Abstract
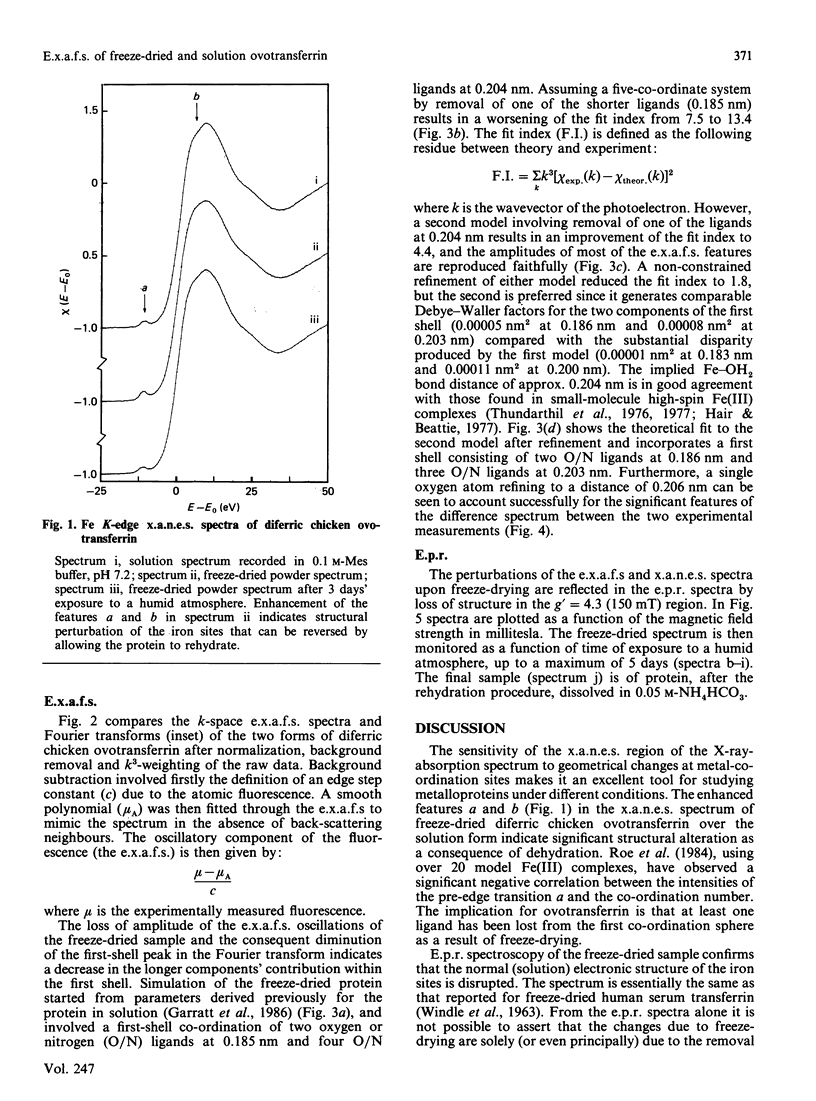
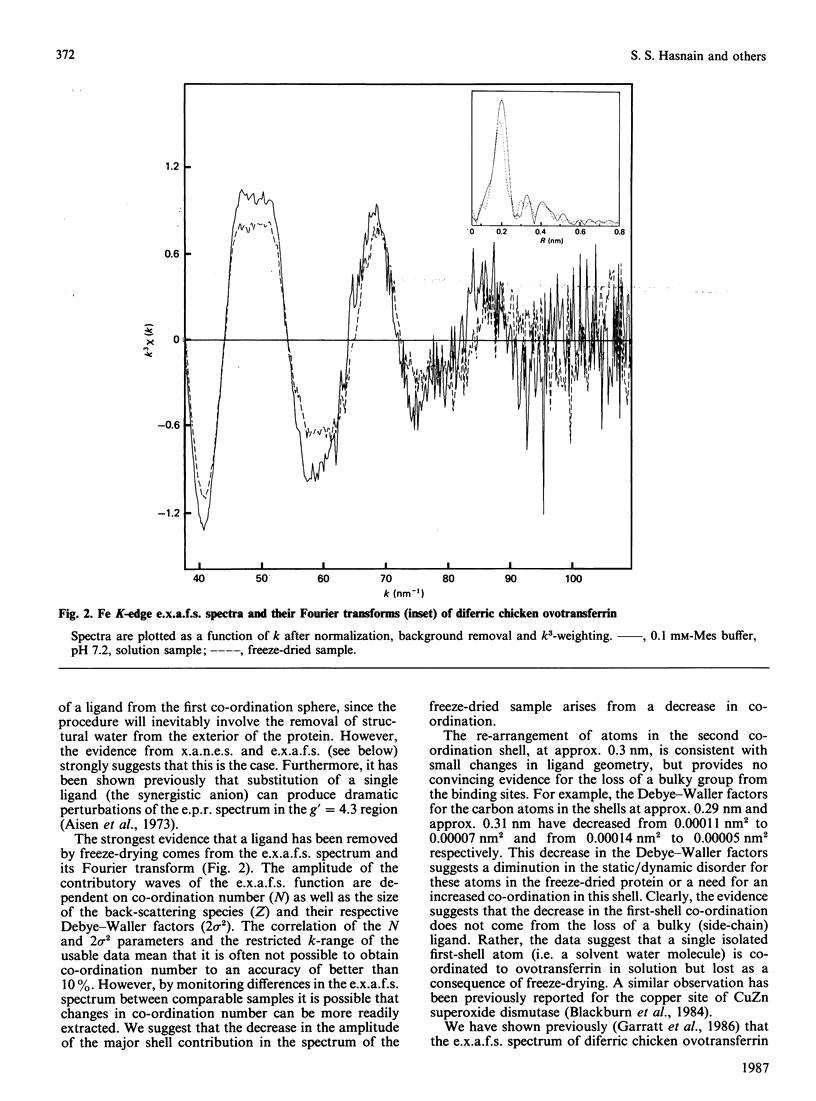
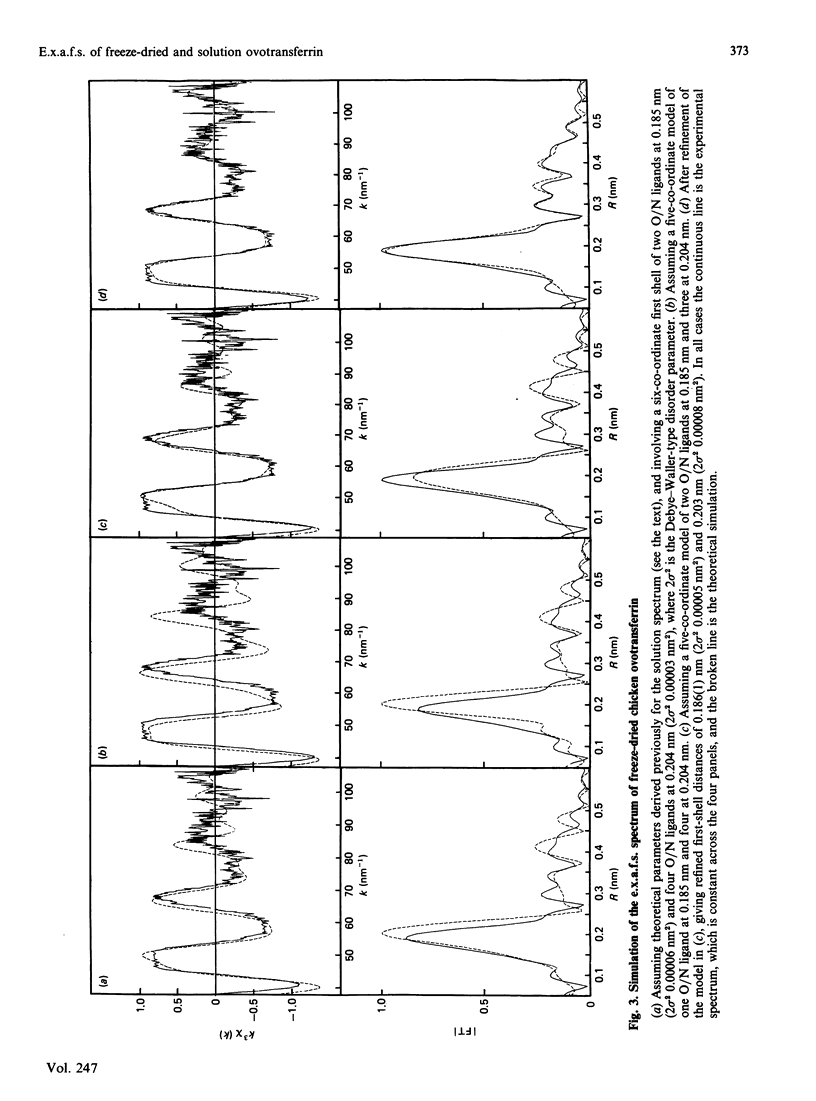
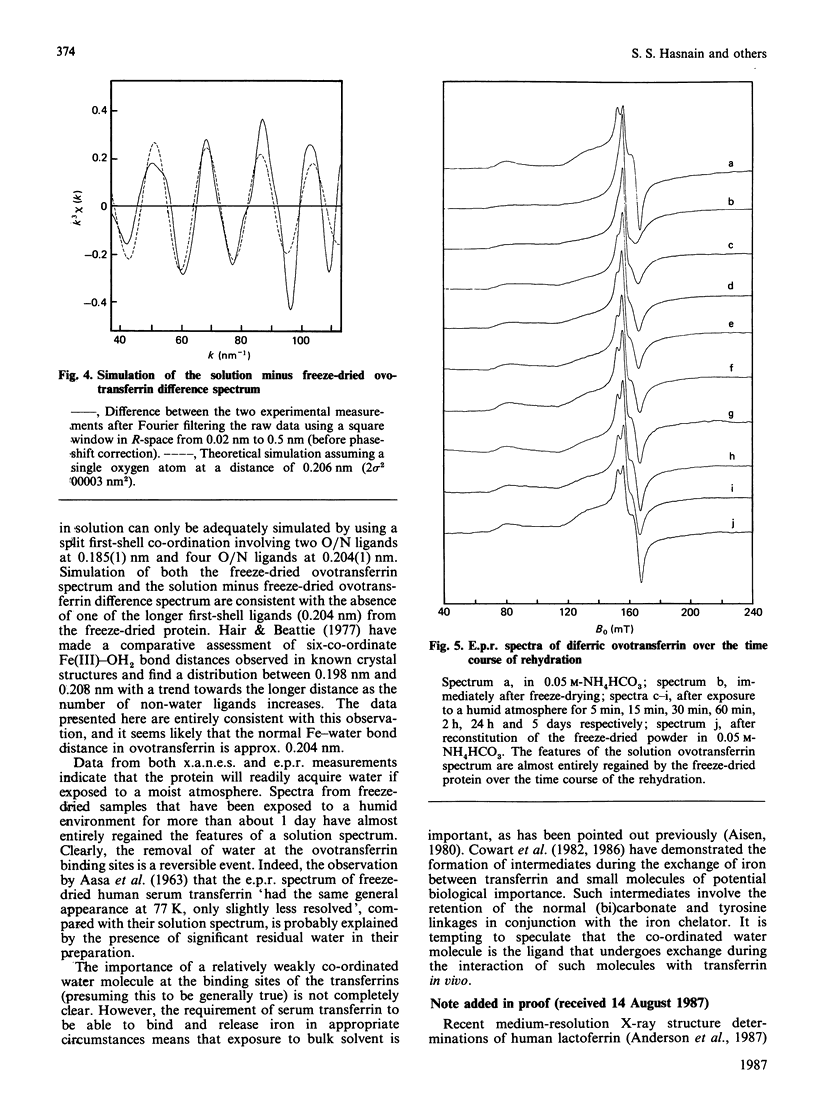
Our previous extended-X-ray-absorption-fine-structure (e.x.a.f.s.) study has shown that the probable iron environment in chicken ovotransferrin involves two low-Z ligands (consistent with phenolate linkages) at 0.185(1) nm and four low-Z ligands at 0.204(1) nm [Garratt, Evans, Hasnain & Lindley (1986) Biochem. J. 233, 479-484]. Herein we provide additional information from the e.x.a.f.s. and near-edge structure suggestive of a decrease in the co-ordination number of ovotransferrin-bound iron upon freeze-drying. These effects are reversible, and exposure of the freeze-dried material to a humid atmosphere results in reversion to the solution spectra. Progressive rehydration was monitored by using e.p.r. spectroscopy and was confirmed by recording the high-resolution X-ray-absorption near-edge structure (x.a.n.e.s.). The results suggest the presence of a labile water molecule at the iron-binding sites of ovotransferrin in solution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Aasa R. Re-interpretation of the electron paramagnetic resonance spectra of transferrins. Biochem Biophys Res Commun. 1972 Nov 1;49(3):806–812. doi: 10.1016/0006-291x(72)90482-2. [DOI] [PubMed] [Google Scholar]
- Aisen P., Aasa R., Redfield A. G. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969 Sep 10;244(17):4628–4633. [PubMed] [Google Scholar]
- Aisen P., Leibman A., Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978 Mar 25;253(6):1930–1937. [PubMed] [Google Scholar]
- Aisen P., Pinkowitz R. A., Leibman A. EPR and other studies of the anion-binding sites of transferrin. Ann N Y Acad Sci. 1973 Dec 31;222:337–346. doi: 10.1111/j.1749-6632.1973.tb15272.x. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Dodson E. J., Norris G. E., Rumball S. V., Waters J. M., Baker E. N. Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I., Briganti F., Koenig S. H., Luchinat C. Magnetic relaxation of solvent protons by Cu2+- and VO2+-substituted transferrin: theoretical analysis and biochemical implications. Biochemistry. 1985 Oct 22;24(22):6287–6290. doi: 10.1021/bi00343a037. [DOI] [PubMed] [Google Scholar]
- Blackburn N. J., Hasnain S. S., Binsted N., Diakun G. P., Garner C. D., Knowles P. F. An extended-X-ray-absorption-fine-structure study of bovine erythrocyte superoxide dismutase in aqueous solution. Direct evidence for three-co-ordinate Cu(I) in reduced enzyme. Biochem J. 1984 May 1;219(3):985–990. doi: 10.1042/bj2190985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. C., Chasteen N. D. Nonequivalence of the metal binding sites in vanadyl-labeled human serum transferrin. Biochemistry. 1975 Oct 21;14(21):4573–4577. doi: 10.1021/bi00692a003. [DOI] [PubMed] [Google Scholar]
- Cowart R. E., Kojima N., Bates G. W. The exchange of Fe3+ between acetohydroxamic acid and transferrin. Spectrophotometric evidence for a mixed ligand complex. J Biol Chem. 1982 Jul 10;257(13):7560–7565. [PubMed] [Google Scholar]
- Cowart R. E., Swope S., Loh T. T., Chasteen N. D., Bates G. W. The exchange of Fe3+ between pyrophosphate and transferrin. Probing the nature of an intermediate complex with stopped flow kinetics, rapid multimixing, and electron paramagnetic resonance spectroscopy. J Biol Chem. 1986 Apr 5;261(10):4607–4614. [PubMed] [Google Scholar]
- Evans G. W. Transferrin function in zinc absorption and transport. Proc Soc Exp Biol Med. 1976 Apr;151(4):775–778. doi: 10.3181/00379727-151-39305. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams J. Studies of the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978 Aug 1;173(2):543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber B. P., Schillinger W. E., Koenig S. H., Aisen P. Nuclear magnetic relaxation dispersion in protein solutions. 3. Copper transferrin and bicarbonate-free copper transferrin. J Biol Chem. 1970 Sep 10;245(17):4251–4255. [PubMed] [Google Scholar]
- Garratt R. C., Evans R. W., Hasnain S. S., Lindley P. F. An extended-X-ray-absorption-fine-structure investigation of diferric transferrins and their iron-binding fragments. Biochem J. 1986 Jan 15;233(2):479–484. doi: 10.1042/bj2330479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer D. G., Monzingo A. F., Matthews B. W. An interactive computer graphics study of thermolysin-catalyzed peptide cleavage and inhibition by N-carboxymethyl dipeptides. Biochemistry. 1984 Nov 20;23(24):5730–5741. doi: 10.1021/bi00319a011. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Gelb M. H. Binding of xylenol orange to transferrin. Demonstration of metal-anion linkage. Biochim Biophys Acta. 1980 May 29;623(1):1–9. doi: 10.1016/0005-2795(80)90002-1. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. II. Transferrin. J Biol Chem. 1969 Dec 10;244(23):6520–6526. [PubMed] [Google Scholar]
- Lipscomb W. N. Structure and catalysis of enzymes. Annu Rev Biochem. 1983;52:17–34. doi: 10.1146/annurev.bi.52.070183.000313. [DOI] [PubMed] [Google Scholar]
- Najarian R. C., Harris D. C. Oxalate and spin-labeled oxalate as probes of the anion binding site of human transferrin. Metal to anion distance. J Biol Chem. 1978 Jan 10;253(1):38–42. [PubMed] [Google Scholar]
- O'Hara P. B., Koenig S. H. Electron spin resonance and magnetic relaxation studies of gadolinium(III) complexes with human transferrin. Biochemistry. 1986 Mar 25;25(6):1445–1450. doi: 10.1021/bi00354a038. [DOI] [PubMed] [Google Scholar]
- Palmer G. The electron paramagnetic resonance of metalloproteins. Biochem Soc Trans. 1985 Jun;13(3):548–560. doi: 10.1042/bst0130548. [DOI] [PubMed] [Google Scholar]
- Schlabach M. R., Bates G. W. The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem. 1975 Mar 25;250(6):2182–2188. [PubMed] [Google Scholar]
- Schneider D. J., Roe A. L., Mayer R. J., Que L., Jr Evidence for synergistic anion binding to iron in ovotransferrin complexes from resonance Raman and extended X-ray absorption fine structure analysis. J Biol Chem. 1984 Aug 10;259(15):9699–9703. [PubMed] [Google Scholar]
- Thundathil R. V., Holt E. M., Holt S. L., Watson K. J. Preparation and properties of iron (III)-amino acid complexes. 2. The crystal and molecular structure of monoclinic. Tri-mu3-oxo-triaquohexakis(glycine)triiron(III) perchlorate. J Am Chem Soc. 1977 Mar 16;99(6):1818–1823. doi: 10.1021/ja00448a024. [DOI] [PubMed] [Google Scholar]
- Trapp G. A. Plasma aluminum is bound to transferrin. Life Sci. 1983 Jul 25;33(4):311–316. doi: 10.1016/s0024-3205(83)80002-2. [DOI] [PubMed] [Google Scholar]
- Villafranca J. J., Pillai R. P., Woodworth R. C. Magnetic resonance studies of Mn(II)-, Mn(III)-, and Fe(III)-conalbumin complexes. Bioinorg Chem. 1976;6(3):233–245. doi: 10.1016/s0006-3061(00)80230-6. [DOI] [PubMed] [Google Scholar]
- WINDLE J. J., WIERSEMA A. K., CLARK J. R., FEENEY R. E. INVESTIGATION OF THE IRON AND COPPER COMPLEXES OF AVIAN CONALBUMINS AND HUMAN TRANSFERRINS BY ELECTRON PARAMAGNETIC RESONANCE. Biochemistry. 1963 Nov-Dec;2:1341–1345. doi: 10.1021/bi00906a028. [DOI] [PubMed] [Google Scholar]
- Williams J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem J. 1968 Jun;108(1):57–67. doi: 10.1042/bj1080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Aisen P. Studies of transferrin with the use of Cu2+ as an electron paramagnetic resonance spectroscopic probe. J Biol Chem. 1977 Sep 10;252(17):6090–6096. [PubMed] [Google Scholar]
- Zweier J. L., Wooten J. B., Cohen J. S. Studies of anion binding by transferrin using carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 Jun 9;20(12):3505–3510. doi: 10.1021/bi00515a031. [DOI] [PubMed] [Google Scholar]


