hypoglycaemia, were more common in women. Stroke and transient ischaemic attack were evenly distributed in both men and women with type 1 and type 2 diabetes; however, these were more commonly reported in men with unspecified diabetes type. In all types of diabetes combined, the prevalence of most of the conditions was higher in men than in women (Additional file 1: Table S3). Years of follow-up in all types of diabetes were slightly higher in women compared to men (mean ± standard deviation 7.4 ± 6.6 years versus 7.2 ± 6.5 years).
Table 2.
Ever recorded conditions by type, duration of diabetes mellitus, and sexa at the last clinical encounter: n (%)
| Type 1 diabetes mellitus | Type 2 diabetes mellitus | Unspecified diabetes mellitus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes duration 1 to 10 years |
Diabetes duration >10 years |
Diabetes duration 1 to 10 years |
Diabetes duration >10 years |
Diabetes duration 1 to 10 years |
Diabetes duration >10 years |
|||||||
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| N=480 | N=603 | N=468 | N=512 | N=5829 | N=6858 | N=3922 | N=4472 | N=4769 | N=3660 | N=1027 | N=957 | |
| Retinopathy | 13 (2.7) | 19 (3.2) | 47 (10.0) | 68 (13.3) | 89 (1.5) | 119 (1.7) | 175 (4.5) | 238 (5.3) | 21 (0.4) | 21 (0.6) | 22 (2.1) | 30 (3.1) |
| Nephropathy | 18 (3.8) | 19 (3.2) | 23 (4.9) | 37 (7.2) | 404 (6.9) | 528 (7.7) | 498 (12.7) | 591 (13.2) | 119 (2.5) | 137 (3.7)** | 76 (7.4) | 77 (8.1) |
| Neuropathy | 23 (4.8) | 29 (4.8) | 36 (7.7) | 47 (9.2) | 449 (7.7) | 565 (8.2) | 409 (10.4) | 551 (12.3)* | 260 (5.4) | 185 (5.1) | 61 (5.9) | 64 (7.7) |
| Hypertension | 57 (11.9) | 117 (19.4)** | 120 (25.6) | 156 (30.5) | 3194 (54.8) | 3784 (55.2) | 2716 (69.2) | 2954 (66.1)* | 1399 (29.3) | 1361 (37.2)** | 499 (48.6) | 454 (47.4) |
| Dyslipidaemia | 53 (11.0) | 87 (14.4) | 102 (21.8) | 105 (20.5) | 2459 (42.2) | 2955 (43.1) | 2022 (51.6) | 2229 (49.8) | 1117 (23.4) | 1006 (27.5)** | 361 (35.2) | 305 (31.9) |
| PVD / PAD | 4 (0.8) | 5 (0.8) | 12 (2.6) | 20 (3.9) | 83 (1.4) | 191 (2.8)** | 145 (3.7) | 278 (6.2)** | 21 (0.4) | 49 (1.3)** | 20 (1.9) | 26 (2.7) |
| CHD | 22 (4.6) | 32 (5.3) | 51 (10.9) | 54 (10.6) | 695 (11.9) | 1292 (18.8)** | 850 (21.7) | 1351 (30.2)** | 281 (5.9) | 456 (12.5)** | 159 (15.5) | 228 (23.8)** |
| Heart failure | 6 (1.3) | 4 (0.7) | 16 (3.4) | 13 (2.5) | 234 (4.0) | 340 (5.0)* | 355 (9.1) | 461 (10.3) | 93 (2.0) | 135 (3.7)** | 65 (6.3) | 77 (8.1) |
| Stroke/TIA | 7 (1.5) | 12 (2.0) | 20 (4.3) | 34 (6.6) | 600 (10.3) | 648 (9.5) | 626 (16.0) | 662 (14.8) | 290 (6.1) | 247 (6.7) | 86 (8.4) | 107 (11.2)* |
| Cancer | 28 (5.8) | 30 (5.0) | 58 (12.4) | 51 (10.0) | 1148 (19.7) | 1236 (18.0)* | 1107 (28.2) | 1380 (30.9)* | 641 (13.4) | 495 (13.5) | 204 (19.9) | 186 (19.4) |
| Metabolicb | 25 (5.2) | 16 (2.7)* | 40 (8.6) | 21 (4.1)* | 26 (0.5) | 31 (0.5) | 33 (0.8) | 33 (0.7) | 14 (0.3) | 6 (0.2) | 9 (0.9) | 4 (0.4) |
| Otherc | 1 (0.2) | 2 (0.3) | 2 (0.4) | 3 (0.6) | 35 (0.6) | 23 (0.3)* | 29 (0.7) | 24 (0.5) | 23 (0.5) | 12 (0.3) | 3 (0.3) | 4 (0.4) |
Abbreviations: CHD Coronary heart disease, PAD Peripheral artery disease, PVD Peripheral vascular disease, TIA Transient ischaemic attack
Sexes were compared in each diabetes duration period in each type of diabetes with ≤0.05 * ≥0.001; **<0.001
aNot included in the table: 108 individuals with unknown sex, and 524 and 470 males and females, respectively, who did not have a known diabetes diagnosis date
bMetabolic conditions included diabetic ketoacidosis (DKA), hyperglycaemic non-ketotic coma (HONK), and hypoglycaemia
cOther included cheiroarthropathy and periodontitis
Sex differences were consistently observed in diabetes management over the period spanning 395 days up to the last clinical encounter, whether for prevalent cases (Table 3) or newly diagnosed ones (Table 4). Approximately 77.4% and 86.7% of prevalent and incident cases, respectively were overweight or obese, with obesity class II and class III or more being significantly more prevalent among women, p < 0.001. BMI ≥ 35 kg/m2 was significantly less prevalent in patients with type 1 diabetes compared to those with type 2 or unspecified diabetes, consistently observed in both sexes (Tables 3 and 4). Women were less likely than men to achieve lipid health targets and less likely to be prescribed with lipid modifying agents over the period of 395 days up to the last clinical encounter (35.7% in women versus 44.7% in men, p < 0.001). Sex differences in lipid management remained after limiting this comparison to those with a confirmed diagnosis of dyslipidaemia (62.3% in women versus 69.0% in men, p < 0.001). Confined to those with a confirmed diagnosis of hypertension, management with blood pressure lowering agents over the period of 395 days up to the last clinical encounter was also significantly lower in women (74.3%) than in men (76.2%), p = 0.003. Similarly, women diagnosed with diabetes were significantly less likely than their male counterparts to receive glucose lowering medications, (56.0% in women versus 59.2% in men, p < 0.001). Sex disparities in lipid, blood pressure, and glucose management remained consistent when the analyses were stratified by prevalent or incident diabetes among patients with at least three years of follow-up as shown in Tables 3 and 4.
Table 3.
Sex-stratified age, follow-up, health targets, clinical management goals, prescription, and screening over a period spanning 395 days up to the last clinical encounter in patients with a prevalent diabetes with at least 3 years of follow-up: n (%) if not otherwise stated
| All types of DM | Type 1 DM | Type 2 DM | Unspecified type DM | |||||
|---|---|---|---|---|---|---|---|---|
| Women N=4,580 |
Men N=5,078 |
Women N=417 |
Men N=493 |
Women N=3,385 |
Men N=3,875 |
Women N=778 |
Men N=710 |
|
| Age at last encounter (years), mean (SD) | 65.5 (16.9) | 65.3 (15.2) | 46.9 (18.3) | 47.3 (16.9) | 68.6 (14.8) | 67.8 (13.1)* | 61.9 (17.7) | 64.2 (15.7)* |
| Adult follow-up (years), mean (SD) | 8.6 (4.9) | 8.2 (4.6)** | 8.9 (5.3) | 8.4 (4.8) | 8.8 (5.0) | 8.4 (4.7)** | 7.4 (4.2) | 7.4 (4.2) |
| HbA1c ≤7.0% (≤53 mmol/mol) | ||||||||
| Yes | 1,496 (32.7) | 1,765 (34.8)** | 64 (15.3) | 76 (15.4) | 1,261 (37.2) | 1,496 (38.6)** | 171 (22.0) | 193 (27.2)* |
| No | 1,293 (28.2) | 1,613 (31.8) | 167 (40.0) | 193 (39.1) | 995 (29.4) | 1,269 (32.7) | 131 (16.8) | 151 (21.3) |
| Nottested | 1,791 (39.1) | 1,700 (33.5) | 186 (44.6) | 224 (45.4) | 1,129 (33.3) | 1,110 (28.6) | 476 (61.2) | 366 (51.5) |
| BP ≤140/90 mm Hg | ||||||||
| Yes | 1,331 (29.1) | 1,567 (30.9)** | 158 (37.9) | 139 (28.2)** | 989 (29.2) | 1,233 (31.8)** | 184 (23.6) | 195 (27.5)** |
| No | 1,760 (38.4) | 2,174 (42.8) | 82 (19.7) | 161 (32.7) | 1,481 (43.7) | 1,780 (45.9) | 197 (25.3) | 233 (32.8) |
| Notmeasured | 1,489 (32.5) | 1,337 (26.3) | 177 (42.4) | 193 (39.1) | 915 (27.0) | 862 (22.2) | 397 (51.0) | 282 (39.7) |
| BP ≤130/80 mm Hg | ||||||||
| Yes | 546 (11.9) | 625 (12.3)** | 80 (19.2) | 66 (13.4)* | 380 (11.2) | 477 (12.3)** | 86 (11.0) | 82 (11.5)** |
| No | 2,545 (55.6) | 3,116 (61.4) | 160 (38.4) | 234 (47.5) | 2,090 (61.7) | 2,536 (65.4) | 295 (37.9) | 346 (48.7) |
| Not measured | 1,489 (32.5) | 1,337 (26.3) | 177 (42.4) | 193 (39.1) | 915 (27.0) | 862 (22.2) | 397 (51.0) | 282 (39.7) |
| Total cholesterol <4.0 mmol/L | ||||||||
| Yes | 697 (15.2) | 1,293 (25.5)** | 39 (9.3) | 58 (11.8) | 581 (17.2) | 1,136 (29.3)** | 77 (9.9) | 99 (13.9)* |
| No | 1,689 (36.9) | 1,655 (32.6) | 141 (33.8) | 158 (32.0) | 1,354 (40.0) | 1,298 (33.5) | 194 (24.9) | 199 (28.0) |
| Not tested | 2,194 (47.9) | 2,130 (41.9) | 237 (56.8) | 277 (56.2) | 1,450 (42.8) | 1,441 (37.2) | 507 (65.2) | 412 (58.0) |
| LDL-Ca | ||||||||
| Yes | 767 (16.7) | 1,140 (22.4)** | 36 (8.6) | 37 (7.5) | 651 (19.2) | 1,013 (26.1)** | 80 (10.3) | 90 (12.7)* |
| No | 1,425 (31.1) | 1,531 (30.2) | 124 (29.7) | 154 (31.2) | 1,134 (33.5) | 1,194 (30.8) | 167 (21.5) | 183 (25.8) |
| Nottested | 2,388 (52.1) | 2,407 (47.4) | 257 (61.6) | 302 (61.3) | 1,600 (47.3) | 1,668 (43.0) | 531 (68.2) | 437 (61.5) |
| HDL-C ≥1.0 mmol/L | ||||||||
| Yes | 1,930 (42.1) | 1,793 (35.3)** | 152 (36.4) | 174 (35.3) | 1,545 (45.6) | 1,425 (36.8)** | 233 (29.9) | 194 (27.3)** |
| No | 326 (7.1) | 984 (19.4) | 10 (2.4) | 23 (4.7) | 291 (8.6) | 877 (22.6) | 25 (3.2) | 84 (11.8) |
| Nottested | 2,324 (50.7) | 2,301 (45.3) | 255 (61.1) | 296 (60.0) | 1,549 (45.8) | 1,573 (40.6) | 520 (66.8) | 432 (60.8) |
| Triglycerides <2.0 mmol/L | ||||||||
| Yes | 1,348 (29.4) | 1,631 (32.1)** | 156 (37.4) | 167 (33.9)* | 1,018 (30.1) | 1,303 (33.6)** | 174 (22.4) | 161 (22.7)* |
| No | 1,028 (22.4) | 1,309 (25.8) | 23 (5.5) | 48 (9.7) | 910 (26.9) | 1,125 (29.0) | 95 (12.2) | 136 (19.1) |
| Nottested | 2,204 (48.1) | 2,138 (42.1) | 238 (57.1) | 278 (56.4) | 1,457 (43.0) | 1,447 (37.3) | 509 (65.4) | 413 (58.2) |
| Non-HDL-C <2.5 mmol/L | ||||||||
| Yes | 80 (1.7) | 153 (3.0)** | 4 (1.0) | 8 (1.6) | 67 (2.0) | 135 (3.5)** | 9 (1.2) | 10 (1.4) |
| No | 263 (5.7) | 290 (5.7) | 22 (5.3) | 30 (6.1) | 202 (6.0) | 218 (5.6) | 39 (5.0) | 42 (5.9) |
| Not tested | 4,237 (92.5) | 4,635 (91.3) | 391 (93.8) | 455 (92.3) | 3,116 (92.0) | 3,522 (90.9) | 730 (93.8) | 658 (92.7) |
| Urine albumin-creatinine ratio (uACR)b | ||||||||
| Yes | 1,285 (28.1) | 1,362 (26.8)** | 113 (27.1) | 146 (29.6) | 1,034 (30.5) | 1,074 (27.7)** | 138 (17.7) | 142 (20.0)* |
| No | 665 (14.5) | 1,120 (22.1) | 38 (9.1) | 58 (11.8) | 561 (16.6) | 974 (25.1) | 66 (8.5) | 88 (12.4) |
| Nottested | 2,630 (57.4) | 2,596 (51.1) | 266 (63.8) | 289 (58.6) | 1,790 (52.9) | 1,827 (47.1) | 574 (73.8) | 480 (67.6) |
| Glucose lowering medications (ever prescription)I | 4,028 (88.0) | 4,545 (89.5)* | 387 (92.8) | 462 (93.7) | 3,024 (89.3) | 3,519 (90.8)* | 617 (79.3) | 564 (79.4) |
| Glucose lowering medications (prescription over a period of 395 days up to last encounter)I | 2,856 (62.4) | 3,496 (68.8)** | 270 (64.7) | 332 (67.3) | 2,269 (67.0) | 2,821 (72.8)** | 317 (40.7) | 343 (48.3)* |
| Lipid modifying agents (ever prescription)II | 3,090 (67.5) | 3,692 (72.7)** | 158 (37.9) | 202 (41.0) | 2,523 (74.5) | 3,054 (78.8)** | 409 (52.6) | 436 (61.4)* |
| Lipid modifying agents (prescription over a period of 395 days up to last encounter)II | 2,159 (47.1) | 2,791 (55.0)** | 97 (23.3) | 141 (28.6) | 1,818 (53.7) | 2,367 (61.1)** | 244 (31.4) | 283 (39.9)* |
| Blood pressure lowering agents (ever prescription)III | 3,842 (83.9) | 4,217 (83.0) | 285 (68.3) | 286 (58.0)* | 2,990 (88.3) | 3,396 (87.6) | 567 (72.9) | 535 (75.3) |
| Blood pressure lowering agents (prescription over a period of 395 days up to last encounter)III | 2,750 (60.0) | 3,227 (63.5)** | 154 (36.9) | 174 (35.3) | 2,270 (67.1) | 2,695 (69.5)* | 326 (41.9) | 358 (50.4)* |
| BMI (kg/m2) | ||||||||
| Underweight: <18.5 | 16 (0.3) | 4 (0.1)** | 7 (1.7) | 2 (0.4)** | 5 (0.1) | 1 (0.0)** | 4 (0.5) | 1 (0.1)** |
| Normal weight: 18.5-24.9 | 360 (7.9) | 384 (7.6) | 91 (21.8) | 89 (18.0) | 212 (6.3) | 237 (6.1) | 57 (7.3) | 58 (8.2) |
| Overweight: 25.0-29.9 | 854 (18.6) | 1,315 (25.9) | 77 (18.5) | 158 (32.0) | 630 (18.6) | 981 (25.3) | 147 (18.9) | 176 (24.8) |
| Obese class I: 30.0-34.9 | 995 (21.7) | 1,386 (27.3) | 88 (21.1) | 94 (19.1) | 763 (22.5) | 1,139 (29.4) | 144 (18.5) | 153 (21.5) |
| Obese class II: 35.0-39.9 | 783 (17.1) | 740 (14.6) | 44 (10.5) | 32 (6.5) | 634 (18.7) | 605 (15.6) | 105 (13.5) | 103 (14.5) |
| Obese class III: ≥40.0 | 842 (18.4) | 561 (11.0) | 27 (6.5) | 14 (2.8) | 699 (20.6) | 484 (12.5) | 116 (14.9) | 63 (8.9) |
| Notmeasured | 730 (15.9) | 688 (13.5) | 83 (19.9) | 104 (21.1) | 442 (13.1) | 428 (11.0) | 205 (26.3) | 156 (22.0) |
| Smoking | ||||||||
| Non-smoker | 2,094 (45.7) | 1,565 (30.8)** | 182 (43.6) | 200 (40.6) | 1,642 (48.5) | 1,179 (30.4)** | 270 (34.7) | 186 (26.2)** |
| Current | 373 (8.1) | 536 (10.6) | 48 (11.5) | 77 (15.6) | 269 (7.9) | 397 (10.2) | 56 (7.2) | 62 (8.7) |
| Past | 1,652 (36.1) | 2,526 (49.7) | 148 (35.5) | 170 (34.5) | 1,192 (35.2) | 1,991 (51.4) | 312 (40.1) | 365 (51.4) |
| Notrecorded | 461 (10.1) | 451 (8.9) | 39 (9.3) | 46 (9.3) | 282 (8.3) | 308 (7.9) | 140 (18.0) | 97 (13.7) |
| Influenza vaccination, ever | 2,252 (49.2) | 2,448 (48.2) | 166 (39.8) | 183 (37.1) | 1,853 (54.7) | 2,053 (53.0) | 233 (30.0) | 212 (29.9) |
| Influenza vaccination, over a period of 395 days up to last encounter | 1,132 (24.7) | 1,179 (23.2) | 70 (16.8) | 71 (14.4) | 944 (27.9) | 1,016 (26.2) | 118 (15.2) | 92 (13.0) |
| Pneumococcal vaccination, ever | 453 (9.9) | 488 (9.6) | 24 (5.8) | 13 (2.6)* | 399 (11.8) | 445 (11.5) | 30 (3.9) | 30 (4.2) |
| Ophthalmological review, ever | 1,776 (38.8) | 1,918 (37.8) | 142 (34.0) | 142 (28.8) | 1,446 (42.7) | 1,584 (40.9) | 188 (24.2) | 192 (27.0) |
| Referral to a podiatrist, ever | 710 (15.5) | 858 (16.9) | 52 (12.5) | 71 (14.4) | 563 (16.6) | 696 (18.0) | 95 (12.2) | 91 (12.8) |
Abbreviations: BMI Body mass index, BP Blood pressure, DM Diabetes mellitus, HbA1c Glycated haemoglobin, HDL High density lipoprotein, LDL Low density lipoprotein
aLDL_C taret: <2.0 mmol/L or <1.8 mmol/L for those with established CVD (in this analysis these included coronary heart disease, cerebrovascular disease/stroke, or heart failure)
bUrine Albumin-creatine ratio (uARC): <3.5 mg/mmol in women and <2.5 mg/mmol in men
IATC code A10; II ATC code C10; III ATC codes C02-C04, C07-C09
Table 4.
Sex-stratified age, follow-up, health targets, clinical management goals, prescription, and screening over a period spanning 395 days up to the last clinical encounter in patients with incident diabetes who have been followed up for at least 3 years following their diabetes diagnosis: n (%) if not otherwise stated
| All types of DM | Type 1 DM | Type 2 DM | Unspecified type DM | |||||
|---|---|---|---|---|---|---|---|---|
| WomenN=3,620 | MenN=3,840 | WomenN=63 | MenN=68 | WomenN=2,610 | MenN=2,997 | WomenN=947 | MenN=775 | |
| Age at last encounter (years), mean (SD) | 66.8 (15.1) | 67.3 (13.6) | 50.9 (17.4) | 54.0 (18.9) | 68.4 (12.9) | 67.7 (13.0) | 63.6 (17.0) | 66.5 (14.6)** |
| Follow-up (years), mean (SD) | 14.4 (5.3) | 14.4 (5.3) | 12.0 (5.0) | 12.5 (5.0) | 14.7 (5.3) | 14.7 (5.3) | 13.8 (5.4) | 13.5 (5.2) |
| HbA1c ≤7.0% (≤53 mmol/mol) | ||||||||
| Yes | 1,595 (44.1) | 1,746 (45.5)** | 8 (12.7) | 13 (19.1) | 1,298 (49.7) | 1,422 (47.4)** | 289 (30.5) | 311 (40.1)** |
| No | 686 (18.9) | 975 (25.4) | 27 (42.9) | 26 (38.2) | 579 (22.2) | 832 (27.8) | 80 (8.5) | 117 (15.1) |
| Nottested | 1,339 (37.0) | 1,119 (29.1) | 28 (44.4) | 29 (42.6) | 733 (28.1) | 743 (24.8) | 578 (61.0) | 347 (44.8) |
| BP ≤140/90 mm Hg | ||||||||
| Yes | 1,066 (29.4) | 1,146 (29.8)** | 20 (31.7) | 19 (27.9) | 784 (30.0) | 908 (30.0) | 262 (27.7) | 219 (28.3)** |
| No | 1,629 (45.0) | 1,868 (48.7) | 16 (25.4) | 21 (30.9) | 1,276 (48.9) | 1,503 (50.1) | 337 (35.6) | 344 (44.4) |
| Notmeasured | 925 (25.6) | 826 (21.5) | 27 (42.9) | 28 (41.2) | 550 (21.1) | 586 (19.6) | 348 (36.7) | 212 (27.3) |
| BP ≤130/80 mm Hg | ||||||||
| Yes | 407 (11.2) | 423 (11.0)** | 9 (14.3) | 8 (11.8) | 277 (10.6) | 332 (11.1) | 121 (12.8) | 83 (10.7)** |
| No | 2,288 (63.2) | 2,591 (67.5) | 27 (42.9) | 32 (47.1) | 1,783 (68.3) | 2,079 (69.4) | 478 (50.5) | 480 (61.9) |
| Not measured | 925 (25.6) | 826 (21.5) | 27 (42.9) | 28 (41.2) | 550 (21.1) | 586 (19.6) | 348 (36.7) | 212 (27.3) |
| Total cholesterol <4.0 mmol/L | ||||||||
| Yes | 504 (13.9) | 1,019 (26.5)** | 4 (6.4) | 9 (13.2) | 434 (16.6) | 861 (28.7)** | 66 (7.0) | 149 (19.2)** |
| No | 1,588 (43.9) | 1,469 (38.3) | 27 (42.9) | 24 (35.3) | 1,225 (46.9) | 1,173 (39.1) | 336 (35.5) | 272 (35.1) |
| Not tested | 1,528 (42.2) | 1,352 (35.2) | 32 (50.8) | 35 (51.5) | 951 (36.4) | 963 (32.1) | 545 (57.5) | 354 (45.7) |
| LDL-Ca | ||||||||
| Yes | 576 (15.9) | 889 (23.1)** | 4 (6.3) | 9 (13.2) | 498 (19.1) | 750 (25.0)** | 74 (7.8) | 130 (16.8)** |
| No | 1,328 (36.7) | 1,371 (35.7) | 23 (36.5) | 21 (30.9) | 1,005 (38.5) | 1,104 (36.8) | 300 (31.7) | 246 (31.7) |
| Nottested | 1,716 (47.4) | 1,580 (41.2) | 36 (57.1) | 38 (55.9) | 1,107 (42.4) | 1,143 (38.1) | 573 (60.5) | 399 (51.5) |
| HDL-C ≥1.0 mmol/L | ||||||||
| Yes | 1,713 (47.3) | 1,590 (41.4)** | 28 (44.4) | 22 (32.3)* | 1,340 (51.3) | 1,278 (42.6)** | 345 (36.4) | 290 (37.4)** |
| No | 240 (6.6) | 768 (20.0) | 1 (1.6) | 9 (13.2) | 201 (7.7) | 654 (21.8) | 38 (4.0) | 105 (13.6) |
| Nottested | 1,667 (46.1) | 1,482 (38.6) | 34 (54.0) | 37 (54.4) | 1,069 (41.0) | 1,065 (35.5) | 564 (59.6) | 380 (49.0) |
| Triglycerides <2.0 mmol/L | ||||||||
| Yes | 1,178 (32.5) | 1,414 (36.8)** | 27 (42.9) | 22 (32.3) | 902 (34.6) | 1,141 (38.1)** | 249 (26.3) | 251 (32.4)** |
| No | 902 (24.9) | 1,071 (27.9) | 4 (6.3) | 11 (16.2) | 748 (28.7) | 891 (29.7) | 150 (15.8) | 169 (21.8) |
| Nottested | 1,540 (42.5) | 1,355 (35.3) | 32 (50.8) | 35 (51.5) | 960 (36.8) | 965 (32.2) | 548 (57.9) | 355 (45.8) |
| Non-HDL-C <2.5 mmol/L | ||||||||
| Yes | 74 (2.0) | 119 (3.1)* | 0 (0.0) | 2 (2.9) | 61 (2.3) | 102 (3.4)* | 13 (1.4) | 15 (1.9) |
| No | 281 (7.8) | 308 (8.0) | 3 (4.8) | 2 (2.9) | 204 (7.8) | 245 (8.2) | 74 (7.8) | 61 (7.9) |
| Not tested | 3,265 (90.2) | 3,413 (88.9) | 60 (95.2) | 64 (94.1) | 2,345 (89.8) | 2,650 (88.4) | 860 (90.8) | 699 (90.2) |
| Urine albumin-creatinine ratio (uACR)b | ||||||||
| Yes | 1,062 (29.3) | 1,144 (29.8)** | 19 (30.2) | 21 (30.9) | 894 (34.2) | 971 (32.4)** | 149 (15.7) | 152 (19.6)** |
| No | 433 (12.0) | 761 (19.8) | 7 (11.1) | 7 (10.3) | 376 (14.4) | 649 (21.6) | 50 (5.3) | 105 (13.5) |
| Nottested | 2,125 (58.7) | 1,935 (50.4) | 37 (58.7) | 40 (58.8) | 1,340 (51.3) | 1,377 (45.9) | 748 (79.0) | 518 (66.8) |
| Glucose lowering medications (ever prescription)I | 2,884 (79.7) | 3,091 (80.5) | 60 (95.2) | 64 (94.1) | 2,163 (82.9) | 2,511 (83.8) | 661 (69.8) | 516 (66.6) |
| Glucose lowering medications (prescription over a period of 395 days up to last encounter)I | 1,894 (52.3) | 2,334 (60.8)** | 43 (68.3) | 46 (67.7) | 1,565 (60.0) | 1,979 (66.0)** | 286 (30.2) | 309 (39.9)** |
| Lipid modifying agents (ever prescription)II | 2,474 (68.3) | 2,866 (74.6)** | 24 (38.1) | 36 (52.9) | 1,943 (74.4) | 2,323 (77.5)* | 507 (53.5) | 507 (65.4)** |
| Lipid modifying agents (prescription over a period of 395 days up to last encounter)II | 1,751 (48.4) | 2,237 (58.3)** | 15 (23.8) | 24 (35.3) | 1,427 (54.7) | 1,841 (61.4)** | 309 (32.6) | 372 (48.0)** |
| Blood pressure lowering agents (ever prescription)III | 3,269 (90.3) | 3,414 (88.9)* | 40 (63.5) | 48 (70.6) | 2,417 (92.6) | 2,698 (90.0)* | 812 (85.7) | 668 (86.2) |
| Blood pressure lowering agents (prescription over a period of 395 days up to last encounter)III | 2,352 (65.0) | 2,676 (69.7)** | 19 (30.2) | 32 (47.1)* | 1,821 (69.8) | 2,151 (71.8) | 512 (54.1) | 493 (63.6)** |
| BMI (kg/m2) | ||||||||
| Underweight: <18.5 | 5 (0.1) | 2 (0.1)** | 0 (0.0) | 1 (1.5)* | 3 (0.1) | 0 (0.0)** | 2 (0.2) | 1 (0.1)** |
| Normal weight: 18.5-24.9 | 211 (5.8) | 144 (3.7) | 20 (31.7) | 8 (11.8) | 122 (4.7) | 105 (3.5) | 69 (7.3) | 31 (4.0) |
| Overweight: 25.0-29.9 | 661 (18.3) | 904 (23.5) | 11 (17.5) | 22 (32.3) | 481 (18.4) | 721 (24.1) | 169 (17.8) | 161 (20.8) |
| Obese class I: 30.0-34.9 | 909 (25.1) | 1,203 (31.3) | 11 (17.5) | 14 (20.6) | 668 (25.6) | 953 (31.8) | 230 (24.3) | 236 (30.4) |
| Obese class II: 35.0-39.9 | 680 (18.8) | 692 (18.0) | 3 (4.8) | 8 (11.8) | 517 (19.8) | 551 (18.4) | 160 (16.9) | 133 (17.2) |
| Obese class III: ≥40.0 | 826 (22.8) | 595 (15.5) | 4 (6.3) | 0 (0.0) | 625 (24.0) | 482 (16.1) | 197 (20.8) | 113 (14.6) |
| Notmeasured | 328 (9.1) | 300 (7.8) | 14 (22.2) | 15 (22.1) | 194 (7.4) | 185 (6.2) | 120 (12.7) | 100 (12.9) |
| Smoking | ||||||||
| Non-smoker | 1,507 (41.6) | 1,087 (28.3)** | 27 (42.9) | 18 (26.5) | 1,158 (44.4) | 873 (29.1)** | 322 (34.0) | 196 (25.3)** |
| Current | 279 (7.7) | 367 (9.6) | 7 (11.1) | 13 (19.1) | 209 (8.0) | 308 (10.3) | 63 (6.7) | 46 (5.9) |
| Past | 1,577 (43.6) | 2,105 (54.8) | 24 (38.1) | 28 (41.2) | 1,086 (41.6) | 1,631 (54.4) | 467 (49.3) | 446 (57.6) |
| Notrecorded | 257 (7.1) | 281 (7.3) | 5 (7.9) | 9 (13.2) | 157 (6.0) | 185 (6.2) | 95 (10.0) | 87 (11.2) |
| Influenza vaccination, ever | 1,881(52.0) | 1,904 (49.6)* | 22 (34.9) | 34 (50.0) | 1,479 (56.7) | 1,557 (52.0)** | 380 (40.1) | 313 (40.4) |
| Influenza vaccination, over a period of 395 days up to last encounter | 871 (24.1) | 883 (23.0) | 10 (15.9) | 14 (20.6) | 682 (26.1) | 725 (24.2) | 179 (18.9) | 144 (18.6) |
| Pneumococcal vaccination, ever | 401 (11.1) | 434 (11.3) | 1 (1.6) | 4 (5.9) | 333 (12.8) | 378 (12.6) | 67 (7.1) | 52 (6.7) |
| Ophthalmological review, ever | 1,289 (35.6) | 1,384 (36.0) | 21 (33.3) | 23 (33.8) | 1,076 (41.2) | 1,186 (39.6) | 192 (20.3) | 175 (22.6) |
| Referral to a podiatrist, ever | 630 (17.4) | 698 (18.2) | 11 (17.5) | 10 (14.7) | 477 (18.3) | 574 (19.1) | 142 (15.0) | 114 (14.7) |
Abbreviations: BMI body mass index, BP Blood pressure, DM Diabetes mellitus, HbA1c Glycated haemoglobin, HDL High density lipoprotein, LDL Low density lipoprotein
aLDL_C taret: <2.0 mmol/L or <1.8 mmol/L for those with established CVD (in this analysis these included coronary heart disease, cerebrovascular disease/stroke, or heart failure)
bUrine Albumin-creatine ratio (uARC): <3.5 mg/mmol in women and <2.5 mg/mmol in men
I ATC code A10; II ATC code C10; III ATC codes C02-C04, C07-C09
The absence of pathology testing among individuals with either prevalent or incident diabetes was notably common, with a higher frequency observed in women compared to men, as indicated in Additional file 1 Table S4. After adjusting for age, BMI, smoking status, SEIFA-IRSD, Indigenous ethnicity, rurality, duration of follow-up, type of diabetes, and cluster effect, women were found to be 24% less likely than men to have their HbA1c tested over the 395-day period leading up to the last clinical encounter. Similarly, women were 23% less likely to undergo cholesterol testing, 35% less likely to undergo kidney function screening, 17% less likely to have their blood pressure measured, and 42% less likely to receive treatment with a lipid-lowering agent (Additional file 1 Table S4).
However, compared to women, men smoked more and were less likely to achieve blood pressure and HbA1c targets (Tables 3 and 4). The multivariable analysis that was limited to those with incident type 2 or unspecified diabetes who had at least three years of follow-up post diagnosis, found that men were 21% less likely than women to achieve the HbA1c target (adjusted OR 0.79, 95% CI 0.69 – 0.91), p = 0.001. The area under the receiver operating characteristic curve of the model was 0.74 (95% CI 0.72 – 0.75) (Table 5).
Table 5.
Multilevel mixed-effects logistic regression modelling “HbA1c ≤7.0% (≤53 mmol/mol)” over a period spanning 395 days up to the last clinical encounter in patients with incident diabetes (type 2 or unspecified diabetes) who had at least 3 years of follow-up post-diabetes
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Men (women as reference) | 0.75 (0.67 – 0.85) | <0.001 | 0.79 (0.69 – 0.91) | 0.001 |
| Age at last encounter (years) | ||||
| 18-49 (reference) | 1.00 | 1.00 | ||
| 50-59 | 1.27 (1.01 – 1.61) | 0.041 | 1.27 (0.89 – 1.65) | 0.069 |
| 60-69 | 1.67 (1.34 – 2.09) | <0.001 | 1.44 (1.12 – 1.86) | 0.004 |
| ≥70 | 2.83 (2.29 – 3.50) | <0.001 | 2.01 (1.57 – 2.59) | <0.001 |
| BMI (kg/m2) | ||||
| ≤24.9 (reference) | 1.00 | 1.00 | ||
| 25.0 – 29.9 | 0.85 (0.59 – 1.22) | 0.383 | 0.90 (0.61 – 1.32) | 0.584 |
| 30.0 – 34.9 | 0.66 (0.47 – 0.94) | 0.022 | 0.78 (0.53 – 1.14) | 0.197 |
| 35.0 – 39.9 | 0.57 (0.40 – 0.82) | 0.002 | 0.71 (0.48 – 1.05) | 0.085 |
| ≥40 | 0.61 (0.43 – 0.87) | 0.007 | 0.80 (0.54 – 1.19) | 0.269 |
| Unknown | 0.55 (0.36 – 0.85) | 0.007 | 0.72 (0.45 – 1.16) | 0.179 |
| Smoking status | ||||
| Non-smoker (reference) | 1.00 | 1.00 | ||
| Past smoker | 1.05 (0.91 – 1.22) | 0.487 | 1.14 (0.97 – 1.34) | 0.101 |
| Smoker | 0.62 (0.50 – 0.78) | <0.001 | 0.92 (0.72 – 1.18) | 0.521 |
| Unknown | 0.78 (0.57 – 1.05) | 0.104 | 0.83 (0.59 – 1.16) | 0.269 |
| SEIFA-IRSD quintiles | ||||
| 1st (Lowest) (Reference) | 1.00 | 1:00 | ||
| 2nd | 0.89 (0.64 – 1.23) | 0.480 | 0.85 (0.60 – 1.19) | 0.346 |
| 3rd | 0.83 (0.60 – 1.14) | 0.249 | 0.80 (0.57 – 1.12) | 0.192 |
| 4th | 0.94 (0.68 – 1.30) | 0.702 | 0.93 (0.66 – 1.31) | 0.679 |
| 5th (Highest) | 0.98 (0.69 – 1.40) | 0.928 | 0.96 (0.66 – 1.39) | 0.831 |
| Unknown | 1.05 (0.46 – 2.43) | 0.904 | 0.88 (0.36 – 2.16) | 0.782 |
| Active statusb | ||||
| Active (reference) | 1.00 | 1.00 | ||
| Inactive | 1.01 (0.87 – 1.18) | 0.870 | 1.06 (0.89 – 1.26) | 0.535 |
| Deceased | 1.51 (1.19 – 1.93) | 0.001 | 1.31 (1.01 – 1.71) | 0.045 |
| Years of follow-up, continuous | 0.99 (0.98 – 1.01) | 0.698 | 0.98 (0.97 – 0.99) | 0.033 |
| Anaemiac, yes | 1.35 (1.11 – 1.64) | 0.002 | 1.29 (1.04 – 1.59) | 0.021 |
| Chronic liver disease, yes | 1.04 (0.63 – 1.73) | 0.867 | 0.98 (0.57 – 1.69) | 0.941 |
| Chronic kidney disease, yes | 1.44 (1.10 – 1.88) | 0.008 | 1.25 (0.93 – 1.68) | 0.143 |
| Hypertriglyceridaemiad, yes | 0.99 (0.72 – 1.37) | 0.961 | 1.14 (0.81 – 1.62) | 0.447 |
| Pregnancye, yes | 0.96 (0.08 – 10.7) | 0.971 | 0.60 (0.05 – 6.45) | 0.641 |
| HbA1c baseline ever first recorded level, continuous | 0.57 (0.54 – 0.60) | <0.001 | 0.59 (0.56 – 0.63) | <0.001 |
| Receiver Operating Characteristic (ROC) curve (95% CI) | 0.74 (0.72 – 0.75) | |||
Abbreviations: BMI Body mass index, HbA1c Glycated haemoglobin, SEIFA-IRSD Socio-Economic Indexes for Areas – Index of Relative Socio-Economic Disadvantage
aThe multivariate model was also adjusted for Indigenous status and intracluster correlations within the participating 39 general practices
bAt the time of data extraction
cAnaemia, chronic or acute over a period spanning 395 days up to the last clinical encounter
dAs coded by MedicineInsight, a yes/no variable
ePregnancy over a period spanning 395 days up to the last clinical encounter
Similar results were found when, in sensitivity analyses, pregnant women were excluded from the model and/or when the model only included patients with type 2 diabetes.
Discussion
This large population-based retrospective study that used routinely collected primary healthcare data validates the overall higher prevalence and incidence of diabetes in men as opposed to women. While discernible sex differences favouring men were observed in diabetes management, women were more likely to achieve blood pressure and HbA1c targets. In contrast, women exhibiting a higher likelihood of obesity were less successful than men to meet blood lipid targets and were also less likely to receive treatment with a lipid lowering or blood pressure lowering or glucose lowering agent. This study highlights a substantially higher prevalence of diabetes-related conditions and comorbidities in men compared to women, including elevated rates of retinopathy, nephropathy, neuropathy, coronary heart disease, and heart failure.
Similar to other studies, we report an overall higher prevalence of diabetes in men compared to women [36], a higher incidence rate in young women (aged ≤ 30 years) [37] but higher incidence rates in men in older patients [38]. In this large sample of Australian adults with a record-based diagnosis of diabetes, there is evidence of sex differences in diabetes incidence diagnosis, with trends increasing in men as they aged. The higher risk of being diagnosed with diabetes in men was not explained by age, BMI, smoking status, socioeconomic status, and years of follow-up. In our sample, women with diabetes were more likely than men to be living with morbid obesity. The information we had on waist circumference was incomplete, precluding its use in the analysis. An explanation for the observed higher risk of diabetes in men compared to women may relate to sex differences in body fat storage. Subcutaneous and lower extremity fat storage is more common in women, while men tend to store fat in the abdominal region. Consequently, men exhibit significantly higher levels of visceral and ectopic fat than premenopausal women, irrespective of BMI and total body fat. The selective accumulation of excess fat in visceral and ectopic tissues in men may accelerate the onset of insulin resistance and diabetes [39]. In contrast, women might need to accumulate more weight, and their metabolic risk factors may need to deteriorate to a greater extent than in men to attain the same levels of visceral and ectopic fat necessary for developing insulin resistance and eventual diabetes [40]. Postmenopausal women tend to store more abdominal visceral fat, similar to patterns typically seen in men [41].
Studies on sex differences in quality-of-care indicators and in diabetes management are inconclusive [42–44]. The National Diabetes Audit, evaluating essential care processes and treatment target attainment in individuals living with diabetes reported that women were less inclined than men to receive screening of risk factors and risk factors control, with women being less likely than men to undergo risk factor assessments for smoking status, BMI, foot surveillance, cholesterol levels, and urine albumin. However, women were more prone to undergo testing for serum creatinine and blood pressure [42]. A large population-based study conducted in Italy, involving 415,294 individuals with type 2 diabetes, indicated that women were less likely to receive recommended care compared to men. Specifically, women were less likely to undergo assessments for kidney function, ophthalmological review, and foot surveillance, with women, who were more likely to have a BMI ≥ 30 kg/m2 than men, facing more challenges in achieving risk factor control for HbA1c and LDL-cholesterol despite drug intervention and were less likely to receive adequate treatment in the presence of micro/macroalbuminuria compared to men [43]. In contrast, a cross-sectional study involving 17,702 individuals with diabetes in the United States, drawn from the Medical Expenditure Panel Survey Household Component, showed that women were more inclined to receive recommended care compared to men over a nine-year study duration [44]. In adjusted analyses, women demonstrated a higher likelihood of undergoing annual tests for dilated eye exams and blood pressure control, as well as visiting a doctor. No disparities were observed in HbA1c testing and foot surveillance compared to men [44].
The RACGP advises to frequently assess HbA1c levels in patients with established diabetes. The HbA1c test is listed on the MBS for subsidy once every 12 months for the diagnosis of diabetes in high-risk individuals, and up to four times per year for monitoring of established diabetes [27]. In our study, overall, 14,843 out of 34,551 individuals (42.9%) did not undergo an HbA1c test over a period spanning 395 days up to their last clinical encounter. The percentages of non-adherence to recommended tests were consistently higher in women compared to men, indicating suboptimal management of established diabetes. This disparity extended beyond HbA1c testing, affecting women's access to essential screenings such as lipid levels, urine-albumin creatine tests, and blood pressure measurements. Additionally, our findings show that women were significantly less likely than men to receive treatment with a lipid lowering or blood pressure lowering or glucose lowering agent.
Men compared to women had more comorbidities and diabetes-associated conditions. Number of consultations did not vary by sex; however, we had no information on compliance with treatment and whether this differed by sex. Non-compliance with long-term medication for conditions like diabetes, hypertension, and dyslipidaemia is not uncommon, leading to compromised health risks [45]. Nonetheless, the reported association of sex/gender with compliance to long-term diabetes medications has not been consistent. Male sex has shown a positive association with compliance [46], a negative association with compliance [47], and no association with compliance [48, 49]. Gender differences in the perception and self-management of the disease have been also reported. Women often take their disease more seriously, reporting a higher impact on their daily life and are more involved in self-management than men [50].
Strengths and limitations
Strengths of this study include its population-based provenance, the longitudinal design, the routinely collected primary healthcare data, and the study’s broaden generalisability. Similarly, our inclusion of all patients irrespective of level of engagement with the health services has made the sample more representative of the wider primary care population. However, the study has limitations. Although MedicineInsight's coverage in Western Australia represents the general population of practices [51], the 39 participating practices in our study may not fully represent all clinics. We had no information on compliance and dispensing data, nor on individuals who may have moved to other general practices where their treatment was resumed. Misclassification of diabetes type could have occurred as adult-onset insulin-dependent diabetes that did not specifically categorise patients as having type 2 diabetes was classified as type 1. The research might have underestimated the percentage of patients undergoing optimal treatment, especially if patients received care in alternative settings (such as different general practices or hospitals), or if the patient's present medication record was incomplete, or if the patient records were not updated at the time of data extraction. While we used reason for consultation, we had no access to the full consultation notes. The multivariable analysis that investigated glycaemic control included only those with a known HbA1c level. Multiple imputations to complete the missingness in HbA1c levels was out of the scope of this study. The aim of this study was not to investigate initial management of diabetes upon diagnosis or change in HbA1c levels over time. The conditions described as “ever recorded” were extracted from any entries in the GP records, both before and after the diabetes diagnosis, as such that these conditions did not necessarily indicate complications that developed post-diabetes. Lastly, we had no information whether women with diabetes and confirmed dyslipidaemia were less treated with lipid modifying agents due to intolerance to statins.
Conclusions
This study used routinely collected primary healthcare data to show sex disparities in the management of diabetes in Australia. Compared to men, women with diabetes were less likely to undergo lipid and kidney function screening but were more likely than men to achieve blood pressure and HbA1c health targets. Men were significantly more likely than women to have retinopathy, nephropathy, neuropathy, coronary heart disease, heart failure, peripheral vascular disease, and peripheral artery disease. Our findings indicate that diabetes management should take into account the sex of the patient.
Supplementary Information
Additional file 1: Tables S1-S4. Table S1. Characteristics of study sample by ever recorded diabetes mellitus status. Table S2. Risk of being diagnosed with diabetes (incident cases): Weibull regression. Table S3. Ever recorded conditions by sex at last clinical encounter, all types of diabetes combined: n (%). Table S4. Adjusted odds ratio of not having a test or measure assessed and not being managed with medications over a period of 395 days up to the last clinical encounter (if not otherwise stated) in individuals with diabetes (all types combined): comparing women to men.
Acknowledgements
The authors thank participating general practices for contributing primary healthcare records to MedicineInsight.
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- CHD
Coronary heart disease
- DKA
Diabetic ketoacidosis
- DM
Diabetes mellitus
- GP
General practitioner
- HbA1c
Glycated haemoglobin
- HDL
High density lipoprotein
- HONK
Hyperglycaemic non-ketotic coma
- IQR
Interquartile range
- LDL
Low-density lipoprotein
- MBS
Medicare Benefits Schedule
- PAD
Peripheral artery disease
- PVD
Peripheral vascular disease
- SD
Standard deviation
- SEIFA-IRSD
Socio-Economic Indexes for Areas – Index of Relative Socio-Economic Disadvantage
- TIA
Transient ischaemic attack
- uACR
Urine albumin-creatinine ratio
Authors’ contributions
GM, CL, GC, JB, RV, SR1, and SR2 were involved in the conception, design, and interpretation of the results. RV acquired the data. GM conducted the analyses and wrote the first draft of the manuscript. GM is the guarantor of this work, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @GMnatzaganian (George Mnatzaganian); @RobinsonSuz (Suzanne Robinson).
Funding
This project was supported by the Western Australian Health Translation Network and the Australian Government’s Medical Research Future Fund (MRFF) as part of the Rapid Applied Research Translation program.
Data availability
All data generated or analysed during this study are included in this published article (and its Additional file 1).
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Curtin University Human Research Ethics Committee (HRE2019-0619). A waiver of consent was granted with no identifying information being available to the researchers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests regarding this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Health Topics. Diabetes. World Health Organization. https://www.who.int/health-topics/diabetes#tab=tab_1.
- 2.International Diabetes Federation . 10th edn. International Diabetes Federation; Brussels: 2021. IDF Diabetes Atlas.
- 3.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. 2023;402:203–34 Erratum in: Lancet 2023;402:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mnatzaganian G, Lee CMY, Robinson S, Sitas F, Chow CK, Woodward M, et al. Socioeconomic disparities in the management of coronary heart disease in 438 general practices in Australia. Eur J Prev Cardiol. 2021;28:400–7. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright AK, Welsh P, Gill JMR, Kontopantelis E, Emsley R, Buchan I, et al. Age-, sex- and ethnicity-related differences in body weight, blood pressure, HbA1c and lipid levels at the diagnosis of type 2 diabetes relative to people without diabetes. Diabetologia. 2020;63:1542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–51. [DOI] [PubMed] [Google Scholar]
- 11.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet. 2014;383:1973–80. [DOI] [PubMed] [Google Scholar]
- 12.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women. BMJ. 2006;332:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara A, Williamson DF, Karter AJ, Thompson TJ, Kim C. Sex differences in quality of health care related to ischemic heart disease prevention in patients with diabetes. Diabetes Care. 2004;27:2974–6. [DOI] [PubMed] [Google Scholar]
- 14.Duarte FG, da Silva Moreira S, Almeida MDCC, de Souza Teles CA, Andrade CS, Reingold AL, et al. Sex differences and correlates of poor glycaemic control in type 2 diabetes: a cross-sectional study in Brazil and Venezuela. BMJ Open. 2019;9:e023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara A, Mangione CM, Kim C, Marrero DG, Curb D, Stevens M, et al. Translating research into action for diabetes study group. Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2008;31:69–74. [DOI] [PubMed] [Google Scholar]
- 16.Australian Institute of Health and Welfare. Australian Government. Diabetes: Australian facts. 2023. https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary.
- 17.Manski-Nankervis JE, Thuraisingam S, Sluggett JK, Lau P, Blackberry I, Ilomaki J, et al. Prescribing for people with type 2 diabetes and renal impairment in Australian general practice: a national cross sectional study. Prim Care Diabetes. 2019;13(2):113–21. [DOI] [PubMed] [Google Scholar]
- 18.Islam SMS, Siopis G, Sood S, Uddin R, Tegegne T, Porter J, et al. The burden of type 2 diabetes in Australia during the period 1990–2019: findings from the global burden of disease study. Diabetes Res Clin Pract. 2023;199:110631. [DOI] [PubMed] [Google Scholar]
- 19.Gettings JV, O’Connor R, O’Doherty J, Hannigan A, Cullen W, Hickey L, et al. A snapshot of type two diabetes mellitus management in general practice prior to the introduction of diabetes Cycle of Care. Ir J Med Sci. 2018;187:953–7. [DOI] [PubMed] [Google Scholar]
- 20.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of Type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busingye D, Gianacas C, Pollack A, Chidwick K, Merrifield A, Norman S, et al. Data resource profile: medicineinsight, an australian national primary health care database. Int J Epidemiol. 2019;48:1741–1741h. [DOI] [PubMed] [Google Scholar]
- 22.Youens D, Moorin R, Harrison A, Varhol R, Robinson S, Brooks C, et al. Using general practice clinical information system data for research: the case in Australia. Int J Popul Data Sci. 2020;5:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havard A, Manski-Nankervis JA, Thistlethwaite J, Daniels B, Myton R, Tu K, et al. Validity of algorithms for identifying five chronic conditions in MedicineInsight, an Australian national general practice database. BMC Health Serv Res. 2021;21:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Australian Bureau of Statistics. Socio-Economic Indexes for Areas. 2023. https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa. Last accessed Oct 2023.
- 25. Colagiuri S, Davies D, Girgis S, Colagiuri R. National Evidence Based Guideline for Case Detection and Diagnosis of Type 2 Diabetes. Canberra: Diabetes Australia and the NHMRC; 2009.
- 26.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S13-27. [DOI] [PubMed] [Google Scholar]
- 27.The Royal Australian College of General Practitioners. Management of type 2 diabetes: A handbook for general practice. East Melbourne, Vic: RACGP; 2020.
- 28.Southern DA, Roberts B, Edwards A, Dean S, Norton P, Svenson LW, et al. Validity of administrative data claim-based methods for identifying individuals with diabetes at a population level. Can J Public Health. 2010;101:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mnatzaganian G, Woodward M, McIntyre HD, Ma L, Yuen N, He F, et al. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katwal PC, Jirjees S, Htun ZM, Aldawudi I, Khan S. The effect of anemia and the goal of optimal HbA1c control in diabetes and non-diabetes. Cureus. 2020;12(6):e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lucca SL, Narciso-Schiavon JL. HbA1c levels as a parameter of glycemic control in patients with liver diseases. Ann Hepatol. 2017;16:469–70. [DOI] [PubMed] [Google Scholar]
- 34.Kang SH, Jung DJ, Choi EW, Cho KH, Park JW, Do JY. HbA1c levels are associated with chronic kidney disease in a non-diabetic adult population: a nationwide survey (KNHANES 2011–2013). PLoS ONE. 2015;10:e0145827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Hng TM. HbA1c: More than just a number. Aust J Gen Pract. 2021;50:628–32. [DOI] [PubMed] [Google Scholar]
- 36.Nordström A, Hadrévi J, Olsson T, Franks PW, Nordström P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J Clin Endocrinol Metab. 2016;101:3740–6. [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: systematic analysis of the global burden of disease study 2019. BMJ. 2022;379:e072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPherson AR, Bancks MP. Assessment for gender differences in trend in age at diagnosis of diabetes among U.S. adults, 1999–2020. Diabetes Care. 2023;46:e76–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7. [DOI] [PubMed] [Google Scholar]
- 40.de Mutsert R, Gast K, Widya R, de Koning E, Jazet I, Lamb H, et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands epidemiology of obesity study. Metab Syndr Relat Disord. 2018;16:54–63. [DOI] [PubMed] [Google Scholar]
- 41.Frank AP, de Souza SR, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019;60:1710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Diabetes Audit - 2012-2013: Report 1, care processes and treatment targets – NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/national-diabetes-audit-2012-2013-report-1-care-processes-and-treatment-targets. Accessed 20 Nov 2023.
- 43.Rossi MC, Cristofaro MR, Gentile S, Lucisano G, Manicardi V, Mulas MF, et al. Sex disparities in the quality of diabetes care: biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the AMD Annals initiative. Diabetes Care. 2013;36:3162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams JS, Bishu KG, St Germain A, Egede LE. Trends in sex differences in the receipt of quality of care indicators among adults with diabetes: United States 2002–2011. BMC Endocr Disord. 2017;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29:1275–86. [DOI] [PubMed] [Google Scholar]
- 47.Wong MCS, Kong APS, So W-Y, Jiang JY, Chan JCN, Griffiths SM. Adherence to oral hypoglycemic agents in 26,782 Chinese patients: a cohort study. J Clin Pharmacol. 2011;51:1474–82. [DOI] [PubMed] [Google Scholar]
- 48.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19:279–84. [DOI] [PubMed] [Google Scholar]
- 49.Tiv M, Viel JF, Mauny F, Eschwège E, Weill A, Fournier C, et al. Medication adherence in type 2 diabetes: the ENTRED study 2007, a French Population-Based Study. PLoS ONE. 2012;7: e32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosnier-Pudar H, Hochberg G, Eschwege E, et al. How do patients with type 2 diabetes perceive their disease? Insights from the French DIABASIS survey. Diabetes Metab. 2009;35:220–7. [DOI] [PubMed] [Google Scholar]
- 51.Busingye D, Myton R, Mina R, Thistlethwaite J, Belcher J, Chidwick K. MedicineInsight report: Validation of the MedicineInsight database: completeness, generalisability and plausibility. Sydney: NPS MedicineWise, 2020. Available from https://www.nps.org.au/assets/MedicineInsight-Validation-completeness-representativeness-plausibility_2020.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Tables S1-S4. Table S1. Characteristics of study sample by ever recorded diabetes mellitus status. Table S2. Risk of being diagnosed with diabetes (incident cases): Weibull regression. Table S3. Ever recorded conditions by sex at last clinical encounter, all types of diabetes combined: n (%). Table S4. Adjusted odds ratio of not having a test or measure assessed and not being managed with medications over a period of 395 days up to the last clinical encounter (if not otherwise stated) in individuals with diabetes (all types combined): comparing women to men.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Additional file 1).