Abstract
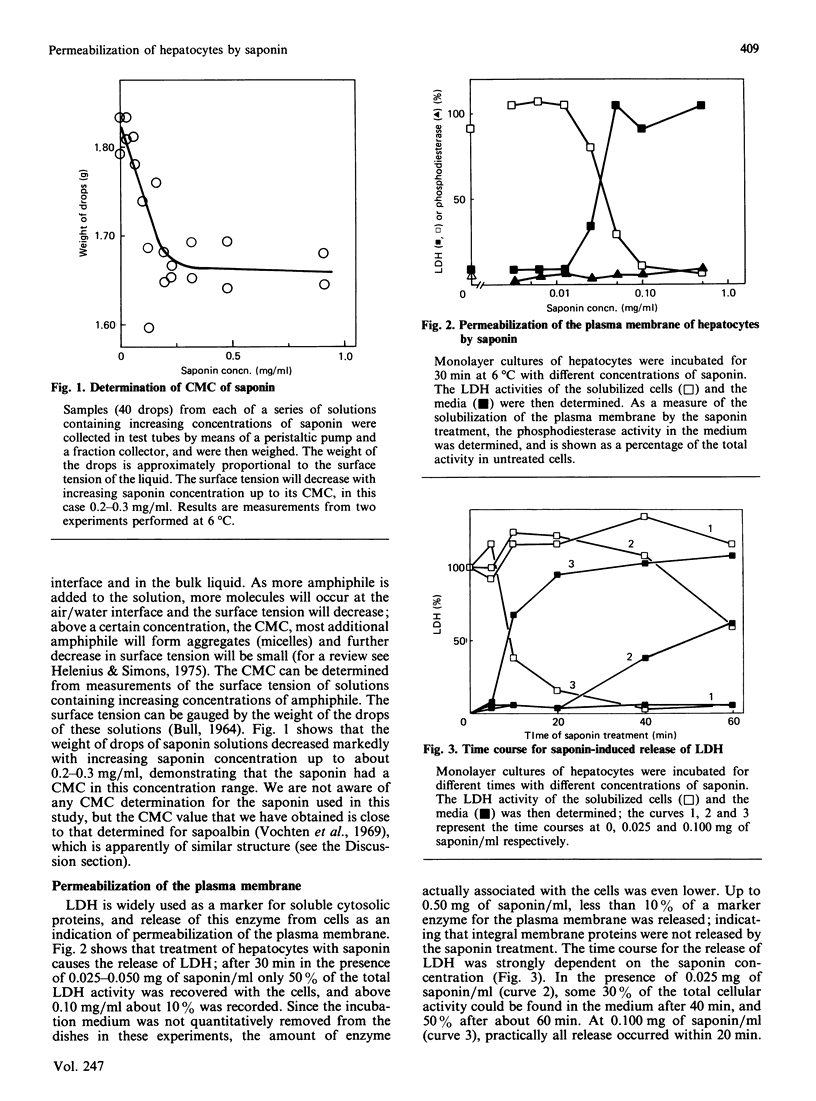
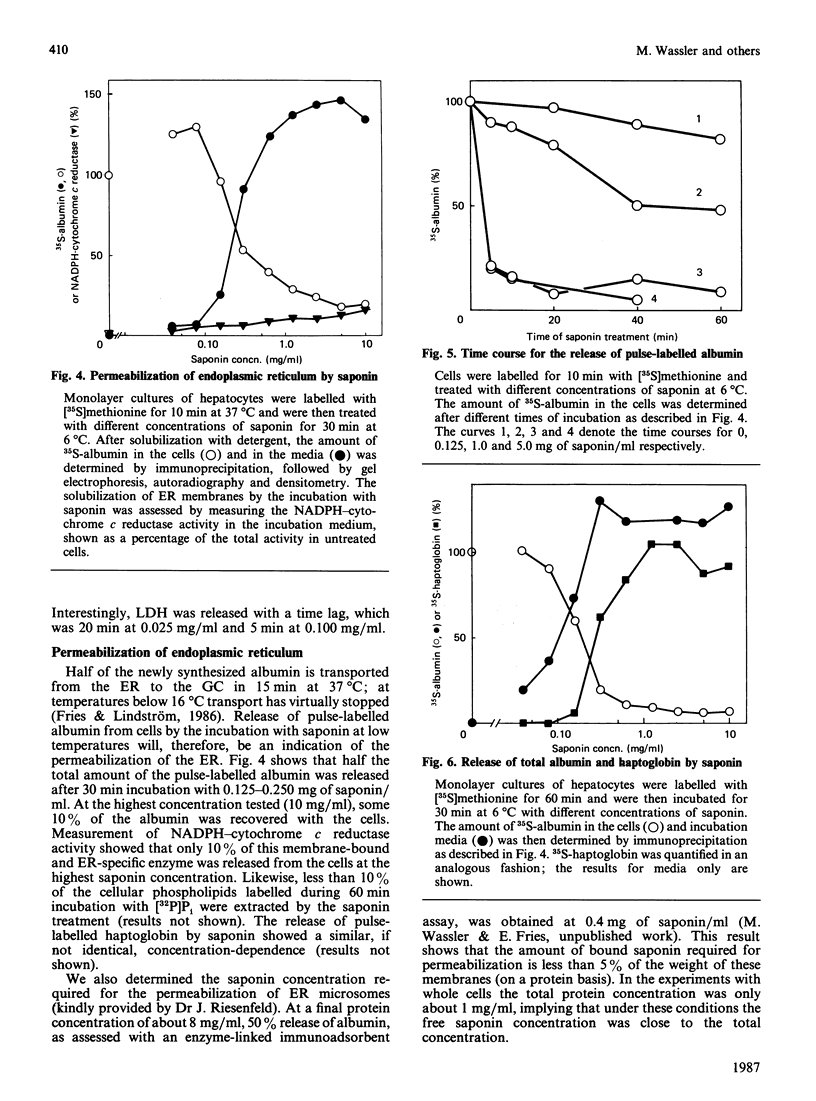
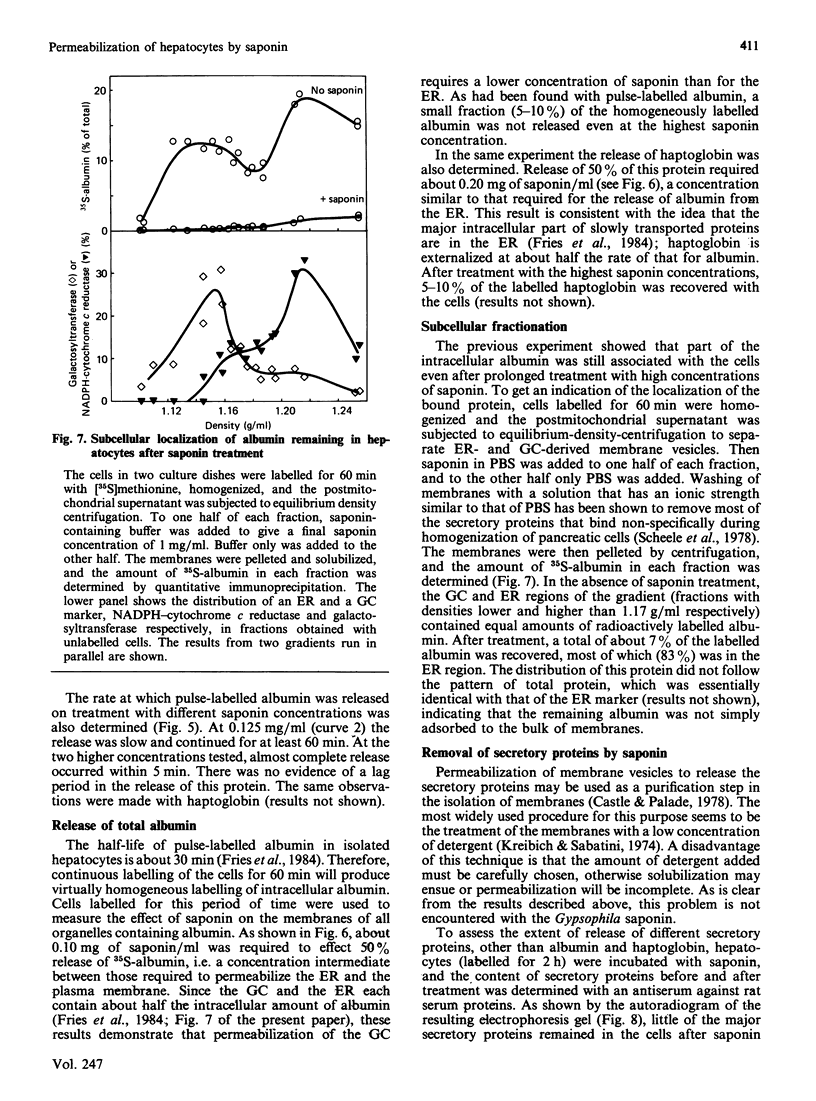
Monolayer cultures of rat hepatocytes were treated with increasing concentrations of saponin (prepared from Gypsophila plants) for 30 min at 6 degrees C. Differential permeabilization of the intracellular membranes could be demonstrated: at 0.040 mg of saponin/ml the plasma membrane was permeabilized, as assessed by the release of 50% of the total cellular amount of lactate dehydrogenase, and at 0.20 mg/ml the endoplasmic reticulum was permeabilized, as measured by the release of 50% of pulse-35S-labelled albumin. The Golgi complex was permeabilized at an intermediate saponin concentration, as indicated by the release of homogeneously 35S-labelled albumin; about half the intracellular albumin is located in this organelle. At 1.0 up to 5.0 mg of saponin/ml 90-95% of the radioactively labelled albumin was released. Even at 5.0 mg/ml less than 10% of the membrane of the endoplasmic reticulum was solubilized, as judged by the degree of release of a membrane-bound enzyme specific for this organelle. These results demonstrate the usefulness of saponin as a tool for investigating the interior of different intracellular compartments.
Full text
PDF

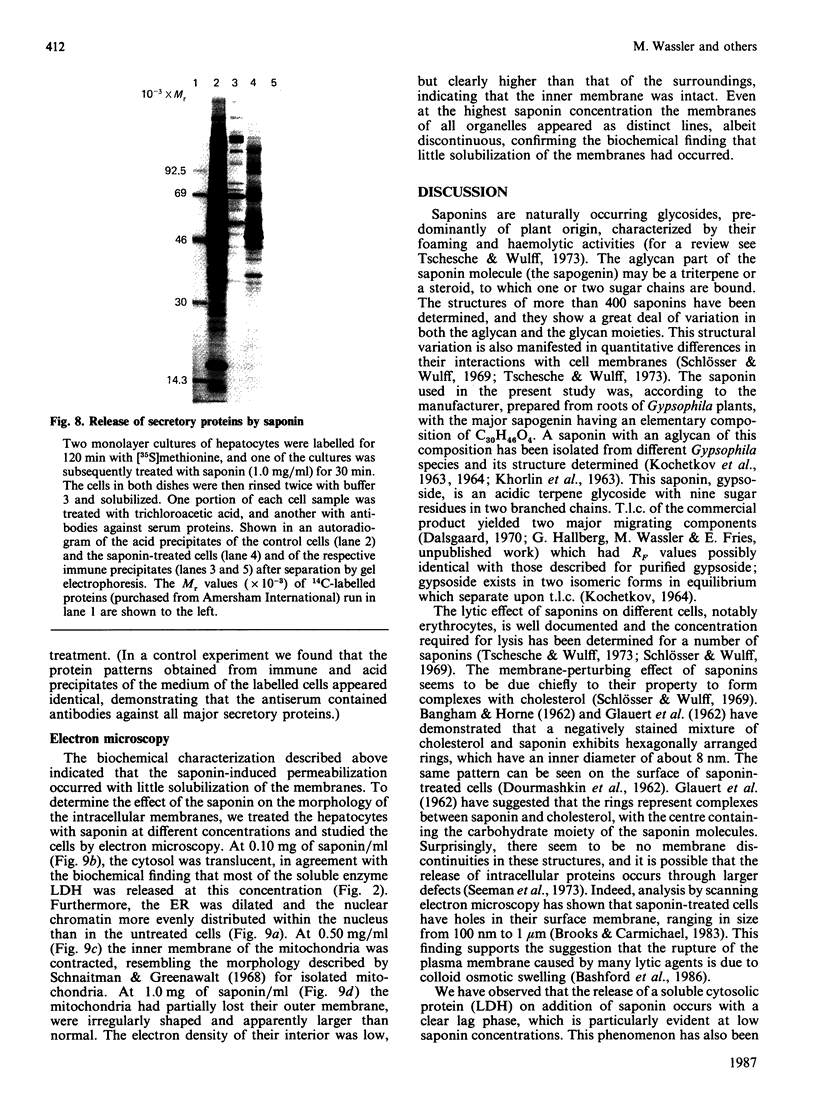
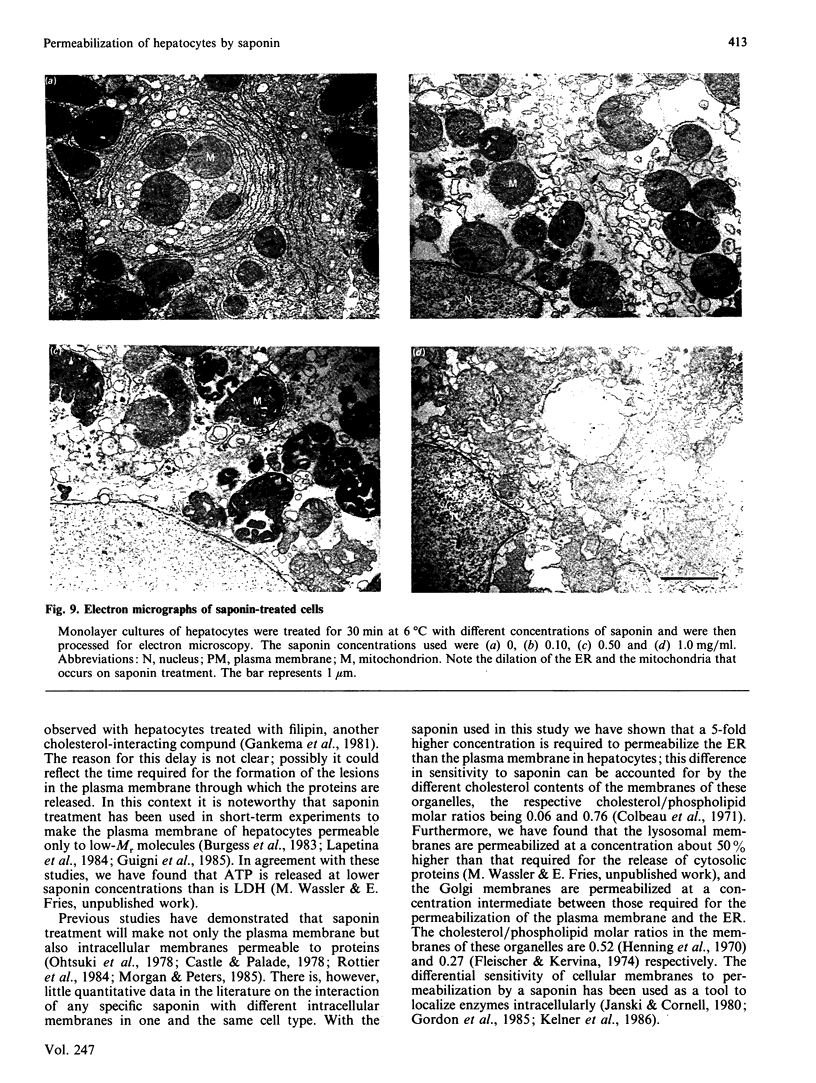


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Boström K., Wettesten M., Borén J., Bondjers G., Wiklund O., Olofsson S. O. Pulse-chase studies of the synthesis and intracellular transport of apolipoprotein B-100 in Hep G2 cells. J Biol Chem. 1986 Oct 15;261(29):13800–13806. [PubMed] [Google Scholar]
- Brooks J. C., Carmichael S. W. Scanning electron microscopy of chemically skinned bovine adrenal medullary chromaffin cells. Mikroskopie. 1983 Dec;40(11-12):347–356. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Castle J. D., Palade G. E. Secretion granules of the rabbit parotid. Selective removal of secretory contaminants from granule membranes. J Cell Biol. 1978 Feb;76(2):323–340. doi: 10.1083/jcb.76.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Gattone V. H., Evan A. P., Harris R. A. Structural changes of isolated hepatocytes during treatment with digitonin. Biochim Biophys Acta. 1983 Dec 19;763(4):356–367. doi: 10.1016/0167-4889(83)90097-6. [DOI] [PubMed] [Google Scholar]
- DEVLIN T. M., LEHNINGER A. L. The preparation of phosphorylating subfragments of rat liver mitochondria with digitonin. J Biol Chem. 1958 Dec;233(6):1586–1588. [PubMed] [Google Scholar]
- DOURMASHKIN R. R., DOUGHERTY R. M., HARRIS R. J. Electron microscopic observations on Rous sarcoma virus and cell membranes. Nature. 1962 Jun 23;194:1116–1119. doi: 10.1038/1941116a0. [DOI] [PubMed] [Google Scholar]
- Dalsgaard K. Thinlayer chromatographic fingerprinting of commercially available saponins. Dan Tidsskr Farm. 1970;44(8):327–331. [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Fries E., Gustafsson L., Peterson P. A. Four secretory proteins synthesized by hepatocytes are transported from endoplasmic reticulum to Golgi complex at different rates. EMBO J. 1984 Jan;3(1):147–152. doi: 10.1002/j.1460-2075.1984.tb01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Lindström I. The effects of low temperatures on intracellular transport of newly synthesized albumin and haptoglobin in rat hepatocytes. Biochem J. 1986 Jul 1;237(1):33–39. doi: 10.1042/bj2370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankema H. S., Laanen E., Groen A. K., Tager J. M. Characterization of isolated rat-liver cells made permeable with filipin. Eur J Biochem. 1981 Oct;119(2):409–414. doi: 10.1111/j.1432-1033.1981.tb05623.x. [DOI] [PubMed] [Google Scholar]
- Giugni T. D., James L. C., Haigler H. T. Epidermal growth factor stimulates tyrosine phosphorylation of specific proteins in permeabilized human fibroblasts. J Biol Chem. 1985 Dec 5;260(28):15081–15090. [PubMed] [Google Scholar]
- Gordon P. B., Tolleshaug H., Seglen P. O. Use of digitonin extraction to distinguish between autophagic-lysosomal sequestration and mitochondrial uptake of [14C]sucrose in hepatocytes. Biochem J. 1985 Dec 15;232(3):773–780. doi: 10.1042/bj2320773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H., Hüby A. Interaction of saponin and digitonin with black lipid membranes and lipid monolayers. Biochim Biophys Acta. 1984 Jun 13;773(1):32–38. doi: 10.1016/0005-2736(84)90547-9. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Henning R., Kaulen H. D., Stoffel W. Biochemical analysis of the pinocytotic process. I. Isolation and chemical composition of the lysosomal and the plasma membrane of the rat liver cell. Hoppe Seylers Z Physiol Chem. 1970 Oct;351(10):1191–1199. doi: 10.1515/bchm2.1970.351.2.1191. [DOI] [PubMed] [Google Scholar]
- Hirata M., Koga T. ATP-dependent Ca2+ accumulation in intracellular membranes of guinea pig macrophages after saponin treatment. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1544–1549. doi: 10.1016/0006-291x(82)91427-9. [DOI] [PubMed] [Google Scholar]
- Janski A. M., Cornell N. W. Subcellular distribution of enzymes determined by rapid digitonin fractionation of isolated hepatocytes. Biochem J. 1980 Feb 15;186(2):423–429. doi: 10.1042/bj1860423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson R. A., Nordlie R. C. Multifunctional glucose-6-phosphatase studied in permeable isolated hepatocytes. J Biol Chem. 1980 Jun 25;255(12):5907–5915. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Studies with digitonin-treated rat hepatocytes (nude cells). J Cell Biochem. 1985;28(3):207–228. doi: 10.1002/jcb.240280304. [DOI] [PubMed] [Google Scholar]
- Kelner K. L., Morita K., Rossen J. S., Pollard H. B. Restricted diffusion of tyrosine hydroxylase and phenylethanolamine N-methyltransferase from digitonin-permeabilized adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2998–3002. doi: 10.1073/pnas.83.9.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Sabatini D. D. Procedure for the selective release of content from microsomal vesicles without membrane disassembly. Methods Enzymol. 1974;31:215–225. doi: 10.1016/0076-6879(74)31023-3. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Watson S. P., Cuatrecasas P. myo-Inositol 1,4,5-trisphosphate stimulates protein phosphorylation in saponin-permeabilized human platelets. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7431–7435. doi: 10.1073/pnas.81.23.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- McEwen B. F., Arion W. J. Permeabilization of rat hepatocytes with Staphylococcus aureus alpha-toxin. J Cell Biol. 1985 Jun;100(6):1922–1929. doi: 10.1083/jcb.100.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. H., Peters T., Jr The biosynthesis of rat transferrin. Evidence for rapid glycosylation, disulfide bond formation, and tertiary folding. J Biol Chem. 1985 Nov 25;260(27):14793–14801. [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Rask L., Valtersson C., Anundi H., Kvist S., Eriksson U., Dallner G., Peterson P. A. Subcellular localization in normal and vitamin A-deficient rat liver of vitamin A serum transport proteins, albumin, ceruloplasmin and class I major histocompatibility antigens. Exp Cell Res. 1983 Jan;143(1):91–102. doi: 10.1016/0014-4827(83)90112-x. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenqvist E., Michaelsen T. E., Vistnes A. I. Effect of streptolysin O and digitonin on egg lecithin/cholesterol vesicles. Biochim Biophys Acta. 1980 Jul 16;600(1):91–102. doi: 10.1016/0005-2736(80)90414-9. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Fries E. Transport of newly synthesized vesicular stomatitis viral glycoprotein to purified Golgi membranes. J Cell Biol. 1981 Apr;89(1):162–168. doi: 10.1083/jcb.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P., Brandenburg D., Armstrong J., van der Zeijst B., Warren G. Assembly in vitro of a spanning membrane protein of the endoplasmic reticulum: the E1 glycoprotein of coronavirus mouse hepatitis virus A59. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1421–1425. doi: 10.1073/pnas.81.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Kjellén L., Obrink B. Intercellular adhesion between juvenile liver cells. A method to measure the formation of stable lateral contacts between cells attached to a collagen gel. Exp Cell Res. 1977 Oct 15;109(2):413–422. doi: 10.1016/0014-4827(77)90021-0. [DOI] [PubMed] [Google Scholar]
- Saraste J., Hedman K. Intracellular vesicles involved in the transport of Semliki Forest virus membrane proteins to the cell surface. EMBO J. 1983;2(11):2001–2006. doi: 10.1002/j.1460-2075.1983.tb01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E., Tartakoff A. M. Cell fractionation studies on the guinea pig pancreas. Redistribution of exocrine proteins during tissue homogenization. J Cell Biol. 1978 Jul;78(1):110–130. doi: 10.1083/jcb.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser E., Wulff G. Uber die Strukturspezifität der Saponinhämolyse. I. Triterpensaponine und -aglykone. Z Naturforsch B. 1969 Oct;24(10):1284–1290. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Cheng D., Iles G. H. Structure of membrane holes in osmotic and saponin hemolysis. J Cell Biol. 1973 Feb;56(2):519–527. doi: 10.1083/jcb.56.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Tschesche V. R., Wulff G. Chemie und Biologie der Saponine. Fortschr Chem Org Naturst. 1973;30:461–606. [PubMed] [Google Scholar]
- Vochten R., Joos P., Ruyssen R. Physico-chemical properties of sapoalbin and their relation to the foam stability. J Pharm Belg. 1969 May-Jun;24(5):213–226. [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Pastan I. Ultrastructural antibody localization of alpha2-macroglobulin in membrane-limited vesicles in cultured cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4359–4363. doi: 10.1073/pnas.75.9.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]