Abstract
Therapeutic delivery of anti-cancer drugs is a major goal of modern medicine. However, developing methods to target cancer cells for more effective treatment and reduced side effects is a significant ongoing challenge. Microrobots have recently been studied for their ability to navigate difficult-to-reach regions in the human body to deliver therapeutics for microscopically localized interventions. Using microrobots for targeted and local therapy therefore, is a promising revolutionary treatment method. In this study, magnetic microrobots were used to target and kill cancer cells via localized magnetic oscillations, resulting in magnetolysis of the cancer cells. The magnetic microrobots were selectively moved to Hepatocarcinoma cells (HepG2 cells) using a custom magnetic system which applied rotating magnetic fields. After internalization of the microrobots by the cancer cells, magnetic oscillation of varying dosages was applied, resulting in cell death.
I. INTRODUCTION
A significant problem with typical cancer treatments, such as chemotherapy and radiation, is the non-specific manner in which they function. This results in unwanted damage to healthy cells and many adverse side effects. Therefore, developing methods of targeted therapy that produce less harmful effects is of significant importance. Although there has been progress in producing targeted treatments using small molecule inhibitors [1], monoclonal antibodies [2], immunotherapy [3], and other methods [4], [5], [6], these treatments have their own side effects and are limited in their range of application.
There are two ways that targeted therapy can be carried out. Either a drug can be delivered systemically while only effecting cancer cells, or an indiscriminate treatment can be delivered to a specific location in the body. The latter method presents technological challenges but can afford the use of less sophisticated and more broad-spectrum treatments. A promising technology for targeted therapy is with the use of microrobots, micron-scale objects that are capable of carrying out desired tasks [7]. Microrobots are an active area of current research and much work is being done with the goal of using them in biomedical applications for tasks such as sensing, targeted drug delivery, or microsurgery [8], [9], [10], [11], [12], [13], [14]. Using microrobots for targeted treatment could provide a significant improvement in cancer or other disease therapies.
Although drug delivery using microrobots is a promising approach for targeted treatment, it requires a means of both carrying the payload and then releasing it at the site, which presents technological challenges. A simpler approach is to use the magnetic properties of the microrobots to disrupt and kill the targeted cells. Driving microrobots with magnetic fields is a common means of control due to its practicality and safety compared to other techniques such as chemically driven microrobots which rely on toxic fuels in their medium [15], [16], [17], [18]. Therefore, microrobots that are used in biomedical applications are likely to possess magnetic properties. Utilizing the already present magnetic nature of the microrobots therefore would provide a simple and more straightforward approach to targeted therapy. Previous work has shown that various magnetic particles such as magnetic disks, iron microparticles, carbon nanotubes, and magnetized-silica spindle-shaped particles can kill cancer cells (magnetolysis) via low frequency magnetic oscillation [19], [20], [21], [22]. While the application of low frequency magnetic fields themselves is known to inhibit cell proliferation and induce apoptosis by a metabolic shift and by affecting gene expression, respectively, [23], [24] the experiments utilizing magnetic particles rely on different mechanisms (either direct cell membrane damage or mechanical stress-induced apoptosis) [22], [25]. Therefore, we sought to use magnetic microrobots, which we can move to specified cells using rotating magnetic fields, to induce cell lysis. Our results demonstrate that magnetic microrobots can be used as an effective means to both target and kill cancer cells by a straightforward application of rotating and alternating magnetic fields, respectively.
II. Materials and Methods
A. Microrobots
The microrobots were made of paramagnetic polystyrene material (diameter= 4.7 μm), which were purchased from Spherotech (Cat. No. FCM-4052-2). Detailed characterization of beads (SEM, magnetization curve, functional group etc.) can be found on Spherotech website. These microrobots are Fluorescein isothiocyanate (FITC)-labeled. We note that although these micron-sized magnetic particles are quite simple, the ability to control and utilize them for desired tasks is what characterizes them as microrobots.
B. Experimental Setup
Experiments were conducted on a Zeiss Axiovert 200 inverted microscope with an Amscope MU903-65 camera as well as on a Ziess Axioplan 2 upright microscope using an Axiovert 503 mono camera. Magnetic fields were applying using custom-built magnetic control systems. We utilized two magnetic systems (Fig. 2). One consisted of four electromagnetic solenoids containing soft iron cores which are arranged in an array along the x and y axes, allowing for magnetic fields to be applied in any orientation in the horizontal plane (Fig. 2a). The other magnetic system consisted of three pairs of coils along three axes, thereby creating fields along any specified direction in three dimensions (Fig. 2b). The magnetic field strength at the sample was approximately 8 mT and 4 mT using the solenoid array and the 3D coil system, respectively. We measured the field strength of the solenoid array while applying the magnetic oscillation at various frequencies used in the experiments and found that it remained approximately constant. Custom python code controlled the amount and direction of current that was sent to each coil. The system is described in detail elsewhere [18]. The fluorescent microrobots were illuminated using an X-cite mini plus from excelitas technologies. Custom software was written in python to detect microrobots and plot their trajectories.
Fig. 2.

Images of the electromagnetic systems used in the experiments. (a) An image of the electromagnetic solenoid array. (b) An image of the 3D coil setup for applying rotating magnetic fields.
C. Cell Cuture
Hepatocellular Carcinoma cells (HepG2) cells were gifted by Richard West (Associate Scientist at Flow Cytometry Core Facility). Cells were cultured in Dulbecco’s Modified Essential Medium (DMEM) (Gibco, BenchStable, USA) media with 5% CO2 and maintained at 37°C in an incubator. All experiments were performed after the third passage of cells.
D. Assessment of Cytotoxicity and Cellular uptake of microrobots
Cytotoxicity of microrobots was evaluated in HepG2 cells. Cells were seeded (2×104 cells/well) in a clear flat bottom 24-well plate (Costar, Corning, USA) and incubated in DMEM media with 5% CO2 at 37°C for 24 hours. Then, cells were treated with microrobots (4.7μm size, 1 mg/mL) and incubated for 24 hours. Cells were then imaged under optical microscope to check cell morphology. Flow cytometry was also performed to quantify exact percentage of cell death after propidium iodide (PI) staining.
Cellular internalization of microrobots was assessed by flow cytometry. Samples for cellular uptake were prepared as described by the aforementioned method in HepG2 cells. 1×105 cells/well were seeded in a 6-well plate and incubated in DMEM with 5% CO2 at 37°C for 24 hours. Then, 20 μL of 1% w/v microrobots solution was added to each well containing 2 mL of the media. After 24 hours, cells were washed and analyzed using a flow cytometer (BD FACS Aria llu).
E. Assessment of Cell Death from Magnetic Vibration
HepG2 cells were treated with microrobots (100 μg/mL) in a 6- well plate and incubated in DMEM media with 5% CO2 at 37°C for 24 hours. After 24 hours, cells were washed and trypsinized to detach cells from the culture dish. Cells with microrobots were sorted and collected in DMEM media. Then, single HepG2 cells with internalized particles were subjected to magnetic oscillation with frequency between 5–15 Hz in xy-plane for 30 minutes.
III. RESULTS AND DISCUSSION
A. Control and transport of Microrobots
Experiments were carried out using our magnetic system, as shown in Fig. 1. The paramagnetic microrobots were transported using our custom-built magnetic controller. To move the microrobots, a rotating magnetic field was applied using the 3D coil system which caused the microrobots to roll along the surface of the substrate. Magnetic oscillation was created by applying a magnetic field in the xy plane and then reversing the direction of the field at various frequencies. The magnetic oscillation was carried out using the magnetic solenoid system while the magnetic rolling was performed using the 3D coil system which can apply the required rotating fields.
Fig. 1.
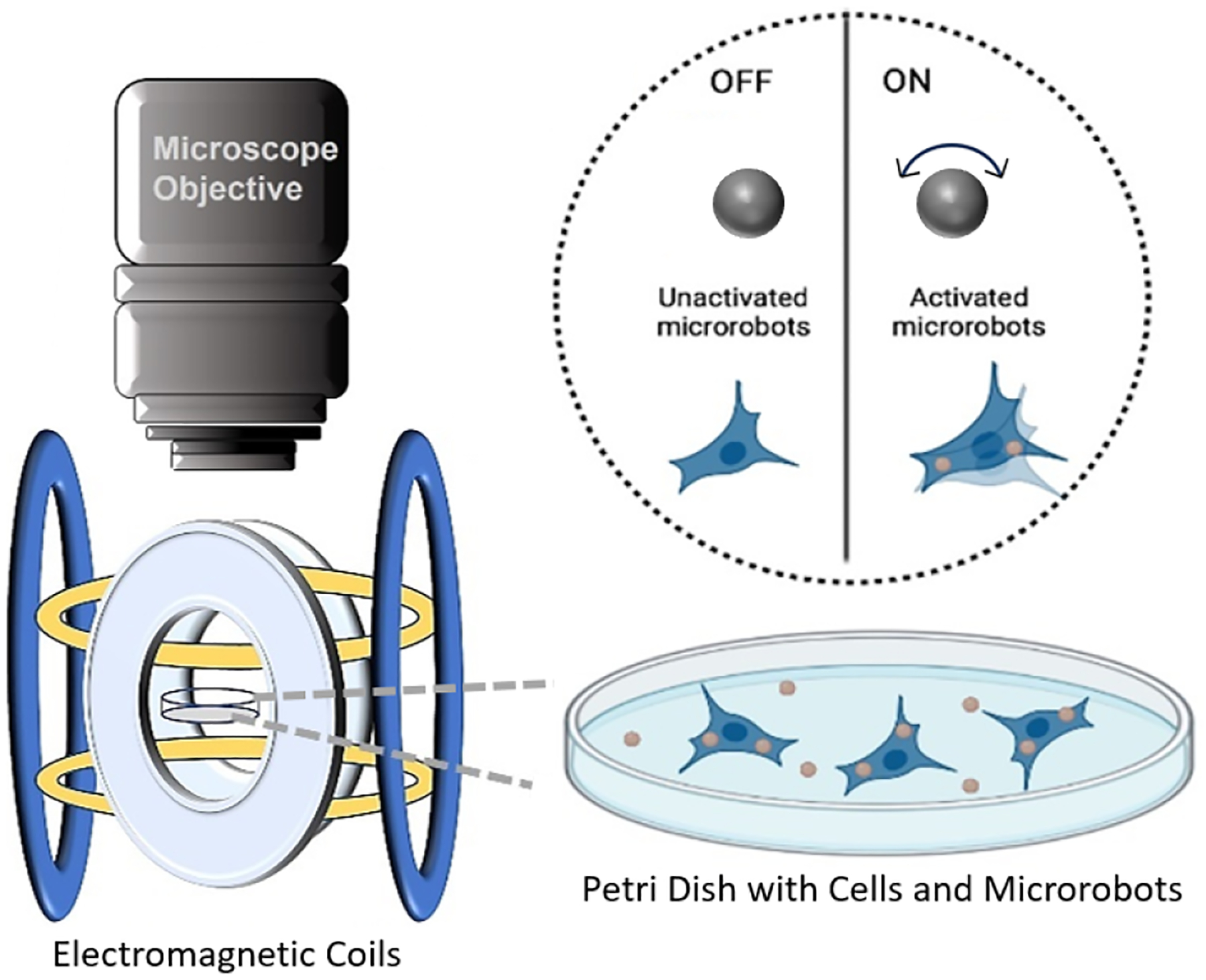
Schematic representation of killing cancer cells via magnetic oscillation.
In this paper we first demonstrate the targeting of cells using the rolling microrobots, then we show that the cells uptake the microrobots, and lastly we apply vibrating magnetic fields and assess the amount of cell death of the cells that internalized the microrobots.
B. Cytotoxicity of Microrobots
Biocompatibility is one of the most important factors in microrobot design. Although the paramagnetic microrobots we use are made of nontoxic polystyrene material, we tested their cytotoxicity to ensure that they are not toxic to the cells. Figure 4 shows brightfield images of the microrobots (a), the cells after internalizing the microrobots (b), and fluorescent images of only the cells (c) and the cells after internalizing the microrobots (d). Cells were incubated with the microrobots for 24 hours, and changes in cell morphology was monitored using an optical and fluorescence microscope. We found that after the 24 hour period the cell monolayer was intact and did not show any effects of toxicity (Fig. 4b and d). Cells were then sorted to separate cell population with microrobots and then cultured again for 24 hours. Figure 5a and b show images of the cells after this period. The cells are adhered to the petridish without any dead suspended cells. Quantitative analysis corroborated this, showing negligible cell death of 3% when compared with untreated cells (Fig. 5c and d).
Fig. 4.
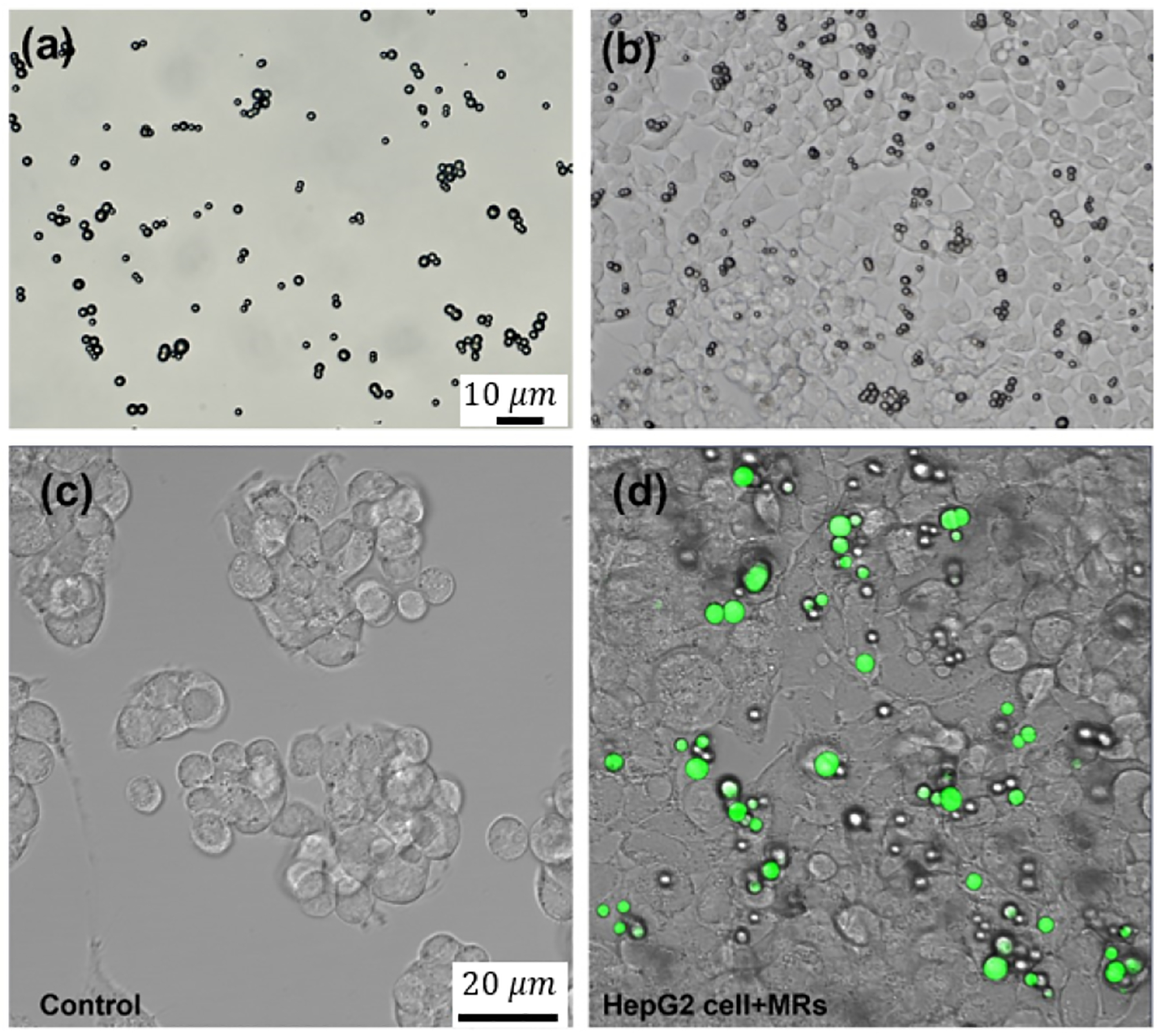
(a) Microscopic image of microrobots, (b) adherent HepG2 cells with microrobots and (c) Fluorescence image without and with (d) microrobots (green) inside the cells.
Fig. 5.
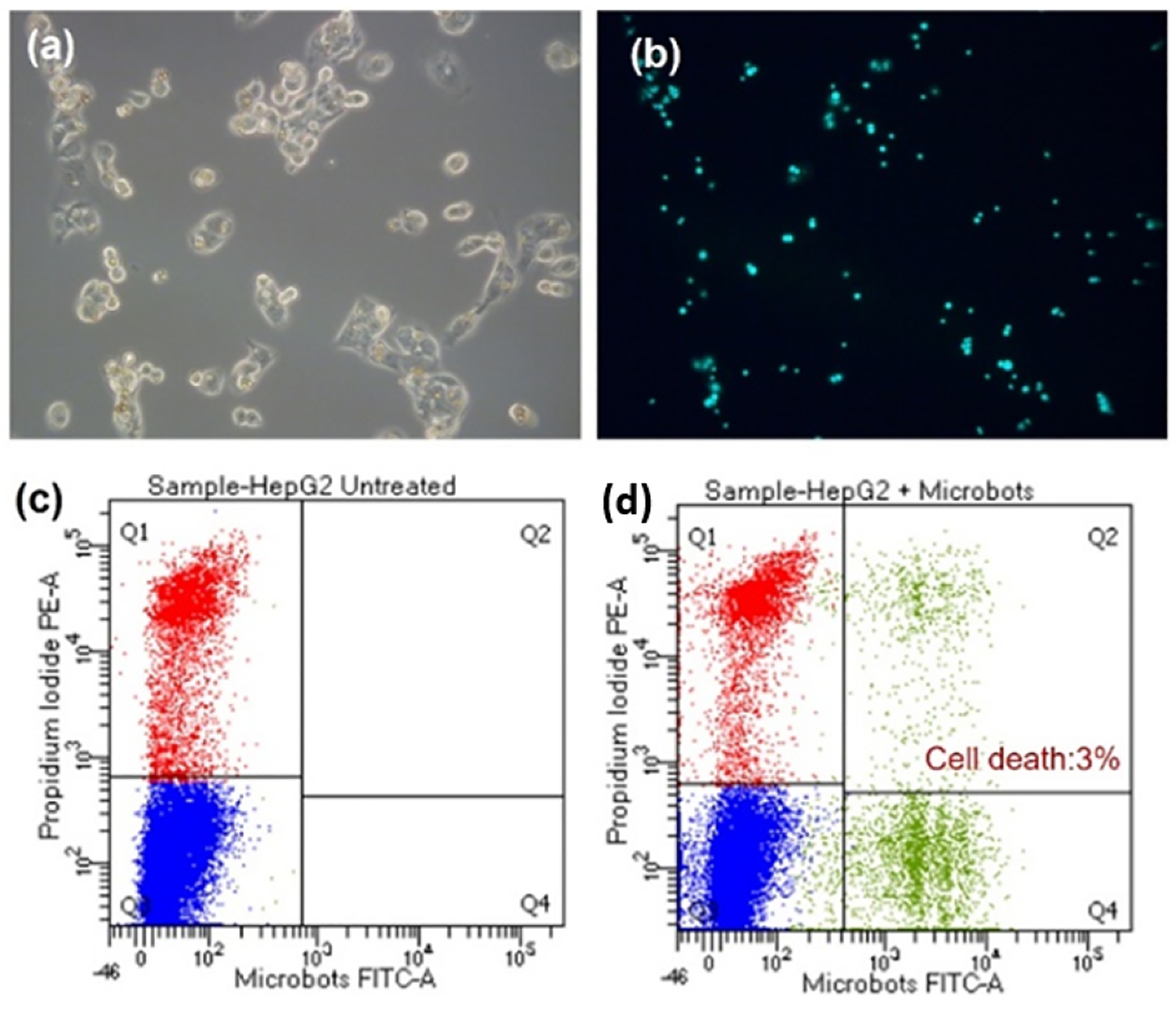
Cytocompatibility of the microrobots. HepG2 Cell treated with microrobots (a) bright field image and (b) corresponding fluorescence image. Flow cytometry data showing cell death in (c) untreated and (d) microrobot-treated cells.
C. Cell-Internalization of Microrobots
The microrobots were completely internalized by the HepG2 cells after 24 hours. There were often multiple microrobots observed inside the cells showing high affinity of the microrobots and cells (Fig. 4b and d). Cell internalization was quantified using flow cytometry which indicates that 16.6% of the cells had microrobots inside (Fig. 6). HepG2 cells with a broad range of intensities demonstrating unequal distribution of microrobots insides the cells. This difference in cell internalization was presumably due to either the initial inhomogeneous distribution of microrobots or to the cluster forming growth pattern of HepG2 cells that limits the surface area of interaction between cells and microrobots.
Fig. 6.
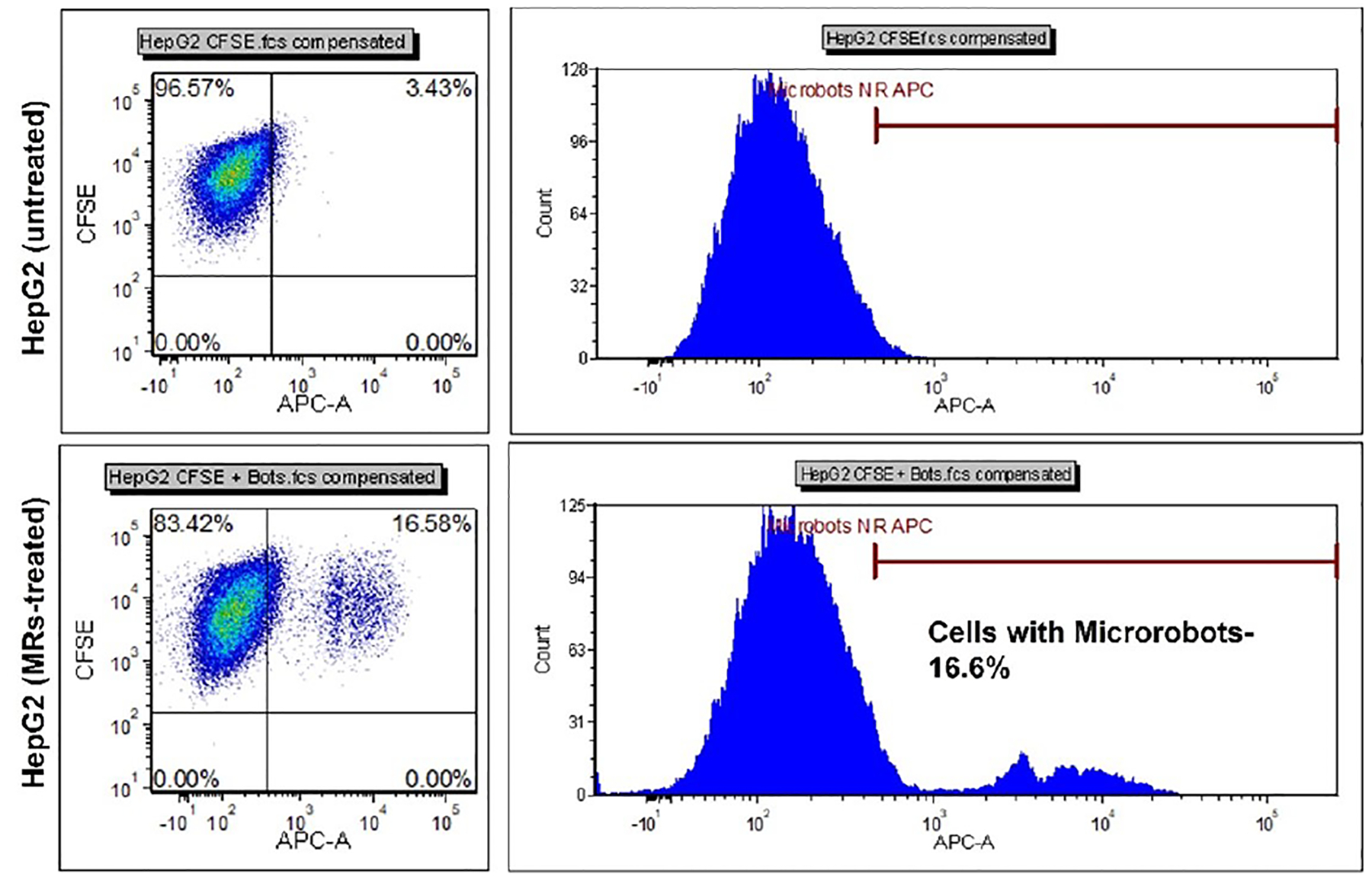
Flow cytometry data showing cell internalization of the microrobots in HepG2 cells.
D. Targeted Delivery of Microrobots
We sought to demonstrate the microrobot’s capability to target individual cells by moving them to a specific cell using a magnetic rolling mechanism. Targeting intracellular structures or organelles using microrobots could be used to not only selectively kill certain cells, but also to potentially alter or interfere with the cell’s biological functionality for other applications. A video of the microrobot being magnetically rolled to the cell is given in Vid. 1, and we show images and the tracked trajectory of the microrobot in Fig. 3. We note that there remains a challenge of moving multiple microrobots to one or more cells, however these results demonstrates a proof of concept of targeting individual cells for selective treatment.
Fig. 3.
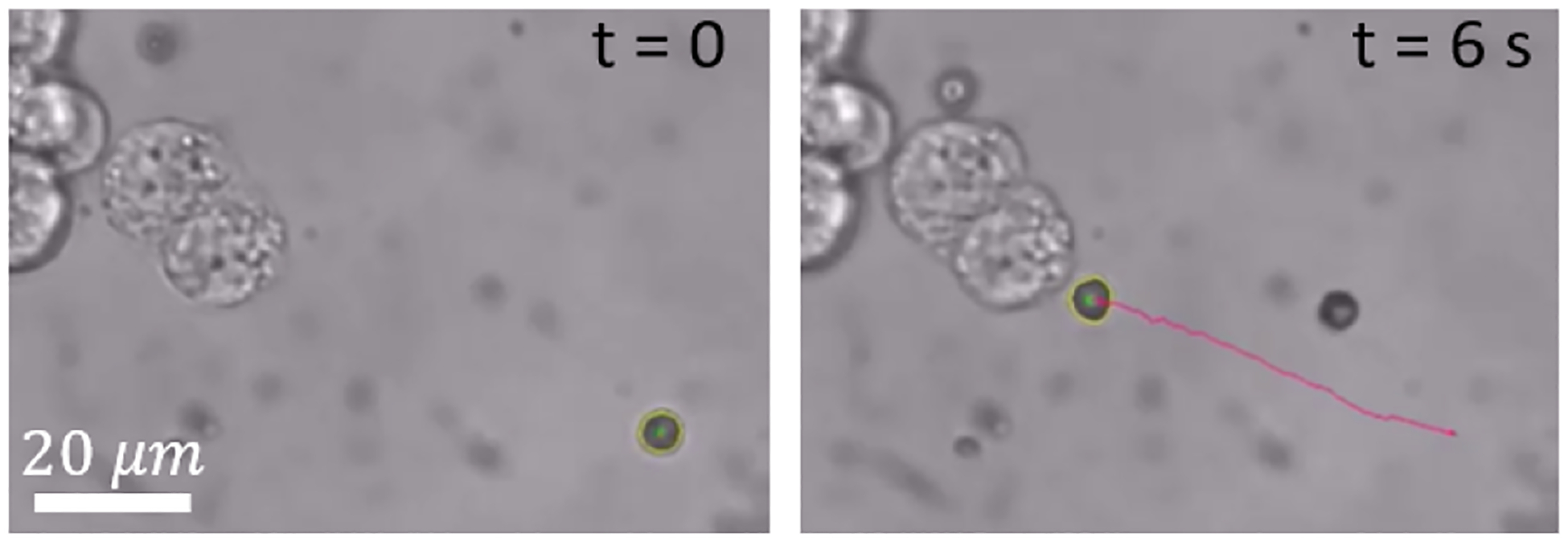
Images showing a microrobot being magnetically rolled to a cell (see Vid. 1).
E. Magnetic Vibration Induced Cell Death
Once the microrobots are internalized, we applied an oscillating magnetic field of predefined frequency to opposite facing electromagnets. The electromagnets were rapidly changed from an on state to an off state asynchronously and with opposite polarity, thereby resulting in a rapid alternating magnetic field along a chosen axis. As shown in Fig. 7 and Vid. 2, the total angular displacement, Δθ, of the microrobots between these cycles of field reversal depended on the applied frequency of the oscillating magnetic field. This is presumably due to the rotational viscous drag on the microrobot which prevents it from fully aligning with the applied magnetic field prior to each field reversal. Vibration was applied for 30 minutes at two different frequencies (5 and 10 Hz) on HepG2 cells and increased cell death was observed just after one application (Fig. 8). We find that the HepG2 cells were responsive to oscillation and a greater number died after 24 hours with microrobots compared to the case without microrobots (Fig. 8c and d). The increase in cell death with magnetic oscillation (Fig. 8c) compared to the case without oscillation (Fig.5c) indicates that the application of the magnetic oscillation does result in some degree of cell death, possibly by a similar mechanism responsible for the cell death under low-frequency magnetic fields in Refs. [23], [24]. We also observed that cells that internalized multiple microrobots tended to die at a higher rate than cells with a single microrobot. This was presumably due to the stronger force collectively generated by the greater number of microrobots. This indicates a potential relationship between internal disruption and biochemical signaling.
Fig. 7.

(a) Graph displaying displacement angle (Δθ) versus frequency and (b) a schematic illustration of magnetic oscillation. As the frequency at which each electromagnet is pulsed increases, the resulting angular displacement of the magnetic microrobot decreases. A frequency of 5 Hz results in the microrobot rotating approximately 60 degrees whereas a frequency of 30 Hz results in very small angular displacement.
Fig. 8.
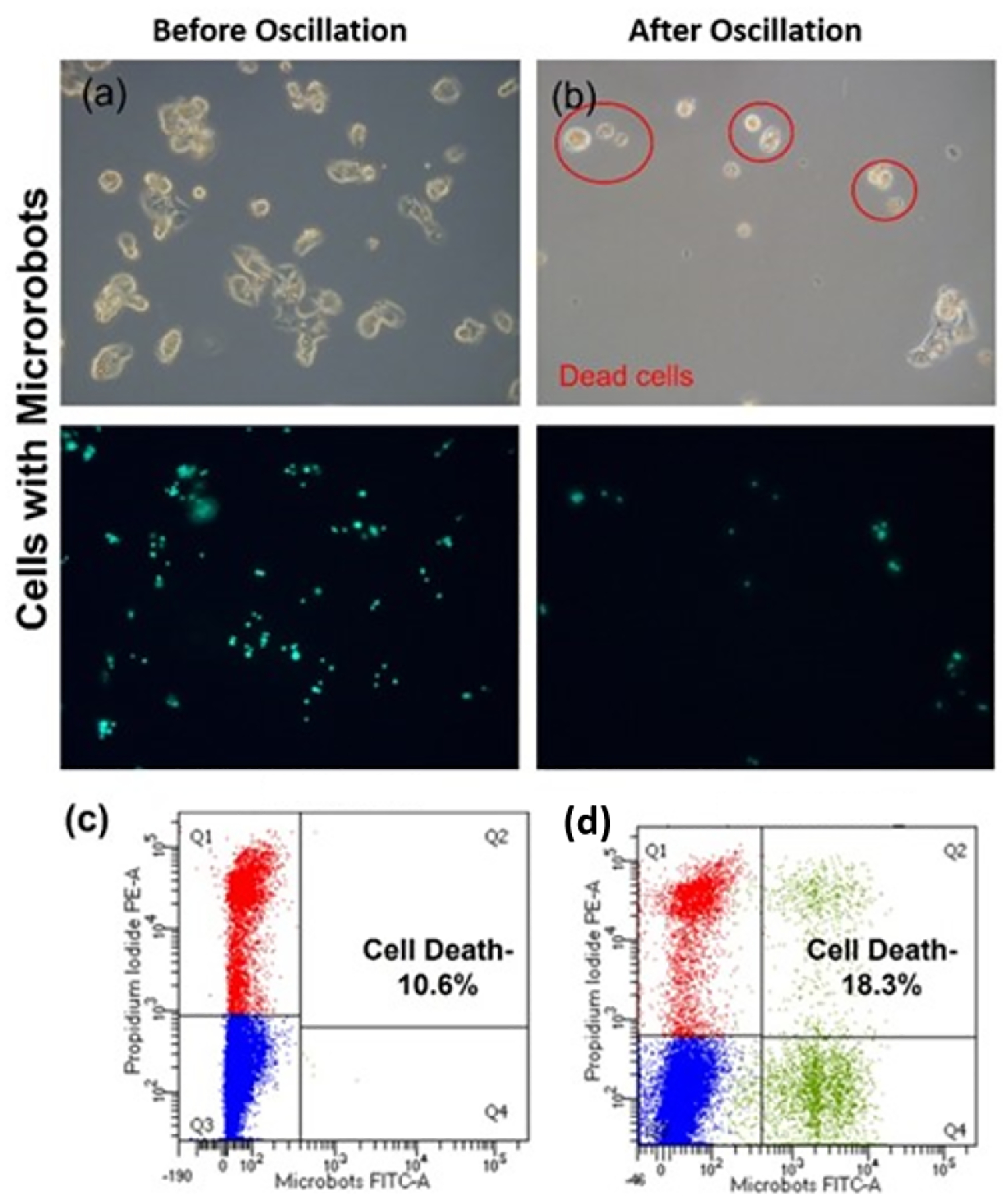
Cell death after oscillation. Fluorescence microscopic images of HepG2 cells (a) before and (b) after oscillation. Cells were trypsinzed and subjected to magnetic oscillation for 30 minutes and transferred to an incubator. Cells that are dead after 24 hours are highlighted with red circles. (c,d) Flow cytometry data showing cell death after culturing for 24 hours following magnetic oscillation without microrobots (c) and with microrobots (d).
We assume the mechanism of internal disruption and cell death is caused by shear stress that is generated in the cytoplasm due to the magnetic oscillation. Chiew et al. have also reported similar findings that shows mechanical stress-induced cancer cell death using microparticles [26], [25]. Moreover, propidium iodide staining during flow cytometry analysis also confirmed that membrane damage could be the possible cell destruction mechanism.
IV. CONCLUSION AND FUTURE WORK
This work demonstrates that microrobots can be an effective means to target and kill cancer cells using simple alternating magnetic fields. Such targeted control could lead to future treatments with reduced side effects, as well as possibly being used to enhance immunotherapy by evoking immune cells activation [27].
Since an increase in temperature is unlikely to be elicited at such a low frequency, the cell death is most likely due to cell membrane or intracellular microstructure damaged-induced necrosis. In the future, we plan to conduct experiments with other cell and tissue types and in animal models as well as to study the underlying mechanism of vibration induced cell death.
Although we demonstrated the ability to move one microrobot to a particular cell, there remains a challenge of moving multiple microrobots to one or more cells. Such control strategies are the focus of much current work of our own and others in the microrobotic community. However, our present study shows promising potential for magnetic oscillation-based therapeutic approaches for cancer treatment, as well as providing a means to better understand cell behavior and related biochemical processes.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the late Richard West for his help with the cell lines. This project was supported by the Delaware INBRE program, with a grant from the National Institute of General Medical Sciences – NIGMS (P20 GM103446) from the National Institutes of Health and the State of Delaware. This work was also supported by NSF grant OIA2020973. This work was also supported by the National Science Foundation under grant GCR 2219101 and the National Health Institute under grant 1R35GM147451. This content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
References
- [1].Bedard PL, Hyman DM, Davids MS, and Siu LL, “Small molecules, big impact: 20 years of targeted therapy in oncology,” The Lancet, vol. 395, no. 10229, pp. 1078–1088, 2020. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0140673620301641 [DOI] [PubMed] [Google Scholar]
- [2].Zahavi D and Weiner L, “Monoclonal antibodies in cancer therapy,” Antibodies, vol. 9, no. 3, 2020. [Online]. Available: https://www.mdpi.com/2073-4468/9/3/34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Farkona S, Diamandis EP, and Blasutig IM, “Cancer immunotherapy: the beginning of the end of cancer?” BMC Medicine, vol. 14, no. 1, p. 73, 2016. [Online]. Available: 10.1186/s12916-016-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Das S, Hunter EE, DeLateur NA, Steager EB, Weiss R, and Kumar V, “Cellular expression through morphogen delivery by light activated magnetic microrobots,” Journal of Micro-Bio Robotics, vol. 15, pp. 79–90, 2019. [Google Scholar]
- [5].Das S, Hunter E, DeLateur NA, Steager EB, Weiss R, and Kumar V, “Controlled delivery of signaling molecules using magnetic microrobots,” in 2018 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS). IEEE, 2018, pp. 1–5. [Google Scholar]
- [6].Shah ZH, Wu B, and Das S, “Multistimuli-responsive microrobots: A comprehensive review,” Frontiers in Robotics and AI, vol. 9, p. 1027415, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schmidt CK, Medina-Sánchez M, Edmondson RJ, and Schmidt OG, “Engineering microrobots for targeted cancer therapies from a medical perspective,” Nature Communications, vol. 11, no. 1, p. 5618, 2020. [Online]. Available: 10.1038/s41467-020-19322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sitti M, Ceylan H, Hu W, Giltinan J, Turan M, Yim S, and Diller E, “Biomedical applications of untethered mobile milli/microrobots,” Proceedings of the IEEE, vol. 103, no. 2, pp. 205–224, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu R, Zhu Y, Cai X, Wu S, Xu L, and Yu T, “Recent process in microrobots: From propulsion to swarming for biomedical applications,” Micromachines, vol. 13, no. 9, 2022. [Online]. Available: https://www.mdpi.com/2072-666X/13/9/1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nelson BJ, Kaliakatsos IK, and Abbott JJ, “Microrobots for minimally invasive medicine,” Annual Review of Biomedical Engineering, vol. 12, no. 1, pp. 55–85, 2010. [Online]. Available: 10.1146/annurev-bioeng-010510-103409 [DOI] [PubMed] [Google Scholar]
- [11].Das S, Steager EB, Stebe KJ, and Kumar V, “Simultaneous control of spherical microrobots using catalytic and magnetic actuation,” in 2017 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS). IEEE, 2017, pp. 1–6. [Google Scholar]
- [12].Beaver LE, Wu B, Das S, and Malikopoulos AA, “A first-order approach to model simultaneous control of multiple microrobots,” in 2022 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS). IEEE, 2022, pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rivas D, Mallick S, Sokolich M, and Das S, “Cellular manipulation using rolling microrobots,” in 2022 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS). IEEE, 2022, pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mallick S, Abouomar R, Rivas D, Sokolich M, Kirmizitas FC, Dutta A, and Das S, “Doxorubicin-loaded microrobots for targeted drug delivery and anticancer therapy.” Advanced Healthcare Materials, pp. e2 300 939–e2 300 939, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ceylan H, Giltinan J, Kozielski K, and Sitti M, “Mobile microrobots for bioengineering applications,” Lab Chip, vol. 17, pp. 1705–1724, 2017. [Online]. Available: 10.1039/C7LC00064B [DOI] [PubMed] [Google Scholar]
- [16].Shah ZH, Sockolich M, Rivas D, and Das S, “Fabrication and open-loop control of three-lobed nonspherical janus microrobots,” MRS Advances, pp. 1–5, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rivas DP, Sokolich M, and Das s., “Spatial patterning of micromotor aggregation and flux,” ChemNanoMat, p. e202300225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sokolich M, Rivas D, Yang Y, Duey M, and Das S, “Modmag: A modular magnetic micro-robotic manipulation device,” MethodsX, vol. 10, p. 102171, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim D-H, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, and Novosad V, “Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction,” Nature materials, vol. 9, no. 2, pp. 165–171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bouchlaka MN, Sckisel GD, Wilkins D, Maverakis E, Monjazeb AM, Fung M, Welniak L, Redelman D, Fuchs A, Evrensel CA, et al. , “Mechanical disruption of tumors by iron particles and magnetic field application results in increased anti-tumor immune responses,” PloS one, vol. 7, no. 10, p. e48049, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu D, Wang L, Wang Z, and Cuschieri A, “Magnetoporation and magnetolysis of cancer cells via carbon nanotubes induced by rotating magnetic fields,” Nano letters, vol. 12, no. 10, pp. 5117–5121, 2012. [DOI] [PubMed] [Google Scholar]
- [22].Wang B, Bienvenu C, Mendez-Garza J, Lançon P, Madeira A, Vierling P, Di Giorgio C, and Bossis G, “Necrosis of hepg2 cancer cells induced by the vibration of magnetic particles,” Journal of magnetism and magnetic materials, vol. 344, pp. 193–201, 2013. [Google Scholar]
- [23].Bergandi L, Lucia U, Grisolia G, Granata R, Gesmundo I, Ponzetto A, Paolucci E, Borchiellini R, Ghigo E, and Silvagno F, “The extremely low frequency electromagnetic stimulation selective for cancer cells elicits growth arrest through a metabolic shift,” Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, vol. 1866, no. 9, pp. 1389–1397, 2019. [DOI] [PubMed] [Google Scholar]
- [24].Xu Y, Wang Y, Yao A, Xu Z, Dou H, Shen S, Hou Y, and Wang T, “Low frequency magnetic fields induce autophagy-associated cell death in lung cancer through mir-486-mediated inhibition of akt/mtor signaling pathway,” Scientific reports, vol. 7, no. 1, p. 11776, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang E, Kircher MF, Koch M, Eliasson L, Goldberg SN, and Renstrom E, “Dynamic magnetic fields remote-control apoptosis via nanoparticle rotation,” ACS nano, vol. 8, no. 4, pp. 3192–3201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takao S, Taya M, and Chiew C, “Mechanical stress-induced cell death in breast cancer cells,” Biology open, vol. 8, no. 8, p. bio043133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mercogliano MF, Bruni S, Mauro F, Elizalde PV, and Schillaci R, “Harnessing tumor necrosis factor alpha to achieve effective cancer immunotherapy,” Cancers, vol. 13, no. 3, p. 564, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


