Abstract
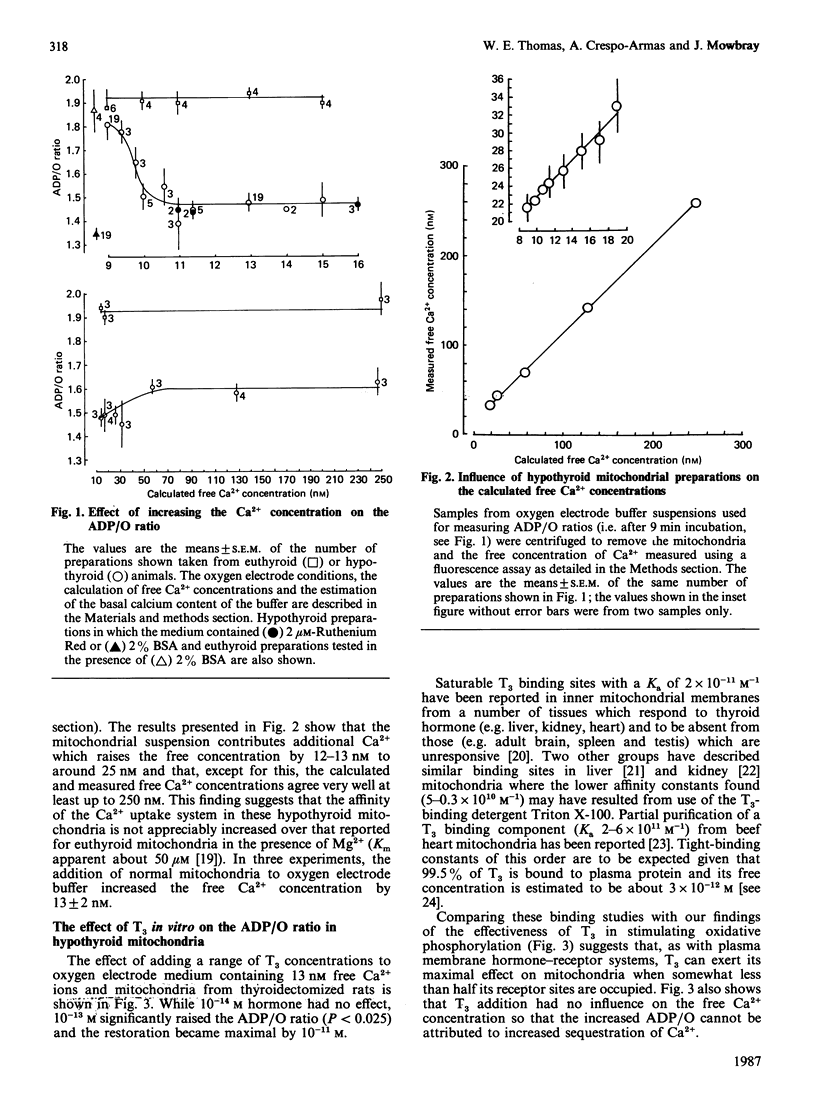
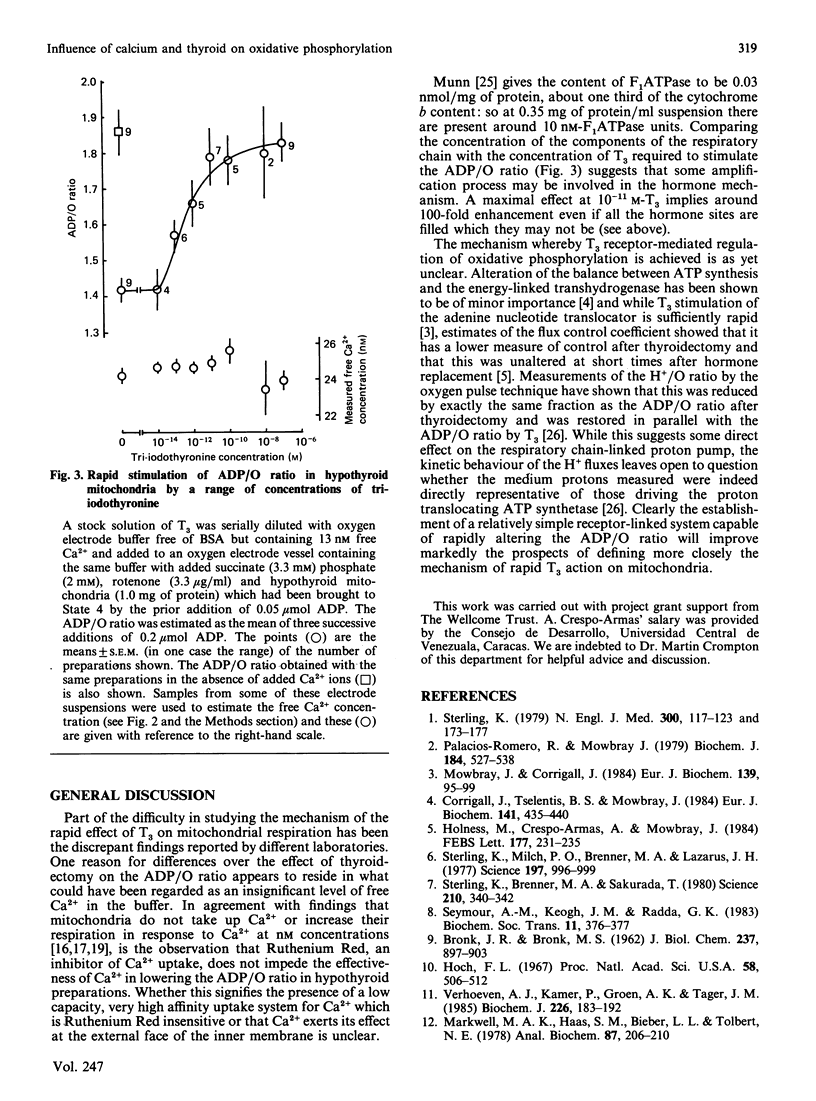
Using different conditions mitochondria from hypothyroid rats can show both unchanged ADP/O ratios and lowered ADP/O ratios without evidence of uncoupling when compared with euthyroid controls. Raising the free Ca2+ concentration to around 25 nM progressively lowered the ADP/O ratio in hypothyroid but not in euthyroid mitochondria. Ruthenium Red did not alter this behaviour and further increasing the Ca2+ concentration to levels below those which stimulate State 3 respiration had no additional effect. Measurements of the free Ca2+ concentration in the mitochondrial suspending medium using a Quin 2 fluorescence assay showed that the mitochondria did not buffer the free Ca2+ at these low concentrations. At 25 nM-free Ca2+, addition of 10-13) M-T3 to hypothyroid mitochondria produced an immediate and significant increase in the ADP/O ratio without altering the free Ca2+ concentration. The hormone effect was maximal by 10(-11) M. The concentration of ATP synthetase can be estimated to lie at about 10 nM in these experiments. Hence it appears possible that a substantial amplification of the hormone signal may have taken place. Comparison with binding studies suggests that T3 may have been maximally stimulating when somewhat less than half its receptor sites had been filled. The possible mechanisms by which this receptor mediated alteration of the ADP/O ratio might be achieved are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRONK J. R., BRONK M. S. The influence of thyroxine on oxidative phosphorylation in mitochondria from thyroidectomized rats. J Biol Chem. 1962 Mar;237:897–903. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Corrigall J., Tselentis B. S., Mowbray J. The efficiency of oxidative phosphorylation and the rapid control by thyroid hormone of nicotinamide nucleotide reduction and transhydrogenation in intact rat liver mitochondria. Eur J Biochem. 1984 Jun 1;141(2):435–440. doi: 10.1111/j.1432-1033.1984.tb08210.x. [DOI] [PubMed] [Google Scholar]
- Crespo-Armas A., Mowbray J. The rapid alteration by tri-iodo-L-thyronine in vivo of both the ADP/O ratio and the apparent H+/O ratio in hypothyroid-rat liver mitochondria. Biochem J. 1987 Feb 1;241(3):657–661. doi: 10.1042/bj2410657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goglia F., Torresani J., Bugli P., Barletta A., Liverini G. In vitro binding of triiodothyronine to rat liver mitochondria. Pflugers Arch. 1981 May;390(2):120–124. doi: 10.1007/BF00590193. [DOI] [PubMed] [Google Scholar]
- Hashizume K., Ichikawa K. Localization of 3,5,3'-L-triiodothyronine receptor in rat kidney mitochondrial membranes. Biochem Biophys Res Commun. 1982 Jun 15;106(3):920–926. doi: 10.1016/0006-291x(82)91798-3. [DOI] [PubMed] [Google Scholar]
- Herd P. A. Thyroid hormone-divalent cation interactions. Effect of thyroid hormone on mitochondrial calcium metabolism. Arch Biochem Biophys. 1978 May;188(1):220–225. doi: 10.1016/0003-9861(78)90375-2. [DOI] [PubMed] [Google Scholar]
- Hoch F. L. Early action of injected L-thyroxine on mitochondrial oxidative phosphorylation. Proc Natl Acad Sci U S A. 1967 Aug;58(2):506–512. doi: 10.1073/pnas.58.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M., Crespo-Armas A., Mowbray J. The influence of thyroid hormone on the degree of control of oxidative phosphorylation exerted by the adenine nucleotide translocator. FEBS Lett. 1984 Nov 19;177(2):231–235. doi: 10.1016/0014-5793(84)81289-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Corrigall J. Short-term control of mitochondrial adenine nucleotide translocator by thyroid hormone. Eur J Biochem. 1984 Feb 15;139(1):95–99. doi: 10.1111/j.1432-1033.1984.tb07981.x. [DOI] [PubMed] [Google Scholar]
- Palacios-Romero R., Mowbray J. Evidence for the rapid direct control both in vivo and in vitro of the efficiency of oxidative phosphorylation by 3,5,3'-tri-iodo-L-thyronine in rats. Biochem J. 1979 Dec 15;184(3):527–538. doi: 10.1042/bj1840527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Bronk J. R. The effects of thyroxine treatment, in vivo and in vitro, on Ca2+ efflux from rat liver mitochondria. FEBS Lett. 1981 Apr 6;126(1):9–12. doi: 10.1016/0014-5793(81)81020-4. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Sakurada T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science. 1980 Oct 17;210(4467):340–342. doi: 10.1126/science.7423197. [DOI] [PubMed] [Google Scholar]
- Sterling K. Direct thyroid hormone activation of mitochondria: the role of adenine nucleotide translocase. Endocrinology. 1986 Jul;119(1):292–295. doi: 10.1210/endo-119-1-292. [DOI] [PubMed] [Google Scholar]
- Sterling K., Lazarus J. H., Milch P. O., Sakurada T., Brenner M. A. Mitochondrial thyroid hormone receptor: localization and physiological significance. Science. 1978 Sep 22;201(4361):1126–1129. doi: 10.1126/science.210507. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (second of two parts). N Engl J Med. 1979 Jan 25;300(4):173–177. doi: 10.1056/NEJM197901253000405. [DOI] [PubMed] [Google Scholar]
- Verhoeven A. J., Kamer P., Groen A. K., Tager J. M. Effects of thyroid hormone on mitochondrial oxidative phosphorylation. Biochem J. 1985 Feb 15;226(1):183–192. doi: 10.1042/bj2260183. [DOI] [PMC free article] [PubMed] [Google Scholar]


