Abstract
Background
The characteristics of electrocardiogram (ECG) abnormalities related to cardiac channelopathies potentially linked to sudden cardiac death (SCD) are not widely recognized in Iran. We examined the prevalence of such ECG patterns and their related factors among adult residents of Tehran, Iran.
Methods
The clinical characteristics and 12-lead ECGs of Tehran Cohort Study participants were examined. Long QT intervals, short QT intervals, Brugada syndrome (BrS) patterns, and early repolarization (ER) were evaluated using computer-based assessment software validated by cardiologists. Logistic regression models were employed to identify the factors associated with the prevalence of different ECG patterns.
Results
Out of 7678 available ECGs, 7350 were included in this analysis. Long QT interval, ER pattern, BrS patterns, and short QT interval were found in 3.08%, 1.43%, 0.31%, and 0.03% of participants, respectively. The prevalence of long QT interval increased with age, opium consumption, and presence of hypertension. Younger age, lower body mass index (BMI), alcohol use and male sex were independently linked to an elevated prevalence of ER pattern. Most individuals with BrS patterns were men (95%) and had lower BMI, high- and low-density lipoprotein, and total cholesterol compared to those without the BrS pattern. At a mean follow-up of 30.2 ± 5.5 months, all-cause mortality in the group exhibiting abnormal ECG patterns (6.3%) was approximately twice as high as that in the group without such patterns (2.96%).
Conclusion
Abnormal ECG patterns corresponding to channelopathies were relatively rare among adult residents of the Tehran population, and their prevalence was influenced by various factors.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04235-w.
Keywords: Electrocardiography, Heart conduction system, Long QT syndrome, Brugada syndrome, Population surveillance, Cross-sectional studies
Introduction
Several cardiac channelopathies have been recognized to date, with the most common being long QT syndrome (LQTS), short QT syndrome (SQTS), Brugada syndrome (BrS), and early repolarization (ER). LQTS and SQTS are characterized by the prolongation and shortening of the QT interval on the electrocardiogram (ECG), respectively. These conditions are typically accompanied by syncopal events, cardiac dysrhythmias, and sudden cardiac death (SCD) [1, 2]. BrS is defined by an elevation of the ST-segment in the right precordial leads, occurring in the absence of ischemia, electrolyte imbalances, or structural heart disease. This syndrome can be associated with ventricular fibrillation (VF) and SCD [3]. The ER pattern, another ECG marker for channelopathies, was previously considered benign but has recently been linked to idiopathic VF and SCD [4].
Cardiac channelopathies leading to SCD are commonly observed in infants, children, and young individuals who lack significant cardiac structural disease or cardiovascular risk factors. Nevertheless, ECG patterns suggestive of these disorders, even without clinical symptoms, can also be present in adulthood, indicating that there may be abnormalities in repolarization and conduction that could lead to SCD without any identifiable cause. SCD poses a challenge for physicians as it might be the only manifestation of a cardiac issue. As such, determining the prevalence of such ECG abnormalities and associated factors in local populations is crucial. This information can help develop cost-effective strategies for prevention, timely detection, and treatment [5].
The frequency of long and short QTc intervals in the general population greatly varies across countries. This variation depends on population characteristics and the established cut-off for interval prolongation and shortening, ranging from 0.17 to 31.6% [6–11] and 0.02–9% [8, 11–15], respectively. A recent meta-analysis reveals that the prevalence of ER patterns also varies by population, ranging from 1.3 to 45.8%, with a pooled prevalence of 11.6% [16]. There is also a significant variation in the prevalence of BrS-type ECG across different populations, ranging from 0.07 to 6% [17–23]. Prior research has also identified links between the aforementioned ECG patterns and various demographic and clinical characteristics; while some of these associations are consistent between studies, the majority vary across populations [6, 7, 12, 24–26].
This study aimed to ascertain the frequency of long QT intervals, short QT intervals, BrS-type ECG patterns, and ER patterns in a random sample of apparently healthy adult residents of Tehran. Additionally, it sought to identify the factors significantly associated with these ECG patterns.
Materials and methods
Study design and participants
This research was a cross-sectional analysis with a short-term follow-up, conducted on participants from the initial phase of the Tehran Cohort Study (TeCS) who had a digital ECG. Each subject provided written consent before participating in the study. The TeCS is primarily focused on investigating the prevalence, incidence, and trends of cardiovascular diseases, psychological symptoms, injuries, and risk factors within the Tehran population. More details about the TeCS protocol can be found in a previously published paper [27].
We examined the demographics, clinical characteristics, and 12-lead standard ECG of individuals aged ≥ 35 years who enrolled between March 2016 and March 2019. A cardiologist reviewed the ECGs and excluded any that fulfilled one or more of the following exclusion criteria: [1] Low ECG quality; [2] Presence of grade 2 or 3 AV blocks; [3] QRS duration of more than 120 ms; [4] Non-sinus rhythms; [5] More than 2 PVCs within 10 s.
We followed individuals identified as high-risk for adverse outcomes based on their ECG through telephone contact to ascertain their survival status. The records of individuals who died during or subsequent to hospitalization were reviewed in person to determine the precise cause of death. SCD was defined as an unexpected mortality due to cardiovascular factors in an individual, regardless of prior cardiac disorders, occurring within one hour following an alteration in clinical state or 24 h of last being seen healthy and alive [28, 29].
Electrocardiogram measurements and interpretation
QT interval measurement
Two M-trace ECG devices (M4Medical, Lublin, Poland) were utilized to record the patients’ ECGs. The QT interval measurement was conducted using a custom automated computer software developed by Nabz Hooshmand Co., based in Tehran, Iran. This software interprets digital ECGs and estimates the QT interval by determining the time between the start of the first QRS complex deflection and the point where the T wave returns to the isoelectric line. The accuracy of the software measurement was evaluated by comparing it with expert manual measurement. Two cardiologists used on-screen caliper software to measure the QT interval in a sample of 200 standard ECGs. According to the guidelines established by the International Electrotechnical Commission (IEC), it is considered acceptable for ECG software to have a mean difference of less than 25 ms and a standard deviation (SD) of less than 30 ms compared to observer measurements in terms of accuracy [30]. Three different methods were employed by automated software to measure the QT interval, which were then compared with manual measurements made by experts: (1) The median value of the QT interval across all beats and leads; (2) The mean value of the QT interval across all beats and leads; (3) Initially, the median value of the QT interval for each lead was calculated from beats that fell within the acceptable range (± SD of the mean). Subsequently, the median QT of all leads was determined for each ECG. Using the last method, the mean difference and SD of differences between the average of experts and software were 9.92 msec and 10.81 msec, respectively, which was acceptable according to IEC standards. The QT interval measurement for the remaining ECGs was performed by the automated software using this third method.
The Bazett formula was utilized to correct the QT measurement based on heart rate (HR). The formula is as follows: Bazett: QTc = QT / (RR)1/2 [31]. The heart rate (HR) was calculated using the following formula in lead II:
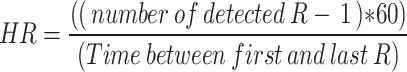 |
In accordance with most studies, a long QTc interval was defined as QTc > 450 ms in men and QTc > 460 ms in women, and a shortened QT interval was defined as QTc < 330 ms in men and QTc < 340 ms in women [9, 11]. Given the discrepancy of 9.92 msec between expert and machine measurements, a bilateral safety margin of 10 msec was established. This safety margin necessitates that experts recheck any ECGs that are suspicious to prevent potential cases from being overlooked.
An expert cardiologist reviewed all ECGs with suspiciously long (QTc > 440 ms in men and > 450 ms in women) and short (QTc < 340 ms in men and < 350 ms in women) QT intervals to verify the accuracy of the QT measurement. Furthermore, if the algorithm reported a QT in fewer than 8 leads, it was considered an unreliable QT report, prompting experts to recheck those ECGs. This rigorous process ensures the accuracy and reliability of the QT measurements.
BrS-type and ER ECG patterns
The ECGs underwent thorough evaluation by two independent cardiologists, who made the diagnosis of BrS-type and ER patterns only after reaching a consensus. In the case of a disagreement between the diagnoses of cardiologists, an experienced electrophysiologist made the ultimate decision.
The BrS-type ECG pattern was identified using the criteria specified by the Heart Rhythm Society and the European Heart Rhythm Association at their 2nd consensus meeting for diagnosing the Brugada pattern [32]. The right precordial leads of the 12-lead standard ECG were analyzed, and the criteria for classifying BrS-type patterns into three types were as follows: [1] Type 1: a coved ST-segment elevation ≥ 2 mm (0.2 mV), followed by a negative T wave; [2] Type 2: a saddle-back ST-segment elevation with a high takeoff ST-segment elevation of ≥ 2 mm (0.2 mV), followed by either a positive or biphasic T wave; [3] Type 3: either a saddle-back or coved appearance with an ST-segment elevation of < 1 mm (0.1 mV) [32]. Type 1 BrS ECG pattern has been associated with an increased risk of adverse outcomes, thereby necessitating careful monitoring. Additionally, longitudinal research has indicated that type 2 and 3 BrS patterns may spontaneously evolve into the type 1 BrS pattern. To identify these cases of type 2 and type 3, a challenge test using specific pharmacological agents, such as procainamide or ajmaline, is used to induce the type 1 BrS pattern [32–34]. Given the observational nature of our study in the general population and the impossibility of injecting drugs, we decided to follow up on all BrS-type ECG patterns, as type 2 and type 3 possess the potential to convert into type 1, making them high-risk cases.
Following the recommendations of the consensus meeting for ERP characterization [35], the ER pattern was defined as an end-QRS notch or slur on the downslope of a prominent R-wave, a J-point elevation of ≥ 0.1 mV in two or more adjacent leads of the 12-lead ECG, excluding leads V1–V3, and a QRS duration < 120 ms. The ER patterns were classified based on the region they covered: inferior (leads II, III, aVF), lateral (V4-V6, I, and aVL), or inferolateral [35]. Evidence indicates that inferolateral ER patterns are associated with a greater risk compared to patterns that are confined to inferior or lateral locations alone [36, 37]. Therefore, we have considered this type of ER pattern as a high-risk population for further follow-up.
Statistical analysis
Continuous variables were shown as mean and standard deviation (SD) or median with 25th and 75th percentiles and compared using the student’s t-test or Mann-Whitney test, as appropriate, based on the variable distribution. Categorical variables were shown with frequency and percentage and compared using the chi-square test or Fisher’s exact test. We used multivariable logistic regression models to show the adjusted association of covariates with ECG abnormality patterns. The results were reported through odds ratios (ORs) and 95% confidence intervals (CIs). Due to a few cases of Brugada patterns, adjusted model was not conducted. There were just 3 cases of short QT, which were unsuitable for any statistical analyses. Inter-observer agreement for evaluating ECGs was assessed using intraclass correlation coefficients (ICC). Statistical analyses were conducted using SPSS software (version 25).
Results
The database contained 7678 individuals with at least one ECG. Of these, 328 were excluded due to incomplete data, low-quality ECG, non-sinus rhythms, grade 2 or 3 AV blocks, or more than 2 PVCs. The ECGs of 7350 patients (46% men; mean age 53 years) were included in this study’s analysis. The mean QTc duration calculated by custom automated computer software and manual method had no significant difference according to IEC standards. A strong agreement was observed between the manual and automatic measure of QT interval (ICC = 0.910, 95%CI: 0.868–0.939, p < 0.01), suggesting the precision of the computer-assessed automated QT interval measurement.
Frequency and characteristics of individuals with long QTc interval
227 individuals (3.08% of the total; 44.9% men) exhibited a long QTc interval in their ECGs based on the established cut-offs. Table 1 presents the demographics and clinical features of individuals with and without long QT interval.
Table 1.
Characteristics of individuals with and without ECG abnormalities
| Variables | Total sample | Long QT interval (n) | BrS-type pattern | ER pattern | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + (227) | - (7123) | P | + (23) | − (7327) | P | + (105) | − (7263) | P | ||
| Age, y | 53 ± 12 | 58 ± 14 | 53 ± 12 | < 0.001 | 55 ± 14 | 53 ± 12 | 0.559 | 48.8 ± 12.08 | 53.03 ± 12.25 | 0.001 |
| Male, n (%) | 3381 (46%) | 102 (44.9) | 3253 (45.7) | 0.827 | 22 (95) | 3358 (45.9) | < 0.001 | 42 (47.7) | 3256 (44.2) | 0.025 |
|
Ethnicity, n(%) Fars Azari Others |
3572 (48.6) 2190 (29.8) 1588 (21.6) |
102 (44.9) 73 (32.2) 52 (22.9) |
34.81 (48.9) 2112 (29.7) 1530 (21.4) |
0.522 |
11 (47.8) 8 (34.8) 4 (17.4) |
3651 (47.9) 2296 (30.1) 1675 (22) |
0.850 |
40 (46) 22 (25.3) 25 (28.7) |
3721 (49.3) 2251 (29.8) 1586 (20.9) |
0.205 |
|
Physical Activity, n (%) Low Intermediate High |
1183 (16.1) 4344 (59.1) 1823 (24.8) |
43 (18.9) 136 (60) 48 (21.1) |
1142 (16) 4218 (59.2) 1763 (24.8) |
0.267 |
5 (22.7) 7 (31.8) 10 (45.5) |
1226 (16.1) 4498 (59) 1898 (24.9) |
0.021 |
13 (14.9) 45 (51.8) 29 (33.3) |
1262 (16.7) 4458 (59) 1838 (24.3) |
0.146 |
| Current cigarette smokers, n (%) | 956 (13) | 33 (14.5) | 919 (13) | 0.495 | 4 (18.2) | 948 (13) | 0.519 | 934 (12.9) | 18 (20.5) | 0.054 |
| Opium, n (%) | 390 (5.3) | 21 (9.3) | 367 (5.2) | 0.007 | 3 (13.6) | 385 (5.3) | 0.409 | 9 (10.2) | 379 (5.2) | 0.051 |
|
Waterpipe, n(%) Never Former Current |
6902 (93.9) 37 (0.5) 411 (5.6) |
218 (96) 1 (0.5) 8 (3.5) |
6628 (93.8) 29 (0.5) 392 (5.7) |
0.376 |
21 (91.4) 1 (4.3) 1 (4.3) |
7168 (94) 38 (0.5) 416 (5.5) |
0.132 |
76 (87.4) 2 (2.3) 9 (10.3) |
7059 (93.3) 37 (0.5) 462 (6.2) |
0.008 |
| Alcohol, n (%) | 684 (9.3) | 12 (5.3) | 664 (9.4) | 0.038 | 2 (9.1) | 674 (9.3) | 0.284 | 22 (25.3) | 654 (9.1) | < 0.001 |
|
History of CAD, n (%) |
617 (8.4) | 29 (12.8) | 584 (8.2) | 0.014 | 1 (4.5) | 612 (8.4) | 0.718 | 3 (3.4) | 610 (8.4) | 0.051 |
| Family history of SCD, n (%) | 507 (6.9) | 12 (9.6) | 299 (7.7) | 0.527 | 1 (4.5) | 504 (6.9) | 0.339 | 3 (3.4) | 502 (6.9) | 0.287 |
| Beta Blocker usage, n (%) | 1147 (15.6) | 45 (19.8) | 1104 (15.5) | 0.077 | 3 (13.6) | 1146 (15.6) | 0.233 | 12 (13.5) | 1137 (15.7) | 0.661 |
| BMI, kg/m2 | 27 ± 4 | 37.9 ± 4.84 | 27.93 ± 4.79 | 0.091 | 25.82 ± 4.00 | 27.94 ± 4.79 | 0.038 | 26.39 ± 4.00 | 27.95 ± 4.79 | 0.002 |
| SBP, mmHg | 121 ± 18 | 128.93 ± 22.56 | 121.1 ± 18.47 | < 0.001 | 118.63 ± 16.31 | 121 ± 18 | 0.243 | 118.63 ± 16.31 | 121.37 ± 18.69 | 0.119 |
| DBP, mmHg | 80 ± 10 | 83.53 ± 12.88 | 80.64 ± 10.62 | 0.001 | 79.33 ± 10.80 | 80 ± 10.71 | 0.379 | 79.33 ± 10.80 | 80.75 ± 10.71 | 0.212 |
| MBP, mmHg | 94 ± 12 | 98.64 ± 14.99 | 94.13 ± 12.16 | < 0.001 | 92.43 ± 11.85 | 94 ± 12.29 | 0.958 | 92.43 ± 11.85 | 94.30 ± 12.29 | 0.153 |
| HDL-C, mg/dl | 43 (36–52) | 43 (35–53) | 43 (36–52) | 0.821 | 36 (31–43) | 43 (36–52) | 0.007 | 41 (33–49) | 43 (36–52) | 0.023 |
| LDL-C, mg/dl | 111 (90–135) | 112 (92–139) | 111 (90–134) | 0.243 | 89.5 (75–124) | 111 (91–135) | 0.013 | 116 (99–138) | 111 (90–135) | 0.433 |
| Total Cholesterol, mg/dl |
170 (145–197) |
175 (148–202) |
170 (145–197) |
0.162 |
145 (125–178) |
170 (145–197) |
0.004 |
172 (143–198) |
170 (145–197) |
0.927 |
| TG, mg/dl |
125 (88–176) |
122 (87–180) |
125 (88–197) |
0.584 |
112 (80–194) |
125 (88–175) |
0.980 |
125 (88–175) |
112 (80–194) |
0.457 |
| FBS, mg/dl |
97 (90–107) |
99 (92–115) |
97 (90–106) |
0.013 |
94 (89–104) |
97 (90–107) |
0.505 |
94 (89–104) |
97 (90–107) |
0.119 |
| Cr, mg/dl |
0.8 (0.7–0.93) |
0.8 (0.63–0.97) |
0.8 (0.7–0.93) |
0.638 |
0.8 (0.7–0.96) |
0.8 (0.7–0.9) |
0.162 |
0.88 (0.78–0.97) |
0.8 (0.7–0.93) |
0.002 |
BMI, body mass index; BrS, Brugada syndrome; CAD, coronary artery disease; Cr, creatinine; DBP, diastolic blood pressure; ER, early repolarization; FBS, fasting blood sugar; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; MBP, mean blood pressure; SBP, systolic blood pressure; SCD, sudden cardiac death; TG, triglyceride
Individuals with a long QT interval were significantly older (58 ± 14 vs. 53 ± 12, p < 0.001), consumed more opium (9.3% vs. 5.2%, p = 0.007) and less alcohol (5.3% vs. 9.4%, p = 0.038), had higher rates of coronary artery diseases (CAD) (12.8% vs. 8.2%, p = 0.014), and had higher levels of systolic blood pressure (SBP) (128.93 ± 22.56 mmHg vs. 121.1 ± 18.47 mmHg, p < 0.001), diastolic blood pressure (DBP) (83.53 ± 12.88 mmHg vs. 80.64 ± 10.62 mmHg, p = 0.001), mean blood pressure (MBP) (98.64 ± 14.99 mmHg vs. 94.13 ± 12.16 mmHg, p < 0.001) and fasting blood sugar (FBS) [99 (92–115) mg/dl vs. 97 (90–106) mg/dl, p = 0.013], compared to those who did not have a long QT interval. Beta-blockers were the only drugs affecting QT interval that a significant number of individuals used on a regular basis; while the frequency of their use was higher in the group with a long QT interval than those with a normal QT interval (19.8% vs. 15.5%), the difference did not reach a significant level (P = 0.077). Other characteristics were comparable between the groups, as shown in Table 1.
Frequency and characteristics of individuals with ER patterns
Based on the ECGs, 105 individuals (1.43%) exhibited ER patterns. These patterns were classified by their distribution across anatomical regions, as shown in Fig. 1. Table 1 presents the demographics and clinical features of individuals with and without ER patterns.
Fig. 1.
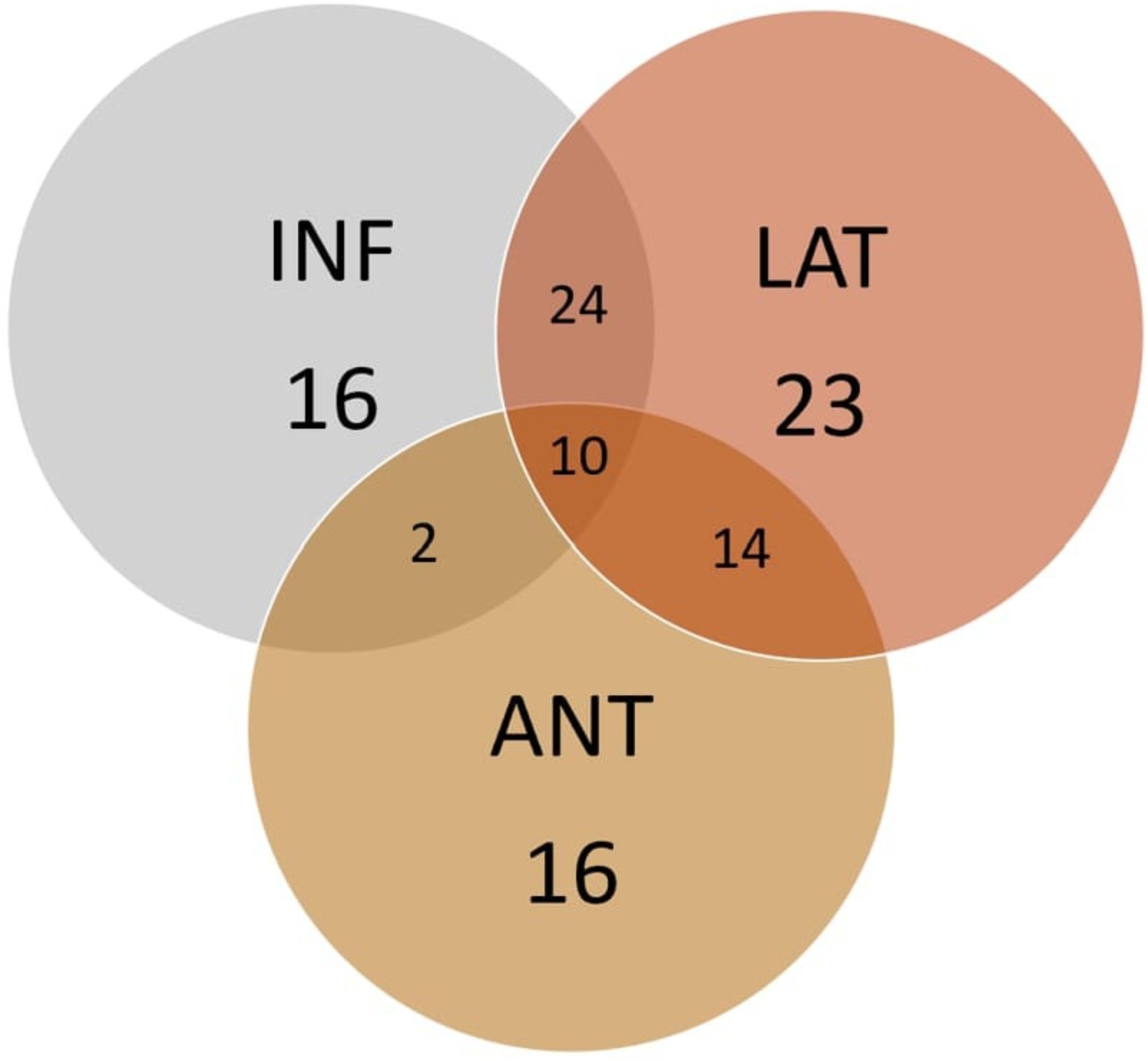
Distribution of early repolarization patterns based on their anatomical region
Individuals exhibiting ER ECG patterns had a lower mean age (48.8 ± 12.08 vs. 53.03 ± 12.25, p = 0.001) and BMI (26.39 ± 4.00 kg/m2 vs. 27.95 ± 4.79 kg/m2, p = 0.002), a higher proportion of males (47.7% vs. 44.2%, p = 0.025), lower levels of HDL-C [41 (33–49) mg/dl vs. 43 (36–52) mg/dl, p = 0.023], and a higher prevalence of water-pipe and alcohol usage compared to those without ER ECG patterns.
Frequency and characteristics of individuals with BrS-type ECG pattern
23 individuals (0.31%) had a BrS-type pattern in their ECGs, of whom only 3 had a type 1 pattern, while the others exhibited either type 2 or 3 patterns. Table 1 shows the demographics and clinical features of individuals with and without BrS-type ECG patterns.
Significant differences were observed between individuals with BrS-type patterns and those without. Specifically, individuals with BrS-type patterns were predominantly male (95% vs. 45.9%, p < 0.001). They also had lower levels of BMI, HDL-C, LDL-C, and total cholesterol (TC) compared to those without BrS-type patterns. In terms of physical activity, both low and high levels were more prevalent among individuals with BrS-type patterns (22.7% vs. 16.1% and 45.5% vs. 24.9%, respectively), while intermediate levels of physical activity were less prevalent (p = 0.021). Other clinical characteristics were similar between the two groups.
Frequency and characteristics of individuals with short QTc interval
Three (0.03%) people exhibited a short QTc interval in their ECGs based on the established cut-offs. Supplementary Table 1 describes the demographics and clinical characteristics of these cases.
Factors associated with ECG abnormality patterns
Tables 2 and 3 show the multivariable logistic regression analysis of different variables associated with the long QTc interval and ER pattern, respectively. The frequency of long QT interval increased with age (OR: 1.015, 95%CI: 1.002–1.029, P = 0.020), current opium usage (OR: 1.741, 95%CI: 1.016–2.984, P = 0.044), and the presence of hypertension (HTN) (OR: 1.788, 95%CI: 1.256–2.545, P = 0.001). Conversely, the frequency of ER patterns decreased with increasing age (OR: 0.974, 95%CI: 0.953–0.996, P = 0.020) and BMI (OR: 0.944, 95%CI: 0.896–0.994, P = 0.029). Males proportion and alcohol consumption were positively and independently associated with an increased frequency of ER pattern (OR: 1.243, 95% CI: 0.896–2.176, P = 0.031, and OR: 2.462, 95% CI: 1.442–4.203, P = 0.001, respectively). Other characteristics were not significantly and independently associated with the frequency of long QTc intervals and ER patterns. Due to the small sample sizes, logistic regression analysis was not feasible for estimating variables linked to the frequency of BrS-type patterns and short QT intervals.
Table 2.
Multivariable logistic regression analysis of different variables associated with long QTc interval
| Variable | Long QT | ||
|---|---|---|---|
| OR | 95%CI | P value | |
| Age, y | 1.015 | 1.002–1.029 | 0.020 |
| BMI, kg/m2 | 0.987 | 0.957–1.018 | 0.399 |
| Cr, mg/dl | 1.245 | 0.829–1.869 | 0.291 |
| Ethnicity | 1.208 | 0.880–1.658 | 0.243 |
| Current cigarette smoking | 1.025 | 0.654–1.607 | 0.913 |
| Waterpipe use | 0.915 | 0.439–1.905 | 0.812 |
| Opium use | 1.741 | 1.016–2.984 | 0.044 |
| Physical activity | 0.991 | 0.627–1.566 | 0.968 |
| Alcohol use | 0.556 | 0.294–1.052 | 0.071 |
| History of CAD | 1.039 | 0.652–1.655 | 0.872 |
| Beta Blocker usage | 0.860 | 0.583–1.269 | 0.448 |
| Family history of SCD | 0.988 | 0.586–1.664 | 0.963 |
| HTN (SBP ≥ 140 and/or DBP ≥ 90) | 1.788 | 1.256–2.545 | 0.001 |
| DM (FBS ≥ 126) | 1.022 | 0.728–1.436 | 0.898 |
BMI, body mass index; CAD, coronary artery diseases; CI, confidence interval; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; FBS, fasting blood sugar; OR, odds ratio; SBP, systolic blood pressure; SCD, sudden cardiac death
Table 3.
Multivariable logistic regression analysis of different variables associated with ER pattern
| Variable | Early repolarization pattern | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age, y | 0.974 | 0.953–0.996 | 0.020 |
| BMI, kg/m2 | 0.944 | 0.896–0.994 | 0.029 |
| Cr, mg/dl | 1.406 | 0.911–2.17 | 0.12 |
| Waterpipe use | 3.03 | 0.669–13.717 | 0.15 |
| Opium use | 1.742 | 0.839–3.614 | 0.136 |
| Alcohol use | 2.462 | 1.442–4.203 | 0.001 |
| History of CAD | 0.553 | 0.116–1.843 | 0.335 |
| HTN(SBP ≥ 140 and/or DBP ≥ 90) | 1.090 | 0.644–1.845 | 0.748 |
| HLP (Total Cholesterol ≥ 200 and/or TG ≥ 150) | 0.822 | 0.477–1.416 | 0.479 |
| Gender (male) | 1.243 | 0.896–2.176 | 0.031 |
BMI, body mass index; CAD, coronary artery diseases; CI, confidence interval; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; HLP, hyperlipidemia; OR, odds ratio; SBP, systolic blood pressure
Follow-up of high risk cases
A short-term follow-up was conducted on 152 patients identified as high-risk for poor outcomes, including 39 individuals with a very long QT duration (QTc > 470 ms), 3 with a very short QT interval (QT < 320 ms), 23 with a BrS-type pattern, and 87 with inferolateral ER. 26 patients (17.1%) could not be contacted and were deemed lost to follow-up. The mean duration of follow-up was 30.2 ± 5.5 months. During the follow-up period, there were 5 (12.8%) and 3 (3.4%) total deaths among individuals who had a very long QT interval and inferolateral ER, respectively, of whom one case with a very long QT interval died of SCD. The mortality rate among individuals not classified as high risk for poor outcomes was 2.96%, much lower than that of high-risk individuals. None of the patients reported experiencing syncope.
Discussion
In this study, we provided detailed characteristics of individuals with ECG abnormalities suggestive of cardiac channelopathies, including long QT intervals, short QT intervals, BrS-type ECG patterns, and ER ECG patterns among the adult general population of Tehran, the capital city of Iran. In line with previous findings, the frequency of these ECG patterns was infrequent, with a male preponderance observed in those with BrS-type ECG patterns. As individuals got older, there was a noticeable rise in the frequency of long QT intervals, whereas the frequency of ER patterns tended to decline. Current opium use and the presence of hypertension were independently associated with an increased frequency of long QT intervals. The frequency of ER pattern was positively influenced by male proportion and alcohol consumption, while BMI exhibited a reverse relationship with the frequency of ER pattern. During the short-term follow-up, it was observed that individuals who had an ECG abnormality indicating high risk for poor outcomes had a mortality rate that was approximately twice as high as those who were not at high risk.
The frequency of a long QT interval in our study, characterized as QTc > 450 ms in males and QTc > 460 ms in females, was 3.08%. This estimate was at least three and ten times lower than that reported by Magodoro et al. [6] in Uganda adults aged 38.4 years, with a QTc cut-off point of 470 ms in females and 450 ms in males, and Ma et al. [7] in a Chinese population aged 56 years, with a cut-off point of 440 ms for both genders, respectively. The high prevalence rate seen in the Ma et al. study is most likely owing to the low and non-specific cut-off used to define QT prolongation in both genders. There were also some reports in which their estimate of long QT interval frequency was significantly lower than ours (≤ 1%) [10, 11], which could be attributed to the different population characteristics they considered, as their sample was primarily made up of young male individuals, whereas ours was middle-aged with the same gender distribution. The frequency our study observed was close to that in middle-aged Finnish people with a similar sex distribution to our sample, wherein the frequency of the long QT interval was 2.6% [9].
Our study found that age and hypertension are correlates of long QTc interval frequency, which is supported by prior research [6, 7, 38]. We also identified a correlation between current opium use and a long QTc interval; however, more research is needed to validate this association. Obesity, diabetes, and female sex have all been linked to longer QTc intervals in prior studies [6, 7, 38]; however, we failed to detect any evidence of a significant association between these factors and long QTc intervals.
In our study, it was found that 1.43% of the participants exhibited ER patterns in their ECGs. This result was in line with that reported by Sun et al. in their study on a Chinese middle-aged general rural population (1.3%) [24] and Uberoi et al. in their study on Caucasian-American middle-aged subjects (1.7%) [39]. Nevertheless, a recent meta-analysis found a wide range of prevalence rates for ERP across different regions, ranging from as low as 1.3% to as high as 45.8%. The pooled frequency in the general population was found to be 11.6%, with certain groups such as blacks, men, and physically active individuals showing a tendency towards higher rates [16]. In our study, younger age and male gender were independent correlates of ER pattern, which was in line with the results of the Framingham Heart Study and the Health 2000 Survey [40]. Moreover, Sun et al. [24], whose sample comprised individuals of comparable age and sex distribution to ours, identified independent associations of ER patterns with male gender and younger ages, which is consistent with our findings. Moreover, our research demonstrated a significant relationship between a lower BMI and the presence of ER patterns. This finding supports the results of the Matta et al. study [25], which examined the adult general population of Latin America and similarly identified this inverse connection. Our study also discovered an independent relationship between alcohol use and the presence of ER patterns; however, this result has not been examined in prior research. In a case series involving 19 patients diagnosed with BrS or ER syndrome with an average follow-up of about 6 years, four of them experienced syncope or aborted SCD after consuming alcoholic beverage [41]. It has been suggested that alcohol consumption may induce repolarization abnormalities and subsequent lethal events by affecting sympathovagal tone and disrupting the balance between outward K+ currents and inward Na+ and Ca2+ currents [42–44].
In our study, the frequency of BrS-type patterns was 0.31%, which was close to a meta-analysis in which the pooled frequency of BrS-type patterns was estimated to be 0.4%, with Asians having a greater rate (0.9%) than those in Europe (0.3%) and North America (0.2%) [45]. Moreover, consistent with the results of this meta-analysis, the frequency of BrS-type patterns in our study was higher in males.
In our population, only three participants (0.03%) had a short QT interval (QTc < 330 ms in males and < 340 ms in females). This was comparable to the study by Kobza et al. [11] that examined QT intervals < 320 ms in Swiss young male citizens and reported a prevalence of 0.02%. A higher prevalence of short QT interval was found in a Finnish middle-aged (0.1% and 0.4% based on 320 ms and 340 ms cut-off points, respectively) [13], a young United Kingdom (UK) (0.1% based on 320 ms cut-off point [14] and a Japanese middle-aged (0.4% based on 362 ms and 369 ms cut-off points for men and women, respectively) population [12]. In another Japanese study, the prevalence of short QTc was 1.25% in males and 1.63% in females, based on a cut-off point that was 2 SDs lower than the mean QTc in males (354 ms) and females (364 ms) [15]. Chandra et al. [8] examined QT interval < 380 ms among young UK subjects, a higher cut-off compared to prior research, and reported a prevalence of 6.9% for non-athletes and 13% for athletes. Overall, these studies indicate a large variance in the frequency estimates of the short QT interval, with the frequency being less than 1% at the 320–340 ms QTc cutoff point and increasing dramatically as the cutoff point climbed to 360–380 ms. Regional and racial disparities may also play a role in the varying results.
The strength of our study was that we for the first time provided an epidemiologic description of ECG abnormalities associated with cardiac channelopathies and SCD in a large adult general population from Iran, highlighting the importance of demographics and clinical factors associated with these ECG patterns. Understanding the range and frequency of ECG abnormalities and the factors that contribute to them is crucial for developing tailored screening programs.
The study had important limitations. First, it had a cross-sectional design, so it could only describe the clinical correlations of ECG abnormalities and not their predictive significance for cardiovascular outcomes. Although we conducted a short-term follow-up on subjects with a high-risk ECG for SCD and discovered higher rates of mortality than those without a high-risk ECG, longitudinal research with long-term follow-up is necessitated to better understand the link between these ECG abnormalities and cardiovascular outcomes in the adult general population. This will help establish whether adding ECG to the present cardiovascular screening program improves its precision. Second, the low frequency of BrS-type patterns and short QT intervals in our sample prevented us from running regression models due to a lack of statistical power. Finally, we excluded individuals under the age of 35, which may have resulted in an underestimation of our results.
Conclusions
Overall, this study found that ECG abnormalities linked to cardiac channelopathies and SCD are not common among the adult general population of Iran. Older age, opium use, and the presence of hypertension were factors independently linked to the frequency of the long QT interval. Younger age, male gender, alcohol consumption, and low BMI were independent correlates of ER pattern frequency. This research would aid in estimating the range of apparently healthy individuals who are at high risk for cardiovascular events by analyzing ECG abnormalities, ultimately providing data for health care decision-makers to optimize screening strategies for the Iranian general population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors kindly thank all of the nurses and administrative staff at Tehran Heart Center who assisted us in carrying out this study.
Author contributions
S.AR. and D.S.: Conceptualization, Methodology, Investigation, Data Curation, Supervision, Writing - Original Draft, Writing - Review & Editing, Visualization; M.F.: Conceptualization, Methodology, Data Curation, Writing - Original Draft, Writing - Review & Editing; A.Sh., A.J. and M.M.: Conceptualization, Methodology, Investigation, Data Curation, Writing - Review & Editing; S.G., E.k., F.A. and S.S.: Conceptualization, Methodology, Data Curation, Writing - Review & Editing; S.S., B.M., M.B. and A.K.: Conceptualization, Methodology, Writing - Review & Editing; F.M. and A.VF.: Conceptualization, Methodology, Investigation, Supervision, Project Administration, Writing - Review & Editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The work received approval from the deputy of research and the ethics committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.017). All participants provided written informed consent prior to their enrollment. All processes were executed in conformance to the Helsinki Declaration and its subsequent revisions.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sajjad Ahmadi-Renani and Danesh Soltani contributed equally to this work.
Contributor Information
Farzad Masoudkabir, Email: farzad.masoudkabir@gmail.com.
Ali Vasheghani-Farahani, Email: avasheghani@tums.ac.ir.
References
- 1.Medeiros-Domingo A, Iturralde-Torres P, Ackerman MJ. [Clinical and genetic characteristics of long QT syndrome]. Rev Esp Cardiol. 2007;60(7):739–52. [PubMed] [Google Scholar]
- 2.Iribarren C, Round AD, Peng JA, Lu M, Klatsky AL, Zaroff JG, et al. Short QT in a cohort of 1.7 million persons: prevalence, correlates, and prognosis. Ann Noninvasive Electrocardiol. 2014;19(5):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vutthikraivit W, Rattanawong P, Putthapiban P, Sukhumthammarat W, Vathesatogkit P, Ngarmukos T, et al. Worldwide Prevalence of Brugada Syndrome: a systematic review and Meta-analysis. Acta Cardiol Sin. 2018;34(3):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holkeri A, Eranti A, Haukilahti MAE, Kerola T, Kenttä TV, Tikkanen JT, et al. Impact of age and sex on the long-term prognosis associated with early repolarization in the general population. Heart Rhythm. 2020;17(4):621–8. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Falgueras A, Sarquella-Brugada G, Brugada J, Brugada R, Campuzano O. Cardiac channelopathies and sudden death: recent clinical and genetic advances. Biology. 2017;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magodoro IM, Albano AJ, Muthalaly R, Koplan B, North CM, Vořechovská D, et al. Population Prevalence and correlates of prolonged QT Interval: cross-sectional, Population-based study from rural Uganda. Glob Heart. 2019;14(1):17–e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Q, Li Z, Guo X, Guo L, Yu S, Yang H, et al. Prevalence and risk factors of prolonged corrected QT interval in general Chinese population. BMC Cardiovasc Disord. 2019;19(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra N, Bastiaenen R, Papadakis M, Panoulas VF, Ghani S, Duschl J, et al. Prevalence of electrocardiographic anomalies in young individuals: relevance to a nationwide cardiac screening program. J Am Coll Cardiol. 2014;63(19):2028–34. [DOI] [PubMed] [Google Scholar]
- 9.Lehtonen AO, Puukka P, Varis J, Porthan K, Tikkanen JT, Nieminen MS, et al. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. J Hypertens. 2016;34(5):959–66. [DOI] [PubMed] [Google Scholar]
- 10.Ng CT, Ong HY, Cheok C, Chua TS, Ching CK. Prevalence of electrocardiographic abnormalities in an unselected young male multi-ethnic south-east Asian population undergoing pre-participation cardiovascular screening: results of the Singapore Armed Forces Electrocardiogram and Echocardiogram screening protocol. Europace. 2012;14(7):1018–24. [DOI] [PubMed] [Google Scholar]
- 11.Kobza R, Roos M, Niggli B, Abächerli R, Lupi GA, Frey F, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm. 2009;6(5):652–7. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto A, Hayashi H, Yoshino T, Kawaguchi T, Taniguchi A, Itoh H, et al. Clinical and electrocardiographic characteristics of patients with short QT interval in a large hospital-based population. Heart Rhythm. 2012;9(1):66–74. [DOI] [PubMed] [Google Scholar]
- 13.Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation. 2007;116(7):714–20. [DOI] [PubMed] [Google Scholar]
- 14.Dhutia H, Malhotra A, Parpia S, Gabus V, Finocchiaro G, Mellor G et al. The prevalence and significance of a short QT interval in 18 825 low-risk individuals including athletes. Br J Sports Med. 2015;50(2):124-9. [DOI] [PubMed]
- 15.Funada A, Hayashi K, Ino H, Fujino N, Uchiyama K, Sakata K, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol. 2008;31(6):270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji HY, Hu N, Liu R, Zhou HR, Gao WL, Quan XQ. Worldwide prevalence of early repolarization pattern in general population and physically active individuals: a meta-analysis. Med (Baltim). 2021;100(22):e25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pecini R, Cedergreen P, Theilade S, Haunsø S, Theilade J, Jensen GB. The prevalence and relevance of the Brugada-type electrocardiogram in the Danish general population: data from the Copenhagen City Heart Study. Europace. 2010;12(7):982–6. [DOI] [PubMed] [Google Scholar]
- 18.Furuhashi M, Uno K, Tsuchihashi K, Nagahara D, Hyakukoku M, Ohtomo T, et al. Prevalence of asymptomatic ST segment elevation in right precordial leads with right bundle branch block (brugada-type ST shift) among the general Japanese population. Heart. 2001;86(2):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher MM, Forleo GB, Behr ER, Magliano G, De Luca L, Morgia V, et al. Prevalence and significance of Brugada-type ECG in 12,012 apparently healthy European subjects. Int J Cardiol. 2008;130(1):44–8. [DOI] [PubMed] [Google Scholar]
- 20.Junttila M, Raatikainen M, Karjalainen J, Kauma H, Kesäniemi Y, Huikuri H. Prevalence and prognosis of subjects with Brugada-type ECG pattern in a young and middle-aged Finnish population. Eur Heart J. 2004;25(10):874–8. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji H, Sato T, Morisaki K, Iwasaka T. Prognosis of subjects with Brugada-type electrocardiogram in a population of middle-aged Japanese diagnosed during a health examination. Am J Cardiol. 2008;102(5):584–7. [DOI] [PubMed] [Google Scholar]
- 22.Gervacio-Domingo G, Isidro J, Tirona J, Gabriel E, David G, Amarillo ML, et al. The brugada type 1 electrocardiographic pattern is common among filipinos. J Clin Epidemiol. 2008;61(10):1067–72. [DOI] [PubMed] [Google Scholar]
- 23.Hermida J-S, Lemoine J-L, Aoun FB, Jarry G, Rey J-L, Quiret J-C. Prevalence of the Brugada syndrome in an apparently healthy population. Am J Cardiol. 2000;86(1):91–4. [DOI] [PubMed] [Google Scholar]
- 24.Sun G-Z, Ye N, Chen Y-T, Zhou Y, Li Z, Sun Y-X. Early repolarization pattern in the general population: prevalence and associated factors. Int J Cardiol. 2017;230:614–8. [DOI] [PubMed] [Google Scholar]
- 25.Matta MG, Gulayin PE, García-Zamora S, Gutierrez L, Rubinstein AL, Irazola VE, et al. Epidemiology of early repolarization pattern in an adult general population. Acta Cardiol. 2020;75(8):713–23. [DOI] [PubMed] [Google Scholar]
- 26.Militz MS, Inacio AS, Wagner HM, Wangenheim AV, Forno A, Moreira DM. Prevalence and related characteristics of patients with Brugada Pattern Electrocardiogram in Santa Catarina, Brazil. Arq Bras Cardiol. 2021;117(2):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafiee A, Saadat S, Shahmansouri N, Jalali A, Alaeddini F, Haddadi M, et al. Tehran cohort study (TeCS) on cardiovascular diseases, injury, and mental health: design, methods, and recruitment data. Global Epidemiol. 2021;3:100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms, and treatment. Am Heart Assoc; 2006. pp. 1134–6. [DOI] [PubMed]
- 29.Yow AG, Rajasurya V, Ahmed I, Sharma S. Sudden Cardiac Death. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Venkat Rajasurya declares no relevant financial relationships with ineligible companies. Disclosure: Intisar Ahmed declares no relevant financial relationships with ineligible companies. Disclosure: Sandeep Sharma declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright ©. 2024, StatPearls Publishing LLC.; 2024.
- 30.Commission IE. Medical electrical equipment. Part 2–51: particular requirements for safety, including essential performance, of recording and analysing single channel and multichannel electrocardiographs. IEC 60601-2-51. Geneva: International Electrotechnical Commission; 2003. [Google Scholar]
- 31.BAZETT HC, AN ANALYSIS OF THE TIME-RELATIONS, OF ELECTROCARDIOGRAMS. Ann Noninvasive Electrocardiol. 1997;2(2):177–94. [Google Scholar]
- 32.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111(5):659 – 70. [DOI] [PubMed]
- 33.El Ouartassi H, El Boussaadani B, Faraj R, Fellat I, Cherti M. Unmasking idiopathic Brugada ECG Pattern: Inducible Type 1 Brugada Pattern in a young patient and clinical implications. Cureus. 2023;15(6):e40739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaita F, Cerrato N, Giustetto C, Martino A, Bergamasco L, Millesimo M, et al. Asymptomatic patients with Brugada ECG Pattern: long-term prognosis from a large prospective study. Circulation. 2023;148(20):1543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV, Potse M, Rosso R, et al. The early repolarization pattern: a Consensus Paper. J Am Coll Cardiol. 2015;66(4):470–7. [DOI] [PubMed] [Google Scholar]
- 36.Georgopoulos S, Letsas KP, Liu T, Kalafateli M, Korantzopoulos P, Bürkle G, et al. A meta-analysis on the prognostic significance of inferolateral early repolarization pattern in Brugada syndrome. EP Europace. 2017;20(1):134–9. [DOI] [PubMed] [Google Scholar]
- 37.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, Roy L, et al. Sudden Cardiac Arrest Associated with early repolarization. N Engl J Med. 2008;358(19):2016–23. [DOI] [PubMed] [Google Scholar]
- 38.Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39:16–25. [DOI] [PubMed] [Google Scholar]
- 39.Uberoi A, Jain NA, Perez M, Weinkopff A, Ashley E, Hadley D, et al. Early repolarization in an ambulatory clinical population. Circulation. 2011;124(20):2208–14. [DOI] [PubMed] [Google Scholar]
- 40.Noseworthy PA, Tikkanen JT, Porthan K, Oikarinen L, Pietilä A, Harald K, et al. The early repolarization pattern in the general population: clinical correlates and heritability. J Am Coll Cardiol. 2011;57(22):2284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talib AK, Sato N, Myojo T, Sugiyama E, Nakagawa N, Sakamoto N, et al. Insight into specific pro-arrhythmic triggers in Brugada and early repolarization syndromes: results of long-term follow-up. Heart Vessels. 2016;31(12):2035–44. [DOI] [PubMed] [Google Scholar]
- 42.Habuchi Y, Furukawa T, Tanaka H, Lu L-L, Morikawa J, Yoshimura M. Ethanol inhibition of Ca2 + and na + currents in the guinea-pig heart. Eur J Pharmacol Environ Toxicol Pharm. 1995;292(2):143–9. [DOI] [PubMed] [Google Scholar]
- 43.Klein G, Gardiwal A, Schaefer A, Panning B, Breitmeier D. Effect of ethanol on cardiac single sodium channel gating. Forensic Sci Int. 2007;171(2–3):131–5. [DOI] [PubMed] [Google Scholar]
- 44.Manolis TA, Apostolopoulos EJ, Manolis AA, Melita H, Manolis AS. The proarrhythmic conundrum of alcohol intake. Trends Cardiovasc Med. 2022;32(4):237–45. [DOI] [PubMed] [Google Scholar]
- 45.Quan X-Q, Li S, Liu R, Zheng K, Wu X-F, Tang Q. A meta-analytic review of prevalence for Brugada ECG patterns and the risk for death. Medicine. 2016;95(50). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


