Abstract
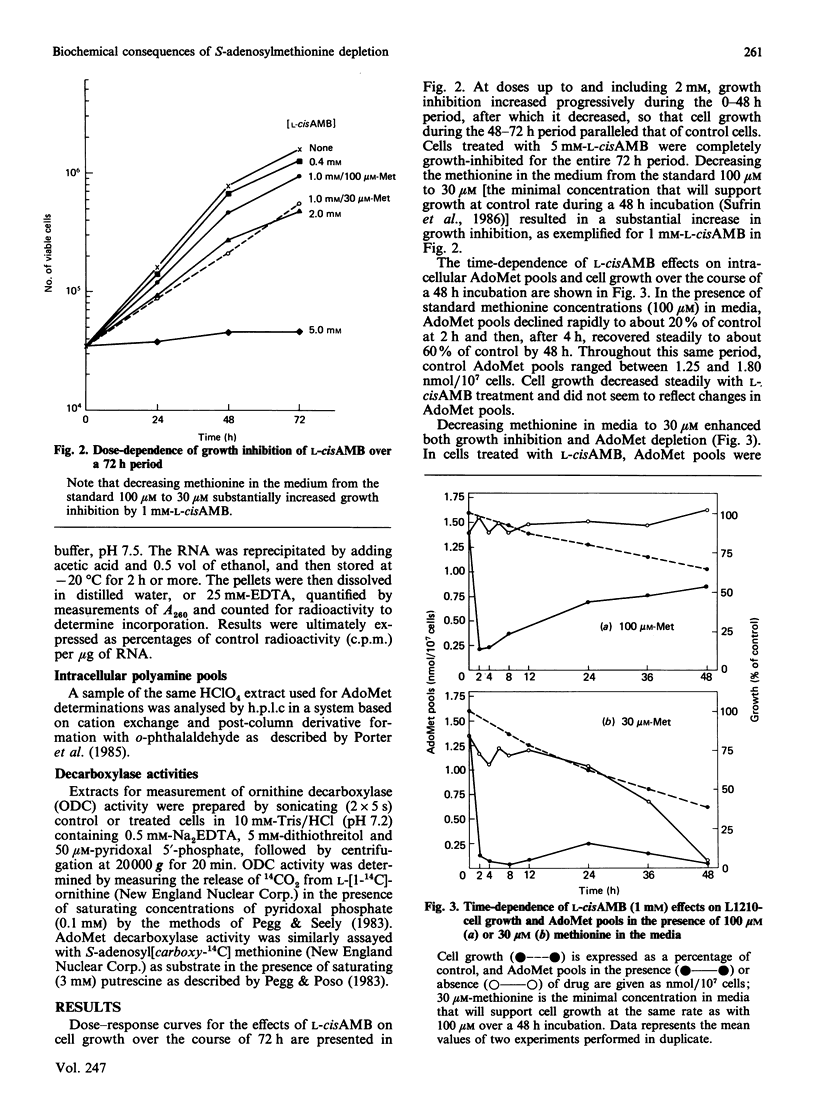
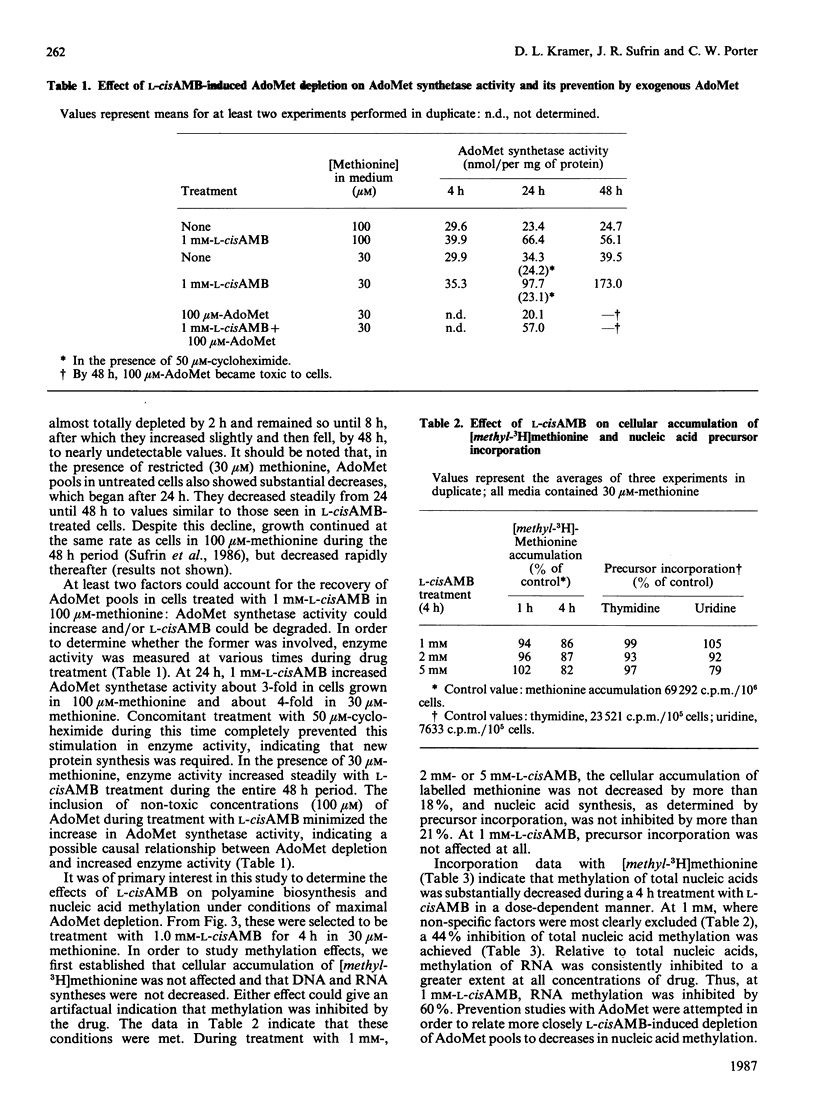
Treatment of cultured L1210 cells with 1 mM-L-2-amino-4-methoxy-cis-but-3-enoic acid (L-cisAMB), a methionine-analogue inhibitor of S-adenosylmethionine (AdoMet) synthetase (EC 2.5.1.6), produced a rapid and near-total depletion of AdoMet by 4 h. After this, the pools recovered to 60% of control by 48 h, apparently because of an increase in AdoMet synthetase activity. Both AdoMet depletion and the accompanying increase in synthetase activity were substantially enhanced by lowering methionine concentrations in the media from 100 microM to 30 microM, the minimal concentration that supports cell growth at control values. During a 4 h incubation in media containing 30 microM-methionine, 1-5 mM-L-cisAMB depleted cellular AdoMet to undetectable values, and inhibited nucleic acid methylation by 44-72% and RNA methylation by 60-87%. Under these same treatment conditions, putrescine pools increased by about 3-fold, whereas spermidine pools decreased by only 20% and spermine pools remained the same. Pool changes were accompanied by a 2-4-fold increase in ornithine decarboxylase activities and AdoMet activities. Thus the rapid depletion of AdoMet pools by L-cisAMB results immediately in a decrease in methyl-transfer reactions involving nucleic acids, whereas, by contrast, biosynthesis of higher polyamines appears to be minimally affected, owing to compensatory increases in key enzyme activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchardt R. T. S-Adenosyl-L-methionine-dependent macromolecule methyltransferases: potential targets for the design of chemotherapeutic agents. J Med Chem. 1980 Apr;23(4):347–357. doi: 10.1021/jm00178a001. [DOI] [PubMed] [Google Scholar]
- Caboche M., Bachellerie J. P. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977 Mar 15;74(1):19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- Caboche M., Hatzfeld J. Methionine metabolism in BHK cells: preliminary characterization of the physiological effects of cycloleucine, an inhibitor of s-adenosylmethionine biosynthesis. J Cell Physiol. 1978 Dec;97(3 Pt 1):361–370. doi: 10.1002/jcp.1040970311. [DOI] [PubMed] [Google Scholar]
- Caboche M. Methionine metabolism in BHK cells: the regulation of methionine adenosyltransferase. J Cell Physiol. 1977 Sep;92(3):407–424. doi: 10.1002/jcp.1040920309. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stolzfus C. M. Cycloleucine blocks 5'-terminal and internal methylations of avian sarcoma virus genome RNA. Biochemistry. 1978 Aug 22;17(17):3627–3632. doi: 10.1021/bi00610a032. [DOI] [PubMed] [Google Scholar]
- Eloranta T. O., Raina A. M. S-adenosylmethionine metabolism and its relation to polyamine synthesis in rat liver. Effect of nutritional state, adrenal function, some drugs and partial hepatectomy. Biochem J. 1977 Nov 15;168(2):179–185. doi: 10.1042/bj1680179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. E., Yoshimura N., Aguirre A., James J. H., Cummings M. G., Abel R. M., Deindoerfer F. Plasma amino acids in patients with hepatic encephalopathy. Effects of amino acid infusions. Am J Surg. 1974 Jan;127(1):40–47. doi: 10.1016/0002-9610(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. J., Hoffman R. M., Erbe R. W. Regulation of methionine adenosyltransferase in normal diploid and simian virus 40-transformed human fibroblasts. J Natl Cancer Inst. 1980 Dec;65(6):1237–1244. [PubMed] [Google Scholar]
- Kajander E. O., Kubota M., Carrera C. J., Montgomery J. A., Carson D. A. Resistance to multiple adenine nucleoside and methionine analogues in mutant murine lymphoma cells with enlarged S-adenosylmethionine pools. Cancer Res. 1986 Jun;46(6):2866–2870. [PubMed] [Google Scholar]
- Kotb M., Kredich N. M. S-Adenosylmethionine synthetase from human lymphocytes. Purification and characterization. J Biol Chem. 1985 Apr 10;260(7):3923–3930. [PubMed] [Google Scholar]
- Lombardini J. B., Coulter A. W., Talalay P. Analogues of methionine as substrates and inhibitors of the methionine adenosyltransferase reaction. Deductions concerning the conformation of methionine. Mol Pharmacol. 1970 Sep;6(5):481–499. [PubMed] [Google Scholar]
- Lombardini J. B., Sufrin J. R. Chemotherapeutic potential of methionine analogue inhibitors of tumor-derived methionine adenosyltransferases. Biochem Pharmacol. 1983 Feb 1;32(3):489–495. doi: 10.1016/0006-2952(83)90528-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Corti A., Williams-Ashman H. G. Paradoxical enhancement of S-adenosylmethionine decarboxylase in rat tissues following administration of the specific inhibitor methyl glyoxal bis(guanylhydrazone). Biochem Biophys Res Commun. 1973 May 15;52(2):696–701. doi: 10.1016/0006-291x(73)90768-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Pösö H. S-adenosylmethionine decarboxylase (rat liver). Methods Enzymol. 1983;94:234–239. doi: 10.1016/s0076-6879(83)94041-7. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R., Keith D. D. Growth inhibition by methionine analog inhibitors of S-adenosylmethionine biosynthesis in the absence of polyamine depletion. Biochem Biophys Res Commun. 1984 Jul 18;122(1):350–357. doi: 10.1016/0006-291x(84)90482-0. [DOI] [PubMed] [Google Scholar]
- Prince D. L., Kotin R. M., Dubin D. T. Evidence that the methylation inhibitor cycloleucine causes accumulation of a discrete ribosomal RNA precursor in hamster mitochondria. Mol Biol Rep. 1986;11(1):51–55. doi: 10.1007/BF00417596. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- Sufrin J. R., Lombardini J. B. Differences in the active-site region of tumor versus normal isozymes of mammalian ATP:L-methionine S-adenosyltransferase. Mol Pharmacol. 1982 Nov;22(3):752–759. [PubMed] [Google Scholar]
- Sufrin J. R., Lombardini J. B., Keith D. D. L-2-Amino-4-methoxy-cis-but-3-enoic acid, a potent inhibitor of the enzymatic synthesis of S-adenosylmethionine. Biochem Biophys Res Commun. 1982 May 31;106(2):251–255. doi: 10.1016/0006-291x(82)91102-0. [DOI] [PubMed] [Google Scholar]
- Zappia V., Galletti P., Porcelli M., Manna C., Ragione F. D. High-performance liquid chromatographic separation of natural adenosyl-sulphur compounds. J Chromatogr. 1980 Mar 7;189(3):399–405. doi: 10.1016/s0021-9673(00)80319-2. [DOI] [PubMed] [Google Scholar]



