Abstract
Subgroups B, D, and E avian leukosis viruses (ALV-B, -D, and -E) share the same chicken receptor, TVBS1, a tumor necrosis factor receptor (TNFR)-related protein. These viruses, however, exhibit nonreciprocal receptor interference (NRI): cells preinfected with ALV-B or ALV-D are resistant to superinfection by viruses of all three subgroups, whereas those pre-infected by ALV-E are resistant only to superinfection by other subgroup E viruses. In this study, we investigated the basis of this phenomenon by characterizing the interaction of TVBS1 with ALV-B Env or ALV-E Env. Sequential immunoprecipitation analysis using surface envelope immunoglobulin fusion proteins revealed the existence of two separate types of TVBS1 that are encoded by the same cDNA clone. One form, designated the type 1 receptor, is specific for ALV-B and ALV-E. The other form, the type 2 receptor, is specific for ALV-B. We show that a protein consisting of only the first and second extracellular cysteine-rich domains of TVBS1 is capable of forming both receptor types. However, the third extracellular cysteine-rich domain is required for efficient formation of the type 1 receptor. We also demonstrate that heterogeneous N-linked glycosylation cannot explain the difference in activities of the two receptor types. The existence of two types of TVBS1 explains the NRI pattern between ALV-B and -E: subgroup B viruses establish receptor interference with both receptor types, whereas subgroup E viruses interfere only with the type 1 receptor, leaving the type 2 receptor available to mediate subsequent rounds of ALV-B entry. The formation of a TVB receptor type that is specific for cytopathic ALV may also have important implications for understanding how some subgroups of ALV cause cell death.
Based on receptor usage in chickens, avian leukosis viruses (ALVs) have been divided into six major subgroups (A through E and J). Subgroups B and D viruses (ALV-B and -D) are cytopathic and share with noncytopathic ALV-E the TVB receptor, a member of the tumor necrosis factor receptor (TNFR) family. TVB is a death receptor that is most structurally related to the human TRAIL receptors, TRAIL-R1 (DR4, APO-2) and TRAIL-R2 (DR5) (7, 13, 15, 17, 19, 23), and is therefore likely to play a direct role in cell killing caused by ALV-B and ALV-D. By comparing TVB with other TNFR-related proteins, we originally proposed that this ALV receptor contains two extracellular cysteine-rich domains (CRDs) that characterize this protein family (6). However, the recently solved structure of TRAIL-R2 has revealed the existence of an additional CRD located at the membrane-distal region of that receptor, (9), making it likely that TVB also contains an extra N-terminal CRD (Fig. 1).
FIG. 1.
Schematic diagram of the TVBS1 constructs used in these studies. The TVBS1 and TVBS1 (ΔDD) proteins were described previously (2). The other TVB proteins were generated specifically for these experiments. The amino acid residues are numbered according to a scheme used previously (6). SP, signal peptide; TM, transmembrane region; DD, cytoplasmic death domain.
Functionally distinct TVB proteins that are encoded by different alleles of the chicken tvb locus (tvbs1 and tvbs3) or instead by the turkey homolog of this gene (tvbt) have been described: TVBS1, a receptor for ALV-B, -D, and -E (2); TVBS3, a receptor specific for ALV-B and -D (6, 20); and TVBT, a subgroup E-specific viral receptor (1). Although ALV-E shares the TVBS1 receptor with ALV-B and ALV-D, these viruses display a nonreciprocal receptor interference (NRI) pattern. Cells preinfected with either ALV-B or ALV-D are resistant to superinfection by ALV-B, -D, and -E presumably because the viral receptor is complexed with newly synthesized Env glycoproteins (24). This interference pattern is expected for viruses that share the same receptor. However, cells preinfected with ALV-E are resistant only to further subgroup E virus infection, remaining susceptible to ALV-B and -D infection.
At least two models have been proposed to explain this NRI pattern. The one-receptor model invokes a single TVBS1 receptor for all three viral subgroups (24). According to this model, the subgroup E Env protein has the lowest affinity for the shared receptor and thus may be displaced by either subgroup B or subgroup D Env proteins contained on incoming virus particles (24). The alternative two-receptor model predicts one receptor specific for ALV-B, -D, and -E and another specific for ALV-B and -D (24). According to this model, there may be two closely linked ALV receptor genes located at the chicken tvbs1 locus that encode the distinct receptors (24).
Previously we identified a tvbs1 cDNA clone that encodes a cellular receptor for ALV-B, -D, and -E (2). We showed that the NRI pattern exhibited by ALV-B and -E could be fully reconstituted by expressing this receptor in mammalian cells along with the respective Env proteins (2). Also, we demonstrated a striking difference in the way that TVBS1 interacts in with ALV-E Env as opposed to ALV-B Env. Specifically, the ALV-E Env interaction is influenced by the first four cysteines of the receptor (Cys-46, Cys-59, Cys-62, and Cys-77), seemingly requiring a putative Cys-46–Cys-59 disulfide bond, whereas ALV-B can use an altered receptor lacking all four of these cysteines (2). Taken together, these data suggested that there is a single type of receptor for ALV-B, -D, and -E and that the NRI pattern among these viruses might be explained by differences in subgroup-specific Env interactions with that common receptor. However, contrary to these expectations, we have now found that the tvbsl cDNA clone encodes two functionally distinct ALV receptors, one that is specific for ALV-B and -E and another that is specific for ALV-B.
MATERIALS AND METHODS
Cells, viruses, and immunoadhesins.
Human 293 cells and transfected 293 cells (S1-5 cells) that express TVBS1(ΔDD), a FLAG epitope-tagged version of the receptor without the cytoplasmic death domain, were described previously (2). Pseudotyped murine leukemia virus (MLV) virions with ALV-B Env proteins, MLV-LacZ (EnvB), or ALV-E Env proteins, MLV-LacZ (EnvE), were produced as described elsewhere (2). The surface envelope (SU)-rabbit immunoglobulin G (rIgG) fusion proteins, SUB-rIgG and SUE-rIgG, were generated and purified as described previously (1, 6, 28).
TVB constructs, transfections, and infections.
PCR amplification was performed using plasmid pHA1 (2) as template DNA to generate (i) the soluble proteins sTVBS1 and sTVBS1(Δ102-143); (ii) TVBS1(ΔDD/Δ102-143), an altered form of the TVBS1(ΔDD) receptor that lacks CRD3; and (iii) TVBS1(ΔDD)-N54A, a mutant TVBs1(ΔDD) protein bearing the N54A mutation (Fig. 1). The authenticity of each construct was validated by DNA sequencing. Human 293 cells were transiently transfected with plasmids encoding different TVB proteins and challenged with pseudotyped MLV virions, and infection was quantitated by β-galactosidase staining as described elsewhere (2).
Flow cytometry.
S1-5 cells were prepared for flow cytometry as described previously (28). Briefly, these studies involved incubating the cells with 5 μM purified SUE-rIgG or SUB-rIgG (in phosphate-buffered saline [PBS] containing 1% fetal bovine serum) and then with a fluorescein isothiocyanate-conjugated secondary antibody. Samples of 5,000 cells were analyzed on a Coulter Epics XL flow cytometer in the Department of Hematologic Oncology at the Dana-Farber Cancer Institute.
Immunoprecipitation and deglycosylation studies.
Human 293 cells were transfected with 10 μg of plasmid DNA expressing the different TVB proteins. Cells that expressed transmembrane forms of TVB proteins were metabolically labeled at 60 h posttransfection. Briefly, cells were starved in Cys- and Met-free Dulbecco modified Eagle medium for 30 min at 37°C and then incubated for 2 to 3 h in medium containing ∼1 mCi of [35S]Cys (ICN) per ml. The labeled cells were then washed in ice-cold PBS and lysed in an NP-40-containing buffer as described elsewhere (1). Cells that expressed soluble TVB proteins were subjected to a similar metabolic labeling procedure that was followed by a 3-h chase period in medium containing unlabeled amino acids to allow for secretion of radioactively labeled proteins into the extracellular supernatant. Unlabeled sTVBS1 was harvested from the extracellular supernatant of transfected human 293 cells at 72 h posttransfection.
Sequential immunoprecipitation experiments were performed at 4°C for 1 h, using 1 mg of total cell lysate (transmembrane TVB receptors) or 50 to 150 μl of extracellular supernatant (soluble TVB receptors) and protein A-Sepharose preloaded with SUB-rIgG or SUE-rIgG. The samples were then pelleted, and the supernatants were subjected to additional rounds of immunoprecipitation. Each pelleted sample was washed in ice-cold lysis buffer, boiled in sodium dodecyl sulfate (SDS) sample buffer under reducing conditions, and electrophoresed on a 12 or 15% polyacrylamide gel containing SDS. Radiolabeled TVB proteins were then detected by autoradiography, and unlabeled TVB proteins were detected by an immunoblotting procedure involving protein transfer to a nitrocellulose membrane followed by incubation with an extracellular supernatant containing SUB-rIgG for 1 h at room temperature. The membrane was then washed with TBSX buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 1% Triton X-100) and incubated for 30 min with TBSX buffer containing a 1:5,000 dilution of a horseradish peroxidase (HRP)-conjugated antibody specific for rabbit immunoglobulins (Amersham). Bound antibodies were detected by enhanced chemiluminescence.
The deglycosylation experiments were performed at 37°C for 2 h by incubating a 50-μl aliquot of 35S-labeled extracellular supernatant containing sTVBs1(Δ102-143) with 1.5 U of N-glycosidase F (Boehringer Mannheim) and 15 U of neuraminidase (Boehringer Mannheim) in an equal volume of PBS containing 20 mM EDTA and 2% NP-40. The deglycosylated proteins were then subjected to sequential immunoprecipitation with the SU-immunoglobulin fusion proteins as described above, and the precipitated proteins were detected by autoradiography following SDS-polyacrylamide gel electrophoresis.
RESULTS
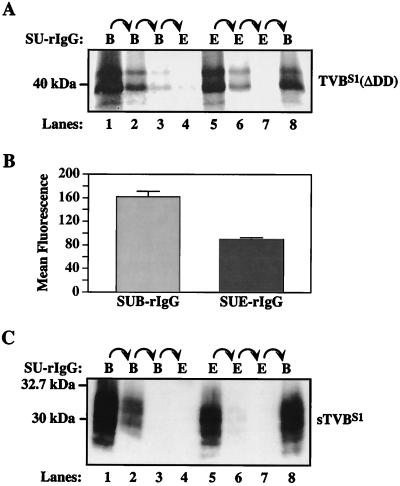
The TVBS1 receptor is produced as two distinct receptor types. To biochemically characterize the interaction between TVBS1 and ALV Env proteins, a radiolabeled protein lysate was prepared from transfected human 293 cells that express a truncated TVB receptor, TVBS1(ΔDD) (2). TVBS1(ΔDD) lacks a cytoplasmic death domain (Fig. 1) and therefore it does not induce any of the nonspecific cell-killing effects that are associated with overexpression of the full-length receptor in transfected human 293 cells (2). Aliquots of the protein lysate were subjected to sequential rounds of immunoprecipitation with SUB-rIgG and with SUE-rIgG (1).
Virtually all of the TVBS1(ΔDD) protein was removed from the lysate after two rounds of precipitation with SUB-rIgG (Fig. 2A, lanes 1 and 2). In fact, only a trace amount of receptor protein was precipitated by a third round of SUB-rIgG precipitation (Fig. 2A, lane 3), and no additional receptor protein bound to SUE-rIgG (Fig. 2A, lane 4). Therefore, as expected, all forms of TVBS1(ΔDD) that were expressed bound to the subgroup B-specific SU-immunoglobulin protein. By contrast, only a fraction of the total receptor protein was precipitated after two rounds of incubation with SUE-rIgG (Fig. 2A, lanes 5 and 6). Although a third round of incubation with SUE-rIgG failed to precipitate more of the TVB protein (Fig. 2A, lane 7), a substantial fraction of the receptor was still present in the lysate, and this form was precipitated specifically by SUB-rIgG (Fig. 2A, lane 8).
FIG. 2.
TVBS1 is produced as two distinct receptor types. (A) Aliquots of [35S]cysteine-labeled protein lysate prepared from transfected human 293 cells that express TVBS1(ΔDD) were subjected to three rounds of sequential immunoprecipitation with protein A-Sepharose beads loaded with either SUB-rIgG (lanes 1 to 3) or SUE-rIgG (lanes 5 to 7) and then with SUE-rIgG (lane 4) or SUB-rIgG (lane 8). The precipitated TVB proteins were visualized by autoradiography. (B) Flow cytometric analysis of 293 cells expressing TVBS1 (ΔDD) was performed with SUB-rIgG or SUE-rIgG, followed by a fluorescein isothiocyanate-conjugated secondary antibody. The results of a representative experiment performed in triplicate are shown as the mean fluorescence values of each cell population with the standard deviations of the data indicated with error bars. (C) An experiment similar to that described for panel A was performed with extracellular supernatant containing sTVBS1. In this case, the immunoprecipitated proteins were detected by immunoblotting with SUB-rIgG and an HRP-conjugated secondary antibody followed by enhanced chemiluminescence.
Taken together, the results of this experiment led to the surprising finding that there are two types of TVBS1 receptor, designated here type 1 and type 2. The type 1 protein is a receptor for ALV-B and ALV-E (Fig. 2A, lanes 1, 2, 5, and 6); the type 2 receptor is specific for ALV-B (Fig. 2A, lane 8).
Flow cytometric analysis performed with the SU-immunoglobulin fusion proteins confirmed that there are more subgroup B than subgroup E viral receptors on the surfaces of transfected 293 cells that express TVBS1(ΔDD) (Fig. 2B). These experiments involved binding the SU-immunoglobulin proteins to cells and then detecting the bound protein by incubation with a fluoresceinated secondary antibody. In these experiments, the mean fluorescence of each cell population is directly related to the amount of each SU-immunoglobuin fusion protein bound, which in turn is a measure of the relative number of ALV-B versus ALV-E receptors. When these experiments were performed under conditions that led to saturable binding of SUE-rIgG (data not shown), a much greater amount of SUB-rIgG was bound to the cells (Fig. 2B). Therefore, we conclude from these studies that there are more subgroup B viral receptors than subgroup E viral receptors present at the surface of these cells, a result that is fully consistent with the existence of the two types of TVBS1 receptor.
To test whether the ability to form both receptor types is a property of the extracellular domain of TVBS1, the sTVBS1 protein (Fig. 1) was produced. This soluble protein was also subjected to the same type of sequential immunoprecipitation analysis that was used to characterize the transmembrane receptor, with the exception that in this case the precipitated proteins were detected by immunoblotting following SDS-polyacrylamide gel electrophoresis with SUB-rIgG and an HRP-coupled secondary antibody. Like the transmembrane receptor, sTVBS1 was produced both as type 1 (Fig. 2C, lanes 1, 2, and 5) and type 2 (Fig. 2C, lanes 1, 2, and 8) forms. Therefore, we conclude that the ectodomain of the TVBS1 receptor is by itself capable of forming both receptor types.
The first two CRDs of TVBS1 are sufficient for forming both receptor types.
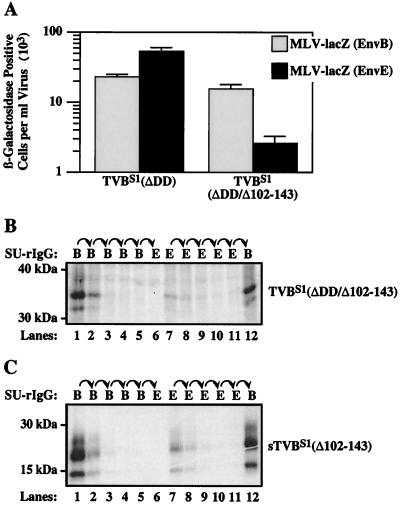
To test whether the formation of both receptor types is a property associated with the first two extracellular CRDs of this protein, the TVBS1(ΔDD/Δ102-143) and sTVBS1(Δ102–143) proteins were generated (Fig. 1). The TVBS1(ΔDD/Δ102-143) receptor was expressed in transfected human 293 cells that were challenged with pseudotyped MLV vectors encoding β-galactosidase (MLV-LacZ) and containing either ALV-B Env (EnvB) or ALV-E Env (EnvE). Compared to wild-type TVBS1(ΔDD), the receptor lacking CRD3 was fully functional for subgroup B viral entry but mediated approximately 30-fold-reduced levels of subgroup E viral entry (Fig. 3A). Nevertheless, the level of subgroup E viral entry mediated by TVBS1(ΔDD/Δ102-143) was significantly (1,000-fold) greater than that seen with nontransfected human 293 cells (data not shown), demonstrating that the protein is indeed a competent subgroup E viral receptor. These data indicated that TVBS1(ΔDD/Δ102-143) might be capable of forming both receptor types but the amount of protein with type 1 receptor activity may be lower than that with type 2 receptor activity.
FIG. 3.
The first two CRDs of TVBS1 are sufficient for formation of the type 1 receptor. (A) Human 293 cells were transiently transfected with a plasmid expressing TVBS1(ΔDD) or TVBS1(ΔDD/Δ102-143) and then challenged with MLV-LacZ (Env B) or MLV-LacZ (Env E). Infected cells were identified by staining for β-galactosidase activity. The data from three independent experiments with standard deviations are shown. Aliquots of [35S]cysteine-labeled protein lysate containing TVBS1(ΔDD/Δ102-143) (B) or supernatant containing sTVBS1 (Δ102-143) (C) were subjected to the sequential immunoprecipitation procedure outlined for Fig. 2A, and the precipitated proteins were visualized by autoradiography following SDS-polyacrylamide gel electrophoresis.
Sequential immunoprecipitation experiments performed with the SU-immunoglobulin fusion proteins confirmed that the type 1 receptor is reduced in level relative to the type 2 receptor in the protein with only two extracellular CRDs. The amount of TVBS1(ΔDD/Δ102-143) that was precipitated specifically by SUE-rIgG (Fig. 3B, lane 7) was much less than that amount precipitated by SUB-rIgG (Fig. 3B, lane 12). Similar results were obtained with sTVBS1(Δ102-143), a soluble form of the receptor lacking CRD3 (Fig. 3C, compare lanes 7 and 8 with lane 12). Taken together, these studies demonstrate that the CRD1 and CRD2 regions of the TVBS1 receptor are sufficient for the formation of both receptor types. However, the CRD3 region is also important either because it is needed for efficient formation of the type 1 receptor or instead because it is required for that receptor to bind strongly to subgroup E Env.
The distinct functional activities of both types of TVBS1 receptor cannot be explained by heterogeneous N-linked carbohydrate additions.
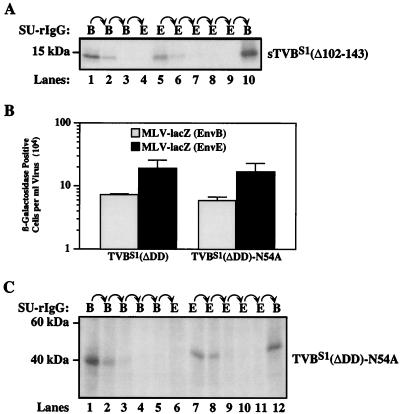
Since N-linked glycosylation gives rise to several distinct forms of TVBS1 (2) (Fig. 2A), we reasoned that subtle differences in the types of carbohydrate added might account for both receptor forms. To test this possibility, the sTVBS1(Δ102-143) protein, which contains a single putative N-linked glycosylation site at residue Asn-54 (Fig. 1), was subjected to enzymatic deglycosylation. Extracellular supernatant containing sTVBS1(Δ102-143) was treated with a combination of N-glycosidase F and neuraminidase prior to sequential immunoprecipitation with the SU-immunoglobulin fusion proteins. This combination of enzymes was more effective than N-glycosidase F alone in deglycosylating the TVB protein (data not shown), presumably because cleavage by neuraminidase allowed N-glycosidase F better access to the sugar moieties. The deglycosylated sTVBS1(Δ102-143) protein, which migrated on SDS-polyacrylamide gels with an apparent molecular mass of 13 kDa was still capable of forming both receptor types (Fig. 4A, lanes 5 and 10). Therefore, differences in N-linked glycosylation do not appear to explain the distinct activities of the two receptor types.
FIG. 4.
The existence of both receptor types cannot be explained by heterogeneous N-linked glycosylation. (A) An aliquot of [35S]cysteine labeled extracellular supernatant containing sTVBS1(Δ102-143) was incubated with N-glycosidase F and neuraminidase and then subjected to serial rounds of immunoprecipitation with SUB-rIgG and SUE-rIgG. The precipitated proteins were subjected to SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. (B) Human 293 cells expressing TVBS1 (ΔDD) and TVBS1(ΔDD)-N54A were challenged with MLV-LacZ (Env B) or MLV-LacZ (Env E) and infected cells were identified and counted after staining for β-galactosidase activity. The data shown represents the average values obtained in three independent experiments with the standard deviations given by error bars. (C) An aliquot of [35S]cysteine-labeled protein lysate containing TVBS1(ΔDD)-N54A was subjected to the sequential immunoprecipitation assay, and proteins were visualized by autoradiography following SDS-polyacrylamide gel electrophoresis.
To formally rule out any possible role for carbohydrates added at residue Asn-54, this residue was changed to an alanine to generate the TVBS1(ΔDD)-N54A receptor (Fig. 1). This altered protein supported subgroup B and subgroup E viral entry at levels comparable to those mediated by wild-type TVBS1(ΔDD) (Fig. 4B). Consistent with this result, the existence of both type 1 and type 2 forms of TVBS1(ΔDD)-N54A receptor was confirmed by sequential immunoprecipitation studies using the SU-immunoglobulin fusion proteins (Fig. 3C, lanes 7, 8, and 12). Together, these data argue that the distinct functional activities of the two classes of TVBS1 receptor cannot be explained by heterogeneous N-linked carbohydrate modifications.
DISCUSSION
In this report, we have provided compelling evidence that there are two distinct types of TVBS1 receptor. The type 1 receptor is specific for ALV-B and ALV-E, whereas the type 2 receptor is specific for ALV-B. Previously we have shown that TVBS1 differs from TVBS3 (a protein which exhibits only type 2 receptor activity) by a single amino acid difference, namely, that residue 62 is a cysteine in the former and a serine in the latter (2). This fact, coupled with our finding that a putative disulfide bond located between residues Cys-46 and Cys-62 seems to be important for subgroup E receptor function (2), raises the possibility that the two types of TVBS1 may differ in their intrachain disulfide bonding patterns, although this idea remains to be tested.
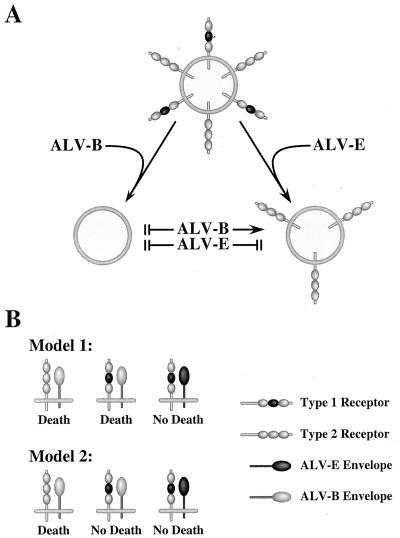
The existence of two types of TVBS1 receptor leads to an attractive model to explain why these viruses exhibit NRI (Fig. 5A). According to this model, following subgroup B viral infection the newly synthesized ALV-B Env proteins interfere with the function of both receptor types leading to a block to superinfection by ALV-B, -D, and -E. Given that subgroup D viruses have the same receptor-interference properties as subgroup B viruses, we fully expect that ALV-D Env will also interact with both types of receptor, although this remains to be formally shown. By contrast to these two viral subgroups, ALV-E infection leads to the production of Env proteins that interfere only with the function of the type 1 receptor, leaving the type 2 receptor still available to mediate subsequent rounds of subgroup B (and presumably also subgroup D) viral entry (Fig. 5A). Thus, the NRI pattern between these viruses is explained by a two-receptor model. The interesting twist to this story is that the two receptor types are derived from precisely the same mRNA species and not from separate genes which are closely linked at tvbs1.
FIG. 5.
Implications of the two receptor types for understanding NRI between ALV-B and ALV-E and for ALV-induced cytopathic effects. (A) NRI between ALV-B and ALV-E; (B) possible role of TVBS1 in cell death induced by ALV-B. These models are discussed in detail in the text.
NRI has also been observed with other retroviruses such as xenotropic and polytropic MLVs (X-MLVs, and P-MLVs, respectively). X-MLVs establish receptor interference to both viral types in Mus dunni cells, whereas P-MLVs only partially interfere with X-MLVs. The Modunni receptor shared by these viruses has been isolated and characterized (3, 21, 27). Although receptor determinants that are specifically involved in X-MLV entry have been defined (14), it is not known yet if, like TVBS1, this cellular protein, is produced as two distinct receptor types (i.e., one type that is specific for X-MLV and P-MLV and a second type specific for X-MLV).
The existence of two types of TVBS1 protein may also have important implications for understanding the mechanism of cell death that is induced by ALV-B and ALV-D (25, 26). The cell-killing events caused by these viruses are associated with massive rounds of viral superinfection which give rise to the accumulation of many copies of unintegrated viral DNA within cells that are destined to die (25, 26). Several lines of evidence support a direct role for the TVB receptor in these viral cytopathic effects. First, the determinants on Env that are required for cell killing are the same as those needed for TVB interaction (8). Second, the TVB receptor is a death receptor of the TNFR family, and this protein can activate avian cell death after binding to either subgroup B or subgroup E SU-immunoglobulin fusion proteins, at least in the presence of cycloheximide which presumably acts to extinguish the expression of cellular survival factors (5, 6).
With the identification of two types of TVBS1, at least two different models can now be envisaged to explain why ALV-B and ALV-D may kill cells whereas ALV-E does not. The first model proposes that subgroup B Env can induce death following infection by interacting with either the type 1 or type 2 receptor (Fig. 5B). If this were the case, then ALV-E might be unable to induce cell death following infection because it interacts with the type 1 receptor in a fundamentally different way that may not activate cell killing unless the action of cellular survival factors is also blocked (Fig. 6B)(2, 5). Mutational studies have in fact shown that subgroup B and subgroup E Env proteins interact with the shared receptor by distinct means (2). In a second model, subgroup B Env can elicit cell death only by interacting with the type 2 receptor (Fig. 5B). If this were the case, the type 1 receptor would be unable to participate in cell killing even when complexed with ALV-B Env (Fig. 5B).
Given that subgroup E viruses are endogenous in the chicken germ line (4), it is tempting to speculate that the two types of TVBS1 evolved in response to selective pressures imposed by these endogenous viruses. For example, there may be a selective pressure to maintain the expression of some form of TVBS1 protein (the type 2 form) in chicken cells that contain endogenous subgroup E viruses and thus exhibit type 1 receptor interference. Indeed, the TVB receptor may play an important role in the host response to viral infection. Support for this idea has come from studies of the highly related TRAIL receptors, which have been shown to mediate ligand-induced apoptosis of cells infected by a variety of viruses including cytomegalovirus (18), measles virus (22), and human immunodeficiency virus type 1 (10, 11).
Additional evidence that TVB receptors may have evolved under the influence of selective pressures imposed by endogenous subgroup E viruses comes from comparing TVBS1 with TVBS3 and with TVBT. TVBS3 is an ALV-B- and ALV-D-specific receptor that is encoded by a different allele of the chicken tvb gene (6, 20). TVBT is an ALV-E-specific receptor encoded by the turkey homolog of tvb (1). The only difference between TVBS3 and TVBS1 is that the former protein contains a serine residue at position 62 whereas the latter protein contains a cysteine at the same position (2). Since the highly related TRAIL receptors also contain a cysteine residue at that corresponding position, it seems intuitive that TVBS3 arose from TVBS1 by accquiring a mutation that selectively abrogates subgroup E Env binding. Further support for this idea is found with the turkey TVBT receptor, which has evolved in a species lacking endogenous subgroup E viruses. TVBT has retained a cysteine residue at position 62 and supports subgroup E viral entry (1). Work is ongoing to characterize the functional consequences of different subgroup-specific ALV Env-TVB interactions with the goals of understanding the mechanisms of entry used by these viruses and establishing how some of these viruses elicit cell death, information that in turn will provide new insights into the coevolution of these avian retroviruses with their hosts.
ACKNOWLEDGMENTS
We thank members of the Young and Blacklow laboratories for stimulating discussions and for constructive criticisms. We especially thank John Naughton for assistance in preparing the figures and Sara Klucking and Dan Knauss for critical reading of the manuscript.
This work was supported by NIH grants CA 70810 (to J.A.T.Y) and RO1-HL61001 (to S.C.B.). S.C.B. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A T. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins H B, Brojatsch J, Young J A T. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J Virol. 2000;74:3572–3578. doi: 10.1128/jvi.74.8.3572-3578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Rasko J E, Miller A D. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–435. [PubMed] [Google Scholar]
- 5.Brojatsch J, Naughton J, Adkins H B, Young J A T. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J Virol. 2000;74:11490–11494. doi: 10.1128/jvi.74.24.11490-11494.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary P M, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 8.Dorner A J, Coffin J M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 9.Hymowitz S G, Christinger H W, Fuh G, Ultsch M, O'Connell M, Kelley R F, Ashkenazi A, de Vos A M. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 10.Jeremias I, Herr I, Boehler T, Debatin K M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristal B S, Reinhart T A, Hoover E A, Mullins J I. Interference with superinfection and with cell killing and determination of host range and growth kinetics mediated by feline leukemia virus surface glycoproteins. J Virol. 1993;67:4142–4153. doi: 10.1128/jvi.67.7.4142-4153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacFarlane M, Ahmad M, Srinivasula S M, Fernandes-Alnemri T, Cohen G M, Alnemri E S. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 14.Marin M, Tailor C S, Nouri A, Kozak S L, Kabat D. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J Virol. 1999;73:9362–9368. doi: 10.1128/jvi.73.11.9362-9368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 16.Reinhart T A, Ghosh A K, Hoover E A, Mullins J I. Distinct superinfection interference properties yet similar receptor utilization by cytopathic and noncytopathic feline leukemia viruses. J Virol. 1993;67:5153–5162. doi: 10.1128/jvi.67.9.5153-5162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider P, Bodmer J L, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 18.Sedger L M, Shows D M, Blanton R A, Peschon J J, Goodwin R G, Cosman D, Wiley S R. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- 19.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 20.Smith E J, Brojatsch J, Naughton J, Young J A. The CAR1 gene encoding a cellular receptor specific for subgroup B and D avian leukosis viruses maps to the chicken tvb locus. J Virol. 1998;72:3501–3503. doi: 10.1128/jvi.72.4.3501-3503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, Goodwin R G, Rauch C T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss R A. The Retroviridae. New York, N.Y: Plenum; 1993. [Google Scholar]
- 25.Weller S K, Joy A E, Temin H M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller S K, Temin H M. Cell killing by avian leukosis viruses. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y L, Guo L, Xu S, Holland C A, Kitamura T, Hunter K, Cunningham J M. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 28.Zingler K, Young J A T. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]