Abstract
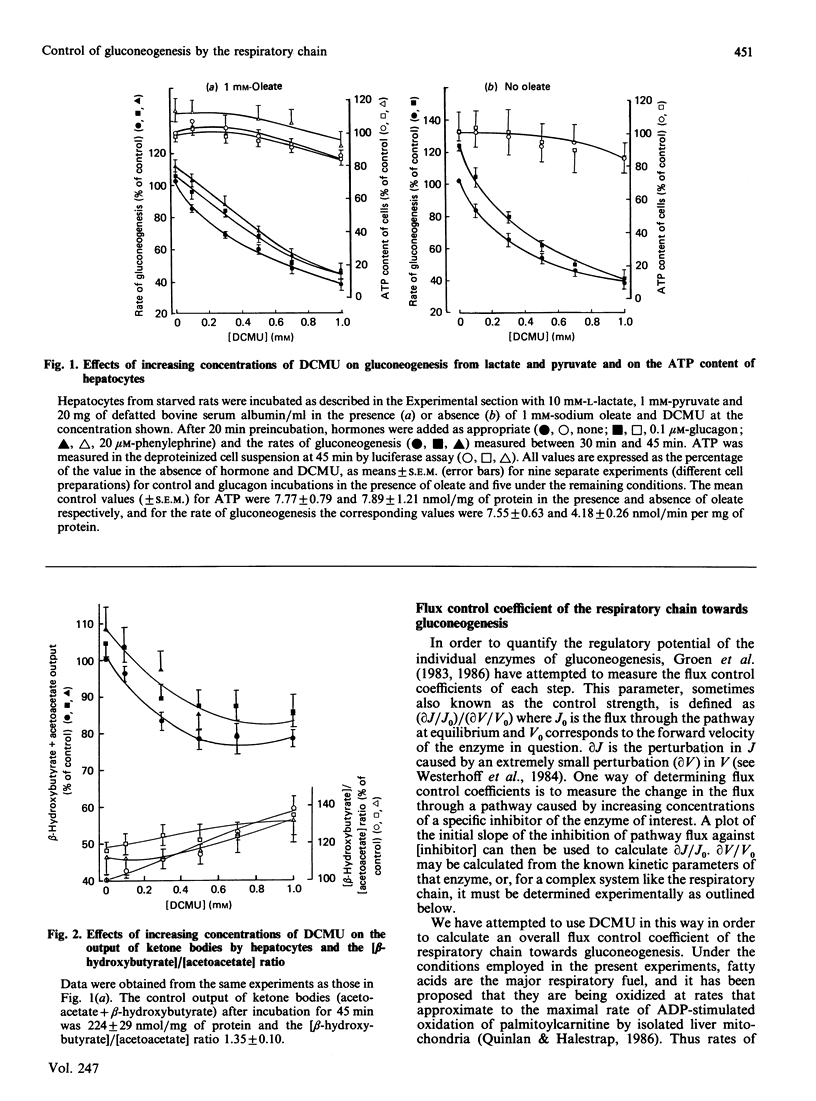
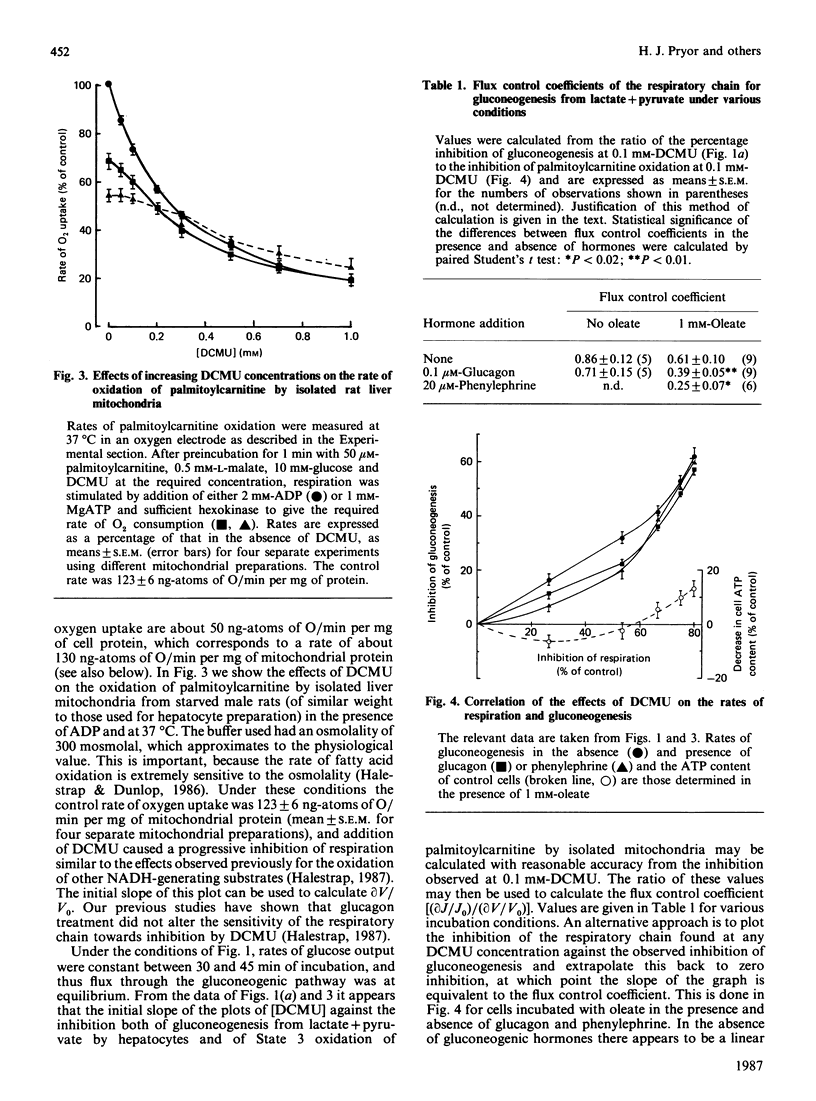
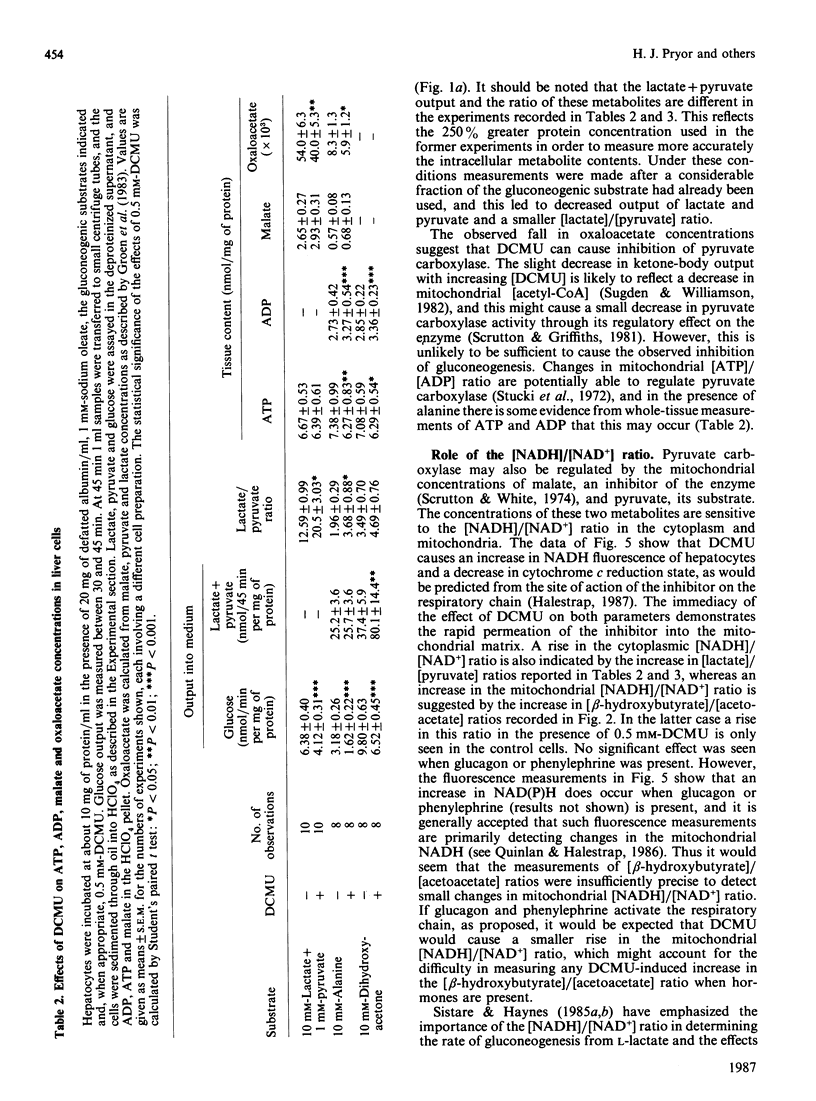
1. Increasing concentrations of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), a mild respiratory-chain inhibitor [Halestrap (1987) Biochim. Biophys. Acta 927, 280-290], caused progressive inhibition of glucose production from lactate + pyruvate by hepatocytes from starved rats incubated in the presence or absence of oleate and gluconeogenic hormones. 2. No significant changes in tissue ATP content were observed, but there were concomitant decreases in ketone-body output and cytochrome c reduction and increases in NADH fluorescence and the ratios of [lactate]/[pyruvate] and [beta-hydroxybutyrate]/[acetoacetate]. 3. The inhibition by DCMU of palmitoylcarnitine oxidation by isolated liver mitochondria was used to calculate a flux control coefficient of the respiratory chain towards gluconeogenesis. In the presence of 1 mM-oleate, the calculated values were 0.61, 0.39 and 0.25 in the absence of hormone and in the presence of glucagon or phenylephrine respectively, consistent with activation of the respiratory chain in situ as previously suggested [Quinlan & Halestrap (1986) Biochem. J. 236, 789-800]. 4. Cytoplasmic oxaloacetate concentrations were shown to decrease under these conditions, implying inhibition of pyruvate carboxylase. 5. Inhibition of gluconeogenesis from fructose and dihydroxyacetone was also observed with DCMU and was accompanied by an increased output of lactate + pyruvate, suggesting that activation of pyruvate kinase was occurring. With the latter substrate, measurements of tissue ADP and ATP contents showed that DCMU caused a small fall in [ATP]/[ADP] ratio. 6. Two inhibitors of fatty acid oxidation, pent-4-enoate and 2-tetradecylglycidate, were shown to abolish and to decrease respectively the effects of hormones, but not valinomycin, on gluconeogenesis from lactate + pyruvate, without changing tissue ATP content. 7. It is concluded that the hormonal increase in mitochondrial matrix volume stimulates fatty acid oxidation and respiratory-chain activity, allowing stimulation of pyruvate carboxylation and thus gluconeogenesis to occur without major changes in [ATP]/[ADP] or [NADH]/[NAD+] ratios. 8. The high flux control coefficient of the respiratory chain towards gluconeogenesis may account for the hypoglycaemic effect of mild respiratory-chain inhibitors.
Full text
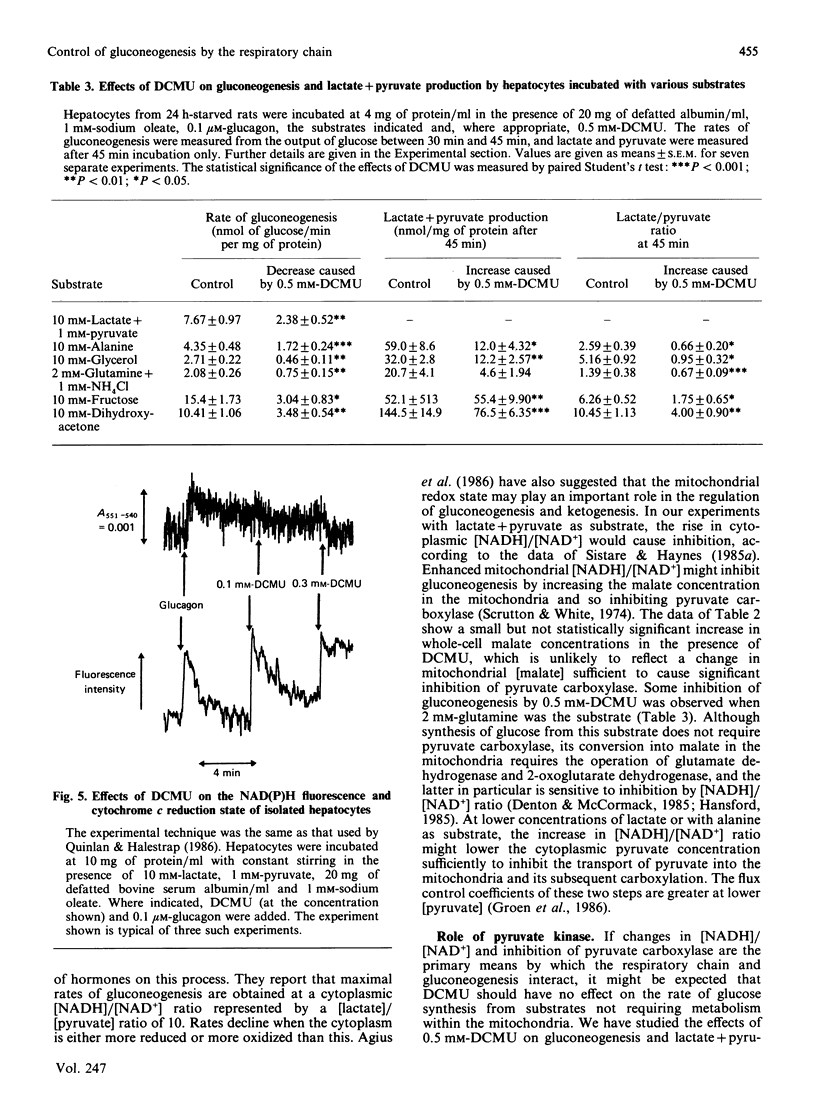
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Chowdhury M. H., Alberti K. G. Regulation of ketogenesis, gluconeogenesis and the mitochondrial redox state by dexamethasone in hepatocyte monolayer cultures. Biochem J. 1986 Nov 1;239(3):593–601. doi: 10.1042/bj2390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armston A. E., Halestrap A. P., Scott R. D. The nature of the changes in liver mitochondrial function induced by glucagon treatment of rats. The effects of intramitochondrial volume, aging and benzyl alcohol. Biochim Biophys Acta. 1982 Sep 15;681(3):429–439. doi: 10.1016/0005-2728(82)90185-2. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Van der Meer R., Tager J. M. Control of gluconeogenesis in rat liver cells. I. Kinetics of the individual enzymes and the effect of glucagon. J Biol Chem. 1983 Dec 10;258(23):14346–14353. [PubMed] [Google Scholar]
- Groen A. K., van Roermund C. W., Vervoorn R. C., Tager J. M. Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. Biochem J. 1986 Jul 15;237(2):379–389. doi: 10.1042/bj2370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Armston A. E. A re-evaluation of the role of mitochondrial pyruvate transport in the hormonal control of rat liver mitochondrial pyruvate metabolism. Biochem J. 1984 Nov 1;223(3):677–685. doi: 10.1042/bj2230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Dunlop J. L. Intramitochondrial regulation of fatty acid beta-oxidation occurs between flavoprotein and ubiquinone. A role for changes in the matrix volume. Biochem J. 1986 Nov 1;239(3):559–565. doi: 10.1042/bj2390559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Glucagon treatment of rats activates the respiratory chain of liver mitochondria at more than one site. Biochim Biophys Acta. 1987 Feb 18;927(2):280–290. doi: 10.1016/0167-4889(87)90145-5. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T. The intramitochondrial volume measured using sucrose as an extramitochondrial marker overestimates the true matrix volume determined with mannitol. Biochem J. 1983 Aug 15;214(2):387–393. doi: 10.1042/bj2140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T., Whipps D. E., Armston A. E. Regulation of the mitochondrial matrix volume in vivo and in vitro. The role of calcium. Biochem J. 1986 Jun 15;236(3):779–787. doi: 10.1042/bj2360779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Patel T. B., Olson M. S. Regulation of gluconeogenesis from pyruvate and lactate in the isolated perfused rat liver. Biochim Biophys Acta. 1986 Oct 10;888(3):316–324. doi: 10.1016/0167-4889(86)90231-4. [DOI] [PubMed] [Google Scholar]
- Quinlan P. T., Halestrap A. P. The mechanism of the hormonal activation of respiration in isolated hepatocytes and its importance in the regulation of gluconeogenesis. Biochem J. 1986 Jun 15;236(3):789–800. doi: 10.1042/bj2360789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Role of pyruvate kinase in the regulation of gluconeogenesis from L-lactate. J Biol Chem. 1977 Mar 25;252(6):1831–1833. [PubMed] [Google Scholar]
- Schudt C., Simon A. Effects of sodium 2-[5-(4-chlorophenyl)pentyl]-oxirane-2-carboxylate (POCA) on carbohydrate and fatty acid metabolism in liver and muscle. Biochem Pharmacol. 1984 Nov 1;33(21):3357–3362. doi: 10.1016/0006-2952(84)90106-0. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C., White M. D. Pyruvate carboxylase. Inhibition of the mammalian and avian liver enzymes by alpha-ketoglutarate and L-glutamate. J Biol Chem. 1974 Sep 10;249(17):5405–5415. [PubMed] [Google Scholar]
- Sistare F. D., Haynes R. C., Jr Estimation of the relative contributions of enhanced production of oxalacetate and inhibition of pyruvate kinase to acute hormonal stimulation of gluconeogenesis in rat hepatocytes. An analysis of the effects of glucagon, angiotensin II, and dexamethasone on gluconeogenic flux from lactate/pyruvate. J Biol Chem. 1985 Oct 15;260(23):12761–12768. [PubMed] [Google Scholar]
- Sistare F. D., Haynes R. C., Jr The interaction between the cytosolic pyridine nucleotide redox potential and gluconeogenesis from lactate/pyruvate in isolated rat hepatocytes. Implications for investigations of hormone action. J Biol Chem. 1985 Oct 15;260(23):12748–12753. [PubMed] [Google Scholar]
- Stucki J. W., Brawand F., Walter P. Regulation of pyruvate metabolim in rat-liver mitochondria by adenine nucleotides and fatty acids. Eur J Biochem. 1972 May;27(1):181–191. doi: 10.1111/j.1432-1033.1972.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Halestrap A. P. Computer stimulation of the effects of alpha-cyano-4-hydroxycinnamate on gluconeogenesis from L-lactate in rat liver cells. Biochem J. 1981 Sep 15;198(3):561–564. doi: 10.1042/bj1980561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutwiler G. F., Dellevigne P. Action of the oral hypoglycemic agent 2-tetradecylglycidic acid on hepatic fatty acid oxidation and gluconeogenesis. J Biol Chem. 1979 Apr 25;254(8):2935–2941. [PubMed] [Google Scholar]
- Tutwiler G. F., Ho W., Mohrbacher R. J. 2-Tetradecylglycidic acid. Methods Enzymol. 1981;72:533–551. doi: 10.1016/s0076-6879(81)72042-1. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Groen A. K., Wanders R. J. Modern theories of metabolic control and their applications (review). Biosci Rep. 1984 Jan;4(1):1–22. doi: 10.1007/BF01120819. [DOI] [PubMed] [Google Scholar]
- Wolf H. P., Engel D. W. Decrease of fatty acid oxidation, ketogenesis and gluconeogenesis in isolated perfused rat liver by phenylalkyl oxirane carboxylate (B 807-27) due to inhibition of CPT I (EC 2.3.1.21). Eur J Biochem. 1985 Jan 15;146(2):359–363. doi: 10.1111/j.1432-1033.1985.tb08661.x. [DOI] [PubMed] [Google Scholar]


