Abstract
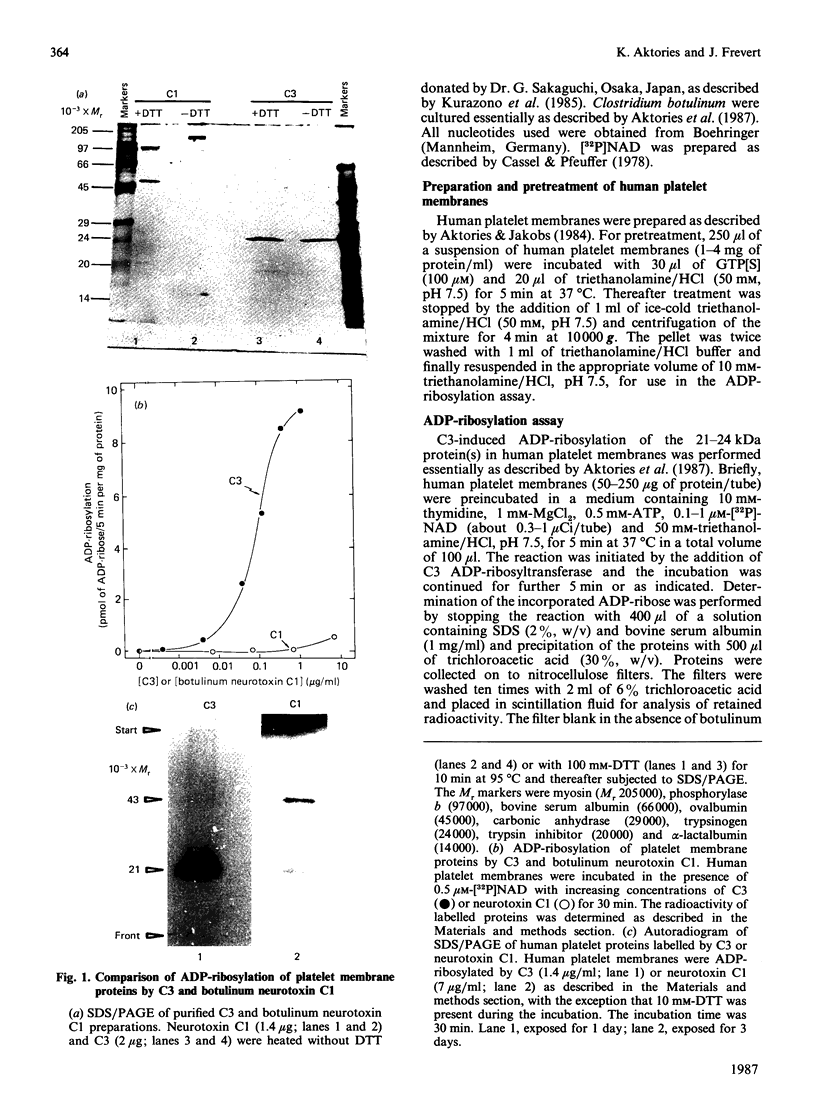
Besides botulinum C2 toxin, Clostridium botulinum type C produces another ADP-ribosyltransferase, which we termed 'C3'. ADP-ribosyltransferase C3 has a molecular mass of 25 kDa and modifies 21-24 kDa protein(s) in platelet and brain membranes. C3 was about 1000 times more potent than botulinum C1 toxin in ADP-ribosylation of membrane proteins. C3-catalysed ADP-ribosylation of the 21-24 kDa protein(s) was decreased by stable guanosine triphosphates, with the potency order GTP[S] much greater than p[NH]ppG greater than p[CH2]ppG. GTP[S] inhibited the ADP-ribosylation caused by C3 by maximally 70-80%, with half-maximal and maximal effects occurring at 0.3 and 10 microM-GTP[S] respectively. The concomitant addition of GTP decreased the inhibitory effect of GTP[S]. GTP[S]-induced inhibition of ADP-ribosylation was resistant to washing of pretreated platelet membranes. The data suggest that the novel botulinum ADP-ribosyltransferase C3 modifies eukaryotic 21-24 kDa guanine nucleotide-binding protein(s).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Ankenbauer T., Schering B., Jakobs K. H. ADP-ribosylation of platelet actin by botulinum C2 toxin. Eur J Biochem. 1986 Nov 17;161(1):155–162. doi: 10.1111/j.1432-1033.1986.tb10136.x. [DOI] [PubMed] [Google Scholar]
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Aktories K., Jakobs K. H. Epinephrine inhibits adenylate cyclase and stimulates a GTPase in human platelet membranes via alpha-adrenoceptors. FEBS Lett. 1981 Aug 3;130(2):235–238. doi: 10.1016/0014-5793(81)81128-3. [DOI] [PubMed] [Google Scholar]
- Aktories K., Jakobs K. H. Ni-mediated inhibition of human platelet adenylate cyclase by thrombin. Eur J Biochem. 1984 Dec 3;145(2):333–338. doi: 10.1111/j.1432-1033.1984.tb08558.x. [DOI] [PubMed] [Google Scholar]
- Aktories K., Weller U., Chhatwal G. S. Clostridium botulinum type C produces a novel ADP-ribosyltransferase distinct from botulinum C2 toxin. FEBS Lett. 1987 Feb 9;212(1):109–113. doi: 10.1016/0014-5793(87)81566-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Evans T., Brown M. L., Fraser E. D., Northup J. K. Purification of the major GTP-binding proteins from human placental membranes. J Biol Chem. 1986 May 25;261(15):7052–7059. [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M. ADP-ribosylating microbial toxins. Crit Rev Microbiol. 1985;11(4):273–298. doi: 10.3109/10408418409105905. [DOI] [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. ADP-ribosylation of Gs promotes the dissociation of its alpha and beta subunits. J Biol Chem. 1984 May 25;259(10):6235–6240. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Kamiya T., Fujiwara M., Narumiya S. ADP-ribosylation by type C1 and D botulinum neurotoxins: stimulation by guanine nucleotides and inhibition by guanidino-containing compounds. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1032–1038. doi: 10.1016/0006-291x(87)91518-x. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Narumiya S. ADP-ribosylation of a Mr 21,000 membrane protein by type D botulinum toxin. J Biol Chem. 1987 Feb 5;262(4):1430–1433. [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuner K. H., Presek P., Boschek C. B., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur J Cell Biol. 1987 Feb;43(1):134–140. [PubMed] [Google Scholar]
- Simpson L. L. Molecular basis for the pharmacological actions of Clostridium botulinum type C2 toxin. J Pharmacol Exp Ther. 1984 Sep;230(3):665–669. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes and the molecular biology of cancer. J Cell Biol. 1983 Dec;97(6):1661–1662. doi: 10.1083/jcb.97.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]