Abstract
Background
Hypertension is a common condition during adolescence with increasing prevalence globally, alongside the epidemic of unhealthy lifestyles and obesity. Health behaviors have been shown to be associated with hypertension risk in adults. Life’s essential 8 (LE8), as a comprehensive indicator to evaluate cardiovascular health (CVH), includes 4 health factors and 4 health behaviors. This study aims to evaluate the association between health behaviors defined in LE8 and hypertension among adolescents.
Methods
Data of this study were extracted from the National Health and Nutrition Examination Surveys (NHANES) 2007–2018. Health behaviors of LE8 including diet, physical activity and tobacco smoke exposure. The outcome was the odd of hypertension in adolescents. The weighted univariate and multivariate logistic regression was unitized to explore the relationship between CVH score and hypertension in adolescents. Subgroup analysis and sensitivity analysis were further conducted to explore the association across different populations.
Results
Totally 3,941 adolescents aged 12–17 years were included, with the mean aged of 14.48 ± 0.04 years. Of whom, 203 (5.15%) had hypertension. After adjusted all covariates, high CVH score was associated with the lower odds of hypertension (OR = 0.32, 95%CI: 0.17–0.61), especially in boys (OR = 0.23, 95%CI: 0.11–0.51) and adolescents with overweight/obesity (OR = 0.24, 95%CI: 0.10–0.56). Sensitivity analysis reported that the association between CVH score and the odds of hypertension was also robust after excluding self-reported hypertension and medication taking (OR = 0.37, 95%CI: 0.18–0.74).
Conclusion
A high CVH score, indicating a greater adherence of health behaviors, was associated with a reduced odds of hypertension, especially among boys and overweight/obesity adolescents. Large-scale prospective cohort studies are needed to further explore the association between health behaviors defined in LE8 and hypertension among adolescents.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04205-2.
Keywords: Life’s essential 8, Health behaviors, Hypertension, Adolescents, NHANES database
Background
Hypertension was a prevalent chronic disease worldwide featured by elevated arterial blood pressure (BP). It affected approximately 1.2 billion people and has become one of the most vital health problem [1]. Recently, with the epidemic of unhealthy lifestyles as well as overweight/obesity in adolescents, the trend of the early onset of hypertension was more obvious [2]. Hypertension in adolescents was associated with long-term negative health effects of hypertension and cardiovascular disease (CVD) in adulthood [3]. Even worse, adolescent hypertension may also lead to irreversible end-organ and vascular damage like hypertension in adults [4]. Control of hypertension in adolescence was becoming a major challenge in primary health care [3].
Previous study reported that daily lifestyle behaviors such as diet and physical activity are considered to be important modifiable factors for the prevention and control of hypertension [5]. Focusing on modifiable risk factors, the American Heart Association (AHA) proposed a cardiovascular health (CVH) quantification tool-life’s essential 8 (LE8). LE8 contains 4 health behaviors (diet, physical activity, tobacco smoke exposure and sleep duration) and 4 health factors [body mass index (BMI), non-high-density-lipoprotein, blood glucose and BP] [6]. In the adult-based cohort studies, adherence to the health behaviors defined in LE8 was associated with the lower risk of hypertension and diabetes [7, 8]. However, the association of adherence to the health behaviors including diet, physical activity and exposure to tobacco smoke defined in LE8 and hypertension among adolescents remains unclear.
Herein, the purpose of this study was to evaluate the relationship between health behaviors defined in LE8 and hypertension among adolescents based on the data from the National Health and Nutrition Examination Surveys (NHANES). The association was further evaluated stratified by gender and body mass index (BMI) Z-score in different subpopulations.
Methods
Study design and population
Data of adolescents for present study were extracted from the NHANES 2007–2018. The NHANES was a large-scale, periodic survey program using a stratified, multistage, and probability-cluster design to collect a nationally representative sample of non-institutionalized US civilians. This survey was conducted at National Center for Health Statistics (NCHS), the Centers for Diseases Control and Prevention (CDC). The requirement of ethical approval for this was waived by the Institutional Review Board of Beijing Chaoyang Hospital, Capital Medical University, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Beijing Chaoyang Hospital, Capital Medical University due to retrospective nature of the study. All methods were performed in accordance with the relevant guidelines and regulations.
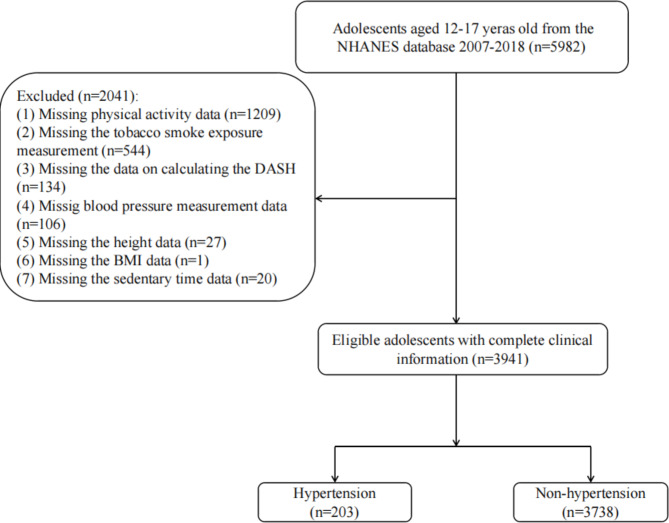
In present study, 5,982 adolescents aged 12–17 years in NHANES 2007–2018 were initial extracted. Then, 1,209 adolescents missing physical activity information, 544 missing tobacco smoke exposure information, 134 missing the complete dietary approach to stop hypertension (DASH) calculation information, 106 missing BP measurement data, 27 missing height data, 20 missing information of sedentary time and 1 missing BMI data were excluded. Finally, 3,941 eligible adolescents were included for further analysis. The screening process was shown in Fig. 1.
Fig. 1.
Subject screening flowchart
BP measurements and definition of hypertension in adolescents
Adolescents were defined as hypertensive if they (1) aged (16–17 years) or their parent/guardian (aged 12–15 years) respond that they were diagnosed as hypertension by clinician and then irrespective their BP value; (2) or taking antihypertensive medication irrespective their BP value; (3) or categorized as having hypertension/elevated according to the 2017 guideline from the American Academy of Pediatrics (AAP) [9]. This guideline recommended that participants with a systolic BP (SBP) ≥ 130 mmHg and/or diastolic BP (DBP) ≥ 80 mmHg were defined as having hypertension. For criteria 3, BP was measured in NHANES as follows: after participants sat quietly for 5 min and after determining the participants’ maximum inflation level (MIL), three times of BP value was measured. If the BP measurement was interrupted or incomplete, a forth BP value was measured. SBP and DBP were taken in the mobile examination center (MEC), and the means value was calculated. The BP measurers were certified for BP test via a training program from Shared Care Research and Education Consulting.
Health behaviors defined in LE8
The CVH status of adolescents was represented by the total score of each health behaviors. In present study, we focused on three health behaviors of LE8 including diet, physical activity and tobacco smoke exposure to evaluate the association between health behaviors and hypertension in adolescents to fit the NHANES database. The detailed algorithms for calculating LE8 score for each item using NHANES data have been reported previously [10]. The definitions and levels of each items have been described in Supplementary Table S1 [10, 11]. Each of the three CVH metrics was scored ranging from 0 to 100 points. The overall CVH score was calculated as the unweighted average of the three metrics. Adolescents with a health behaviors score of 80–100 were considered to be at good CVH status; 50–79 were moderate CVH status; and 0–49 were poor CVH status [10].
Dietary intake information was obtained from 24 h interview. The interview was conducted at Mobile Test Center through face-to-face communication. Adolescents were asked to recall all the types and amount of food and drink as well as the supplements consumed in the 24 h prior the interview. The United States Department of Agriculture’s (USDA) Food and Nutrient Database for Dietary Studies and NHANES Dietary Supplement Database were used to calculate daily intakes of energy, nutrients, and other food components [12].
Physical activity was measured by the ActiGraph GT1M accelerometer (ActiGraph, LLC, Penscola, FL). Adolescents was asked to wear accelerometers while they were awake for 7 days, with exception of water activities such as bathing ad swimming. Physical activity volume was calculated as mean accelerometer counts per minute, and physical activity intensity was calculated using established thresholds [13].
Tobacco smoke exposure was evaluated by serum cotinine level which measured via the isotope-dilution liquid chromatography-tandem mass spectrometry method. According to the analysis in the NAHNES 1999–2004, the optimal serum cotinine cut point to distinguish adolescents between smoke and non-smoke as 2.99 ng/mL [14].
Potential covariates
The potential covariates included in present study were demographic information, physical examination and laboratory values. Age, gender and race were self-reported demographic information. The birth weight below 2,500 g was defined as a low birth weight. In 2007–2026, the sedentary time was assessed as the total time of a participant spent lying or sitting at school, at work, or at home during the day. In 2017–2018, the sedentary time was divided as < 5 h and ≥ 5 h according to screen time. The trained research assistants measured child height and weight without shoes and in light clothing using a Seca scale and a stadiometer. From these measurements, child BMI, and age- and sex-specific BMI Z-score and percentile categories were calculated, basing the CDC guidelines. Percentile categories were defined as normal (≥ 5th to < 85th percentile), overweight (≥ 85th to < 95th percentile), obesity (≥ 95th percentile), and severe obesity (≥ 120% of the 95th percentile) [15]. Insulin resistance status was expressed by HOMA-IR and HOMA-IR = fasting blood glucose (mmol/L) 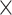 fasting insulin (µU/mL). Based on the National Cholesterol Education Program guidelines, abnormal serum total cholesterol ≥ 200 mg/dL, high-density lipoprotein cholesterol ≤ 35 mg/dL, low-density lipoprotein cholesterol ≥ 130 mg/dL, triglyceride ≥ 150 mg/dL, glucose ≥ 100 mg/dL and HOMA-IR ≥ 4.39 [16].
fasting insulin (µU/mL). Based on the National Cholesterol Education Program guidelines, abnormal serum total cholesterol ≥ 200 mg/dL, high-density lipoprotein cholesterol ≤ 35 mg/dL, low-density lipoprotein cholesterol ≥ 130 mg/dL, triglyceride ≥ 150 mg/dL, glucose ≥ 100 mg/dL and HOMA-IR ≥ 4.39 [16].
Statistical analysis
All statistical analyzes were performed using R v 4.20 (R Foundation for Statistical Computing, Vienna, Austria) and SAS v. 9.4 (SAS Institute, Cary, North Carolina) software. The finally sample size was weighted with SDMVSTRA, SDMVPSU and WTMEC2YR. SDMVSTRA means the CI being applied to assess the reliability of an estimate. SDMVPSU was the masked variance unit pseudo-substrate. WTMEC2YR was the MEC exam weight used for weighting.
Continuous data were expressed as mean and standard error (S.E.), and the weighted t-test was used for comparison between groups. Categorical variables were described as the number and percentage [n (%)], and comparisons between groups used the weighted chi-squared test. The weighted univariate logistics regression was unitized to screen the covariates that associated with the risk of hypertension (Supplementary Table S2). Then, weighted multivariate logistics regression was used to explore the relationship between health behaviors represented by CVH score and the risk of hypertension in adolescents, with odd ratios (ORs) and 95% confidence intervals (CIs). Model 1 was the crude model without adjusted for covariates. Model 2 adjusted for age, gender, low birth weight, BMI Z-score and HDL-C abnormal. Subgroup analysis was conducted to explore the association based on the gender and BMI Z-score. Sensitivity analysis was further used to evaluate these association excluding the self-reported hypertension and taking hypertension medication. Two-sided P-value < 0.05 was considered statistically significant.
Results
Description of study population
Totally, 3,941 eligible adolescents were included, with the mean age of 14.48 ± 0.04 years. Of whom, 203 (5.15%) had hypertension. Characteristics of included participants were shown in Table 1. The proportion of adolescents with higher CVH in hypertension group was lower than in the non-hypertension group (13.27% vs. 27.82%). Difference was found in age, gender, the level of PIR, low birth weight and BMI Z-score and HDL-C abnormal between two groups (all P < 0.05).
Table 1.
Characteristics of study adolescents
| Variables | Total (n = 3961) | Non-hypertension (n = 3738) | Hypertension (n = 203) | P |
|---|---|---|---|---|
| Age, years, Mean (S.E) | 14.48 (0.04) | 14.45 (0.04) | 15.05 (0.14) | < 0.001 |
| Gender, n (%) | 0.005 | |||
| Female | 1931 (49.94) | 1863 (50.57) | 68 (37.81) | |
| Male | 2010 (50.06) | 1875 (49.43) | 135 (62.19) | |
| Race, n (%) | 0.166 | |||
| Mexican American | 903 (13.43) | 859 (13.44) | 44 (13.23) | |
| Non-Hispanic Black | 956 (13.63) | 891 (13.35) | 65 (19.14) | |
| Non-Hispanic White | 1125 (57.88) | 1069 (57.94) | 56 (56.66) | |
| Other Hispanic | 473 (7.08) | 454 (7.12) | 19 (6.27) | |
| Other Race-Including Multi-Racial | 484 (7.98) | 465 (8.15) | 19 (4.70) | |
| PIR, n (%) | 0.016 | |||
| ≤1.85 | 1980 (37.46) | 1874 (37.16) | 106 (43.16) | |
| >1.85 | 1645 (56.58) | 1568 (57.11) | 77 (46.45) | |
| Unknown | 316 (5.96) | 296 (5.73) | 20 (10.39) | |
| Low birth weight, n (%) | < 0.001 | |||
| No | 2236 (57.82) | 2159 (58.93) | 77 (36.43) | |
| Yes | 330 (7.62) | 315 (7.58) | 15 (8.41) | |
| Unknown | 1375 (34.56) | 1264 (33.49) | 111 (55.16) | |
| BMI Z-score, n(%) | < 0.001 | |||
| Underweight | 81 (2.13) | 80 (2.21) | 1 (0.65) | |
| Healthy weight | 2241 (59.07) | 2169 (60.49) | 72 (31.70) | |
| Overweight | 713 (17.76) | 676 (17.65) | 37 (19.77) | |
| Obesity | 906 (21.04) | 813 (19.65) | 93 (47.88) | |
| Sedentary time, mins, n (%) | 0.809 | |||
| <300 | 346 (8.12) | 330 (8.15) | 16 (7.61) | |
| ≥300 | 3595 (91.88) | 3408 (91.85) | 187 (92.39) | |
| TC abnormal, n (%) | 0.311 | |||
| No | 3308 (83.73) | 3147 (83.93) | 161 (80.02) | |
| Yes | 234 (6.15) | 218 (6.17) | 16 (5.80) | |
| Unknown | 399 (10.12) | 373 (9.90) | 26 (14.18) | |
| HDL-C abnormal, n (%) | 0.016 | |||
| No | 3308 (83.70) | 3153 (84.18) | 155 (74.49) | |
| Yes | 399 (10.12) | 373 (9.90) | 26 (14.18) | |
| Unknown | 2297 (58.74) | 2175 (58.61) | 122 (61.27) | |
| LDL-C abnormal, n (%) | 0.756 | |||
| No | 1558 (39.13) | 1482 (39.24) | 76 (37.08) | |
| Yes | 86 (2.13) | 81 (2.15) | 5 (1.65) | |
| Unknown | 86 (2.13) | 81 (2.15) | 5 (1.65) | |
| TAG abnormal, n (%) | 0.426 | |||
| No | 3054 (76.40) | 2907 (76.61) | 147 (72.43) | |
| Yes | 473 (13.25) | 443 (13.25) | 30 (13.39) | |
| Unknown | 414 (10.35) | 388 (10.15) | 26 (14.18) | |
| Fasting glucose abnormal, n (%) | 0.426 | |||
| No | 1309 (33.33) | 1251 (33.56) | 58 (28.86) | |
| Yes | 380 (8.90) | 354 (8.79) | 26 (11.00) | |
| Unknown | 2252 (57.77) | 2133 (57.64) | 119 (60.14) | |
| HOMA-IR abnormal, n (%) | 0.253 | |||
| No | 1244 (32.83) | 1193 (33.13) | 51 (26.99) | |
| Yes | 388 (8.15) | 359 (7.97) | 29 (11.59) | |
| Unknown | 2309 (59.03) | 2186 (58.90) | 123 (61.42) | |
| Energy, kcal, Mean (S.E) | 2083.59 (22.16) | 2081.95 (22.82) | 2115.33 (75.47) | 0.670 |
| Health behaviors CVH, n (%) | < 0.001 | |||
| Low CVH | 793 (19.11) | 735 (18.36) | 58 (33.53) | |
| Moderate CVH | 2122 (53.79) | 2016 (53.82) | 106 (53.20) | |
| High CVH | 1026 (27.10) | 987 (27.82) | 39 (13.27) | |
| Per 10 points increase, Mean (S.E) | 66.37 (0.42) | 66.83 (0.43) | 57.38 (2.06) | < 0.001 |
| DASH score, n (%) | < 0.001 | |||
| High CVH | 1040 (26.91) | 994 (27.26) | 46 (20.10) | |
| Low CVH | 1918 (49.88) | 1794 (49.02) | 124 (66.51) | |
| Moderate CVH | 983 (23.21) | 950 (23.72) | 33 (13.39) | |
| DASH 10 increase, Mean (S.E) | 40.82 (0.85) | 41.33 (0.89) | 31.00 (2.86) | 0.001 |
| Physical activity score, n (%) | ||||
| High CVH | 2337 (63.25) | 2209 (63.37) | 128 (60.85) | |
| Low CVH | 1378 (30.35) | 1310 (29.99) | 68 (37.22) | |
| Moderate CVH | 226 (6.40) | 219 (6.64) | 7 (1.94) | |
| Physical activity score 10 increase, Mean (S.E) | 72.29 (0.77) | 72.54 (0.81) | 67.38 (4.14) | 0.236 |
| Tobacco smoke exposure score, n (%) | < 0.001 | |||
| High CVH | 3414 (85.18) | 3260 (85.96) | 154 (70.09) | |
| Low CVH | 243 (7.13) | 216 (6.58) | 27 (17.80) | |
| Moderate CVH | 284 (7.69) | 262 (7.46) | 22 (12.11) | |
| Tobacco smoke exposure score 10 increase, Mean (S.E) | 86.98 (0.58) | 87.61 (0.55) | 74.83 (3.40) | < 0.001 |
t: t-test; χ2: chi-square test; S.E.: standard error; PIR: poverty-to-income ratio; BMI: body mass index; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; TAG: triglyceride; HOMA-IR: homeostasis model assessment of insulin resistance; CVH: cardiovascular health; DASH: dietary approach to stop hypertension
Association between health behaviors in LE8 and hypertension in adolescents
We employed two logistic regression models to explore the association between health behaviors and hypertension in adolescents, as presented in Table 2. After adjustment for age, gender, low birth weight, BMI Z-score and HDL-C abnormal in model 2, we observed that high CVH score was associated with the lower odds of adolescents with hypertension (OR = 0.32, 95%CI: 0.17–0.61). The finding also indicated that with each 10 score increase in CVH, the odds of hypertension diminished by 2% (OR = 0.98, 95%CI: 0.97–0.99).
Table 2.
Association between health behaviors in LE8 and hypertension in adolescents
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Health behaviors score | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.54 (0.35–0.84) | 0.006 | 0.60 (0.39–0.92) | 0.021 |
| High CVH | 0.26 (0.14–0.48) | < 0.001 | 0.32 (0.17–0.61) | < 0.001 |
| Per 10 points increase | 0.98 (0.97–0.99) | < 0.001 | 0.98 (0.97–0.99) | < 0.001 |
| DASH score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.42 (0.24–0.73) | 0.003 | 0.47 (0.26–0.85) | 0.012 |
| High CVH | 0.54 (0.35–0.84) | 0.006 | 0.63 (0.40–0.98) | 0.039 |
| DASH 10 increase, Mean (S.E) | 0.99 (0.98–0.99) | 0.003 | 0.99 (0.98–0.99) | 0.016 |
| Physical activity score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.24 (0.10–0.58) | 0.002 | 0.30 (0.12–0.75) | 0.010 |
| High CVH | 0.77 (0.50–1.21) | 0.255 | 0.75 (0.47–1.18) | 0.212 |
| Physical activity score 10 increase, Mean (S.E) | 1.00 (0.99-1.00) | 0.215 | 1.00 (0.99-1.00) | 0.165 |
| Tobacco smoke exposure score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.60 (0.25–1.44) | 0.248 | 0.59 (0.26–1.37) | 0.218 |
| High CVH | 0.30 (0.18–0.52) | < 0.001 | 0.37 (0.22–0.63) | < 0.001 |
| Tobacco smoke exposure score 10 increase, Mean (S.E) | 0.99 (0.98–0.99) | < 0.001 | 0.99 (0.98–0.99) | < 0.001 |
OR: odd ratios; CI: confidence interval; Ref: reference
LE8: life’s essential 8; CVH: cardiovascular health
Model 1: crude model
Model 2: adjusted age, gender, low birth weight, BMI Z-score and HDL-C abnormal
Association between health behaviors in LE8 and hypertension in adolescents based on different gender and BMI Z-score
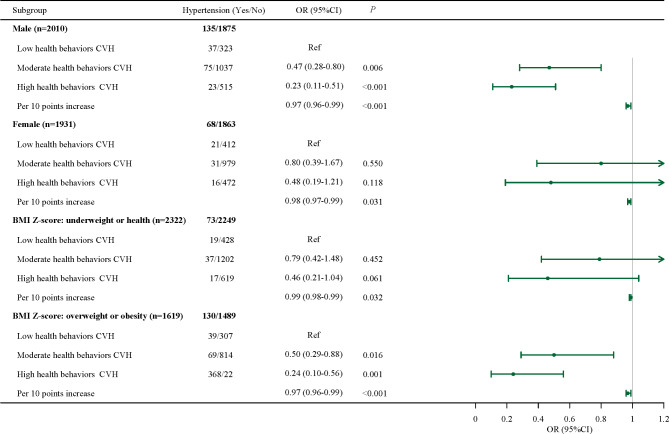
Further association between health behaviors and hypertension in adolescents was explored based on different gender and BMI Z-score. As shown in Fig. 2, after adjustment for age, gender, low birth weight, BMI Z-score and HDL-C abnormal in model 2, high CVH was associated with the lower odds of adolescents with overweight or obesity, especially in boys (OR = 0.23, 95%CI: 0.11–0.51) and adolescents with overweight/obesity (OR = 0.24, 95%CI: 0.10–0.56). We also observed that in girls (OR = 0.98, 95%CI: 0.97–0.99) and underweight or normal adolescents (OR = 0.99, 95%CI: 0.98–0.99), each 10 scores increase in CVH was associated with the lower odds of hypertension.
Fig. 2.
Association between health behaviors in LE8 and hypertension in adolescents based on different gender and BMI Z-score
Sensitivity analysis of association between health behavior in LE8 and hypertension in adolescents
After excluding self-reported hypertension and medication use, the association between health behaviors and hypertension in adolescents was further explored. Table 3 reports that after adjustment for age, gender, low birth weight, BMI Z-score and HDL-C abnormal, the association between health behaviors and hypertension in adolescents was unchanged. High CVH score was related to lower odds of hypertension in adolescents (OR = 0.37, 95%CI: 0.18–0.74) and with each 10 score increase in CVH, the odds of hypertension decreased by 2% (OR = 0.98, 95%CI: 0.97–0.99). Taken together, given the two definitions of hypertension among adolescents in present study, the results were robust that higher health CVH score was associated with lower odds of hypertension among adolescents.
Table 3.
Sensitivity analysis of association between health behavior in LE8 and hypertension in adolescents
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Health behaviors score | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.61 (0.39–0.94) | 0.026 | 0.64 (0.40–1.01) | 0.053 |
| High CVH | 0.31 (0.16–0.61) | < 0.001 | 0.37 (0.18–0.74) | 0.006 |
| Per 10 points increase | 0.98 (0.97–0.99) | < 0.001 | 0.98 (0.97–0.99) | 0.002 |
| DASH score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.37 (0.20–0.70) | 0.003 | 0.30 (0.10–0.86) | 0.025 |
| High CVH | 0.56 (0.36–0.87) | 0.011 | 0.92 (0.57–1.50) | 0.744 |
| DASH 10 increase, Mean (S.E) | 0.99 (0.98–0.99) | 0.005 | 1.00 (0.99–1.01) | 0.716 |
| Physical activity score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.24 (0.09–0.67) | 0.007 | 0.30 (0.10–0.86) | 0.025 |
| High CVH | 0.97 (0.61–1.53) | 0.891 | 0.92 (0.57–1.50) | 0.744 |
| Physical activity score 10 increase, Mean (S.E) | 1.00 (0.99–1.01) | 0.894 | 1.00 (0.99–1.01) | 0.716 |
| Tobacco smoke exposure score, n (%) | ||||
| Low CVH | Ref | Ref | ||
| Moderate CVH | 0.84 (0.31–2.23) | 0.717 | 0.82 (0.31–2.14) | 0.676 |
| High CVH | 0.40 (0.22–0.74) | 0.004 | 0.44 (0.24–0.81) | 0.009 |
| Tobacco smoke exposure score 10 increase, Mean (S.E) | 0.99 (0.98–0.99) | < 0.001 | 0.99 (0.98–0.99) | < 0.001 |
OR: odd ratios; CI: confidence interval; Ref: reference
LE8: life’s essential 8; CVH: cardiovascular health
Model 1: crude model
Model 2: adjusted for age, gender, low birth weight, BMI Z-score and HDL-C abnormal
Discussion
In the present study, we investigated the association between health behaviors in LE8 and hypertension among adolescents. Our findings showed that high CVH score, representing greater adherence the health behaviors defined in LE8, was associated with the lower odds of hypertension in adolescents, especially in boys and overweight/obesity adolescents. Moreover, the association remained robust after excluding adolescents with self-reported hypertension and medication uses.
Hypertension was an ongoing clinical and public health challenge that has been proved to be related to health behaviors such as anti-inflammatory diet intake, reduced tobacco smoke exposure and adequate physical activity. However, there is no standardized or recognized behaviors intervention strategies as yet. Although initially considered a disease of adults, hypertension is increasing in adolescents. To our knowledge, previous studies on the association between health behaviors and hypertension have focused on adults’ cohorts with inconsistent conclusions. A study from Brazilian Longitudinal Study of Aging based on general population suggested that health behaviors were associated with the lower risk of hypertension and the prevalence of hypertension control was 50.7%. Moreover, the effect of health behaviors on hypertension control showed a significant gender difference [17]. Similarly, a symposium presentation interested on the relationship between health behaviors and hypertension among females stated that addressing the barriers to adherence to health behaviors may have a substantial impact on reducing hypertension related cerebral vasospasm and improving heart diseases survival among female [18]. A study from Jordanian population aimed to evaluate the decisional balance of individuals in smoking, weight control, and physical exercise behaviors among hypertensive patients. In conclusion, that study showed that Jordanian hypertensive patients with higher physical exercise decisional balance and higher weight decisional balance had lower smoking decisional behaviors. Designing multidimensional interventions might be effective for modifying different types of health behaviors and decrease the burden of hypertension [19]. COOK WK et al. [20] reported that among White, Black, Hispanic, and Asian American adults, four unhealthy behaviors included alcohol misuse, smoking, poor diet, and physical inactivity were associated with the hypertension, although the results differed by the participants’ race. However, a study included two large cohorts from Coronary Artery Risk Development in Young Adults and Jackson Heart Study suggested that no relationship was found between health behaviors score and nocturnal hypertension or non-dipping SBP [21].
Our study found that health behaviors defined in LE8 was associated with the hypertension among adolescents. However, few studies have focused the association between health behaviors and hypertension among adolescents. Several studies have focused the association between health diet, physical activity and tobacco smoke exposure with adolescent health. A study based on the nationally representative sample showed that the fast food consumption was a predictor of weight gain from adolescence to adulthood. It was lined with both the risk of obesity in youth and the later risk of developing diseases such as hypertension and CVD in adulthood [22]. Another cross-sectional study of Swedish adolescents reported that dietary diversity and health eating index were related to health dietary habits [23]. However, adolescents in the U.S. do not meet dietary recommendations and adolescent diet quality was becoming a public concern problem. In recent study, the overall Healthy Eating Index (HEI)-2015 score for adolescents 12–18 yeas was 52.0. Although this score has increased significantly over time, the current score was still very low [24]. The HEI-2015 scoring for fatty acids was based on a ratio of polyunsaturated and monounsaturated fatty acid relative to saturated fatty acid intake and the low HEI-2015 score suggested that adolescents were not meeting recommendations for fatty acids [25]. In generally population, high diet quality assessed by the HEI-2015 was inversely associated with risk of hypertension and other diseases [26]. Moreover, the overall unhealthy diet quality of adolescents was driven by the inadequate consumption of components considered more healthful, such as fresh fruits, vegetables, and whole grains [27]. Interventions aimed at improving diet quality in adolescents are important to maintain their health now and into adulthood.
As in adults, the main risk factors for adolescent with primary hypertension were excess adiposity and suboptimal lifestyles and physical activity was commonly recommended as an important factor of lifestyle modification [28]. A study reported that low physical activity level and long screen time were associated with higher odds of hypertension among children and adolescents [28]. A meta-analysis reported that many children and adolescents can successfully lower BP through nonpharmacologic lifestyles included physical activity changes [29]. The exact molecular basis of the beneficial effects of physical activity on BP was not fully understood, as the regulation of BP was complex and multifactorial. First, the main mechanism by which physical activity can affect BP was the regulation of endothelial function. Endothelial regulates vascular health and resistance. Nitric oxide (NO) was a key mediator of endothelial function and clinical and preclinical studies have confirmed that exercise can improve No-dependent endothelial vasodilation [30]. Second, hypertension was featured by microvascular rarefaction caused by impaired angiogenesis, and sustained physical exercise has been shown to induce vascular adaptation and increase flow reserve [31]. However, data showed that 80% of children and adolescents in 105 countries do not meet the recommended physical activity level, that is, at least 60 min of moderate to vigorous physical activity daily [32]. Our finding showed that greater adherence to regular physical activity as one of the health behaviors defined in LE8 may has a positive effect on maintain BP among adolescents.
The association between tobacco smoke exposure as a risk factor and the incidence of developing hypertension has been evaluated in previous epidemiological studies and have shown that tobacco smoke exposure is linked with the high incidence of hypertension among adults [33, 34]. Liu et al. [35] showed that tobacco smoke exposure was associated with the DBP of U.S. adolescents. Another study also found the relationship between hair nicotine and higher BP in urban young children [36]. A study among the German preschool population showed an association between exposure to parental smoking and adolescents BP [37]. Tobacco smoke exposure as an element was defined in health behaviors in LE8 and smoking can product harmful substances such as NO, which may cause organ hypoxia and damage the endothelial cells of the arterial wall. Moreover, the nicotine in tobacco stimulated the heart and adrenaline to release large amounts of catecholamines, which cause blood vessels to constrict and raise BP [38]. Reducing tobacco smoke exposure, preferably in completely smoke-free environment, has potential benefits in the prevention and treatment of hypertension in adolescents.
Herein, we provided reference for the prevention and treatment of hypertension among adolescents on the relationship between health behaviors defined in the LE8 and the odds of hypertension. Hypertension among adolescents with increasing prevalence globally, alongside the epidemic of obesity and unhealthy lifestyle. As a comprehensive indicator of CVH, better compliance with the health behaviors defined in LE8 is of positive significance for the CVH and the lower risk of hypertension in adolescents. Eating more fruits and vegetables, replacing saturated fats and trans fats with unsaturated fats, reducing carbohydrate and red meat intake, more than 60 min of moderate-to-vigorous intensity physical activity daily primarily to strengthen muscle and bone, and living in a smoke-free environment have a positive impact on children’s health. The behavior habits of adolescents are largely affected by the environment in which they are grown. Bad tendencies from parents and neglect of exposure problems may have adverse consequences on the development of healthy behavior habits of adolescents. The combination of these approaches with family and school-based multicomponent interventions and skill-building health education programs warrants further investigation. However, several limitations of this study should be considered. First, the cross-sectional study design of this study could not establish a causal relationship between health behaviors defined in LE8 and hypertension among adolescents. Second, the American Heart Association added the factor of sleep health factor to Life’s Simple 7 and proposed the LE8 in 2020. Due to the database limitations, sleep information of adolescents was not recorded in the NHANES database, so the relationship between sleep health and the risk of hypertension in adolescents needs to be proved by further studies. Third, the NAHNES survey only represents the U.S. population, and considering the specificity of race, the applicability of LE8 in other populations need to be confirmed by more large-scale prospective cohort studies in the future.
Conclusion
Our analysis of the NHANES 2007–2018 data suggested that high CVH score of health behaviors defined in LE8 was related to the lower odds of hypertension among adolescents. This relationship suggested that health diet, appropriate physical activity and non-tobacco smoke exposure may have a beneficial effect for maintain a stable BP in adolescents, especially among boys and adolescents with overweight/obesity. Further longitudinal studies are essential to validate these findings and explore the underlying mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CVD
cardiovascular disease
- AHA
American Heart Association
- CVH
cardiovascular health
- LE8
life’s essential 8
- BMI
body mass index
- NHANES
National Health and Nutrition Examination Surveys
- NCHS
National Center for Health Statistics
- CDC
Centers for Diseases Control and Prevention
- BP
Blood pressure
- SBP
systolic BP
- DBP
diastolic BP
- USDA
United States Department of Agriculture’s
- S.E.
standard error
- ORs
odd ratios
- CIs
confidence intervals
Author contributions
Weiming Li designed the study, Zhiyong Zhang wrote the manuscript, Xuejiao Wu, Yu Qu and Dapeng Zhang collected, analyzed and interpreted the data, Weiming Li critically reviewed the manuscript, all authors read and approved the manuscript.
Funding
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The requirement of ethical approval for this was waived by the Institutional Review Board of Beijing Chaoyang Hospital, Capital Medical University, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Beijing Chaoyang Hospital, Capital Medical University due to retrospective nature of the study. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cai Y, Chen M, Zhai W, Wang C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005–2018. BMC Public Health. 2022;22:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson SL, Zhang Z, Wiltz JL, Loustalot F, Ritchey MD, Goodman AB, et al. Hypertension among youths - United States, 2001–2016. MMWR Morb Mortal Wkly Rep. 2018;67:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Simone G, Mancusi C, Hanssen H, Genovesi S, Lurbe E, Parati G, et al. Hypertension in children and adolescents. Eur Heart J. 2022;43:3290–301. [DOI] [PubMed] [Google Scholar]
- 4.Seeman T, Hamdani G, Mitsnefes M. Hypertensive crisis in children and adolescents. Pediatr Nephrol. 2019;34:2523–37. [DOI] [PubMed] [Google Scholar]
- 5.Unda Villafuerte F, Llobera Cànaves J, Lorente Montalvo P, Moreno Sancho ML, Oliver Oliver B, Bassante Flores P, et al. Effectiveness of a multifactorial intervention, consisting of self-management of antihypertensive medication, self-measurement of blood pressure, hypocaloric and low sodium diet, and physical exercise, in patients with uncontrolled hypertension taking 2 or more antihypertensive drugs: the MEDICHY study. Med (Baltim). 2020;99:e19769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of Cardiovascular Health in US adults and Children Using the American Heart Association’s New Life’s essential 8 Metrics: Prevalence Estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–35. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure Guideline with Risk of Heart failure and Atrial Fibrillation. Circulation. 2021;143:2244–53. [DOI] [PubMed] [Google Scholar]
- 8.Tong TY, Wareham NJ, Khaw KT, Imamura F, Forouhi NG. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-mediterranean population: the EPIC-Norfolk study. BMC Med. 2016;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.lynn JT, Kaelber DC, Baker-Smith CM et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents [published correction appears in Pediatrics. 2017;140(6):e20173035. doi: 10.1542/peds.2017-3035] [published correction appears in Pediatrics. 2018;142(3):e20181739. doi: 10.1542/peds.2018-1739]. Pediatrics. 2017;140(3):e20171904. 10.1542/peds.2017-1904 [DOI] [PubMed]
- 10.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of Cardiovascular Health: a Presidential Advisory from the American Heart Association. Circulation. 2022;146:e18–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Ning H, Labarthe D et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association’s New Life’s Essential 8 Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 Through 2018 [published correction appears in Circulation. 2022;146(20):e298. doi: 10.1161/CIR.0000000000001113]. Circulation. 2022;146(11):822–835. 10.1161/CIRCULATIONAHA.122.060911 [DOI] [PubMed]
- 12.Jun S, Cowan AE, Dodd KW, Tooze JA, Gahche JJ, Eicher-Miller HA, et al. Association of food insecurity with dietary intakes and nutritional biomarkers among US children, National Health and Nutrition Examination Survey (NHANES) 2011–2016. Am J Clin Nutr. 2021;114:1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–65. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–48. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–20. [DOI] [PubMed] [Google Scholar]
- 16.Cai H, Huang J, Xu G, Yang Z, Liu M, Mi Y, et al. Prevalence and determinants of metabolic syndrome among women in Chinese rural areas. PLoS ONE. 2012;7:e36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firmo JOA, Peixoto SV, Loyola Filho AI, Souza-Júnior PRB, Andrade FB, Lima-Costa MF, et al. Health behaviors and hypertension control: the results of ELSI-BRASIL. Cad Saude Publica. 2019;35:e00091018. [DOI] [PubMed] [Google Scholar]
- 18.Krousel-Wood M. Hypertension and Health behaviors in females across the Lifespan. Am J Med Sci. 2015;350:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eshah NF, Mosleh SM, Al-Smadi A. The Decisional Balance toward Health behaviors among patients with hypertension. Clin Nurs Res. 2021;30:977–84. [DOI] [PubMed] [Google Scholar]
- 20.Cook WK, Li L, Tam CC, Mulia N, Kerr WC. Associations of clustered health risk behaviors with diabetes and hypertension in White, Black, Hispanic, and Asian American adults. BMC Public Health. 2022;22:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakhuja S, Booth JN, Lloyd-Jones DM, Lewis CE, Thomas SJ, Schwartz JE, et al. Health behaviors, nocturnal hypertension, and non-dipping blood pressure: the coronary artery risk development in young adults and Jackson Heart Study. Am J Hypertens. 2019;32:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyström J, Benskin JP, Plassmann M, Sandblom O, Glynn A, Lampa E, et al. Healthy eating index and diet diversity score as determinants of serum perfluoroalkyl acid (PFAA) concentrations in a national survey of Swedish adolescents. Environ Res. 2022;212:113170. [DOI] [PubMed] [Google Scholar]
- 23.Moraeus L, Lindroos AK, Warensjö Lemming E, Mattisson I. Diet diversity score and healthy eating index in relation to diet quality and socio-demographic factors: results from a cross-sectional national dietary survey of Swedish adolescents. Public Health Nutr. 2020;23:1754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson JL, Tussing-Humphreys LM, Goodman MH, Landry AS. Diet quality in a nationally representative sample of American children by sociodemographic characteristics. Am J Clin Nutr. 2019;109:127–38. [DOI] [PubMed] [Google Scholar]
- 25.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet Quality as assessed by the healthy eating index, alternate healthy eating Index, Dietary approaches to stop hypertension score, and Health outcomes: a second update of a systematic review and Meta-analysis of Cohort studies. J Acad Nutr Diet. 2020;120:1998–e203115. [DOI] [PubMed] [Google Scholar]
- 27.Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet. 2013;113:297–306. [DOI] [PubMed] [Google Scholar]
- 28.Wyszyńska J, Podgórska-Bednarz J, Dereń K, Mazur A. The relationship between physical activity and screen time with the risk of hypertension in children and adolescents with intellectual disability. Biomed Res Int. 2017;2017:1940602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anglum A. Primary care management of childhood and adolescent hypertension. J Am Acad Nurse Pract. 2009;21:529–34. [DOI] [PubMed] [Google Scholar]
- 30.Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2014;173:361–8. [DOI] [PubMed] [Google Scholar]
- 31.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol (1985). 1989;67:1140–9. [DOI] [PubMed] [Google Scholar]
- 32.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Yang S, He Y, Liu M, Wang Y, Wang J, et al. Association between passive smoking and hypertension in Chinese non-smoking elderly women. Hypertens Res. 2017;40:399–404. [DOI] [PubMed] [Google Scholar]
- 34.Park YS, Lee CH, Kim YI, Ahn CM, Kim JO, Park JH, Survey V, et al. BMJ Open. 2018;8:2010–2. [Google Scholar]
- 35.Liu SH, Liu B, Sanders AP, Saland J, Wilson KM. Secondhand smoke exposure and higher blood pressure in children and adolescents participating in NHANES. Prev Med. 2020;134:106052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groner JA, Huang H, Joshi MS, Eastman N, Nicholson L, Bauer JA. Secondhand smoke exposure and preclinical markers of Cardiovascular Risk in toddlers. J Pediatr. 2017;189:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wühl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation. 2011;123:292–8. [DOI] [PubMed] [Google Scholar]
- 38.Skipina TM, Soliman EZ, Upadhya B. Association between secondhand smoke exposure and hypertension: nearly as large as smoking. J Hypertens. 2020;38:1899–908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.