Abstract
Introduction and importance
Spontaneous coronary artery dissection is a rare cause of acute coronary syndromes not related to atherosclerosis. It involves the sudden tearing of the coronary artery wall, separating the inner intimal lining from the outer vessel wall, typically affecting a single coronary vessel. In 20% of cases, the cause of spontaneous coronary artery dissection is unknown. The other cases often occur in pregnant or postpartum women or in individuals with conditions such as connective tissue disorders or vasculitis.
Case presentation
Here, we describe a case of a 69-year-old African female presenting with non-ST-segment elevation myocardial infarction. Coronary angiography revealed an unusual triple-vessel spontaneous coronary artery dissection affecting peripheral segments, with further investigations suggesting polyarteritis nodosa.
Conclusion
While triple-vessel spontaneous coronary artery dissection and polyarteritis nodosa (PAN) are individually rare, their coexistence is exceptionally uncommon and presents diagnostic and therapeutic challenges. Clinicians should be alert to vasculitic causes in patients with spontaneous coronary artery dissection, especially with atypical clinical features.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13256-024-04841-4.
Keywords: Spontaneous coronary artery dissection, Polyarteritis nodosa, Vasculitis, Acute coronary syndrome, Coronary angiogram, Case report
Introduction
SCAD is a rare but significant cause of acute myocardial infarction (AMI), characterized by a nontraumatic, noniatrogenic separation of the coronary arterial wall. Although infrequent, SCAD accounts for approximately 0.1–4% of acute coronary syndromes (ACS) cases in the general population [1]. It predominantly affects younger individuals, with a marked predilection for women, who comprise 87–95% of SCAD cases, typically presenting between the ages of 44 and 53 years [1]. Multivessel SCAD, however, remains an unusual presentation. Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that primarily targets medium-sized muscular arteries, with potential involvement of small arteries. The prevalence of PAN is estimated to range from 2 to 33 per million people [2]. While overt myocardial infarction is a rare consequence of PAN, myocardial ischemia may occur due to the narrowing or occlusion of coronary arteries.
The intersection of SCAD and PAN is exceptionally rare. The first documented case of PAN presenting as AMI with coronary dissection was reported in 1998 [3]. Up to October 2020, only ten cases of triple-vessel SCAD have been reported [4]. Here, we present what we believe to be the first documented case of triple-vessel SCAD secondary to PAN, contributing to the limited body of literature on this uncommon, yet clinically significant, overlap.
Our paper was written according to the CARE guidelines [5].
Case presentation
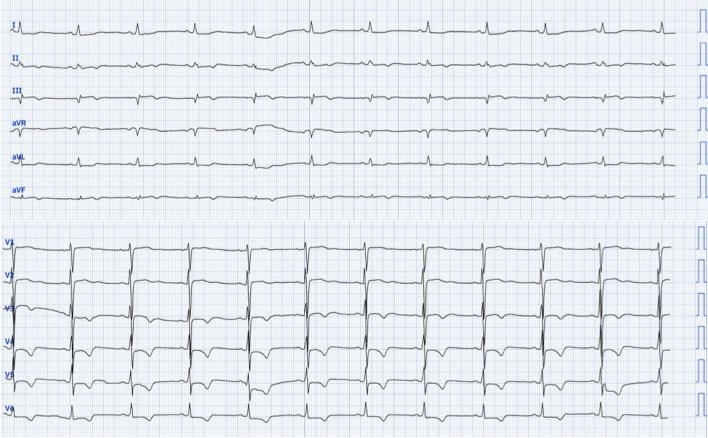
A 69-year-old African female presented to our department with complaints of exertional dyspnea and episodes of prolonged anginal pain occurring both during exertion and at rest. Her medical history includes hypertension and an undocumented left total nephrectomy performed over 20 years ago. Upon clinical examination at admission, her heart rate was 88 bpm, and her blood pressure was 153/77 mmHg, with no signs of heart failure. The electrocardiogram revealed Q waves, a suspended ST segment, and negative T waves in the inferior leads, along with ST segment depression and negative T waves from V3 to V6 (Fig. 1).
Fig. 1.
Electrocardiogram findings: Q waves, a suspended ST segment, and negative T waves in the inferior leads, along with ST segment depression and negative T waves from V3 to V6
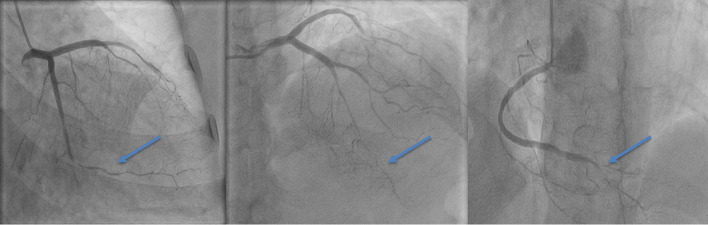
Biologically, she presented a normochromic and normocytic anemia with a hemoglobin level at 11 g/dl (reference range 12.1–15.1 g/dl), ferritin at 244 ng/ml (reference range 24–307 ng/ml), white blood cell count at 7200 elements/mm3 (reference range 4500–11,000/mm3), and C-reactive protein at 127 mg/l (reference range < 5 mg/dl). Troponin level was 12,100 ng/ml, more than 700 times the normal value, with a glycated hemoglobin of 6% and low-density lipoprotein (LDL) cholesterol level of 0.89 g/l (reference range 1–1.2 g/l). Echocardiography showed moderately reduced left ventricular ejection fraction at 48%, with hypokinesia of the apex and apical segments, as well as the mid segments of the inferior and inferolateral wall (Video 1). The patient was transferred to the catheterization laboratory. Coronary angiography revealed large-caliber arteries in the proximal segments with triple vessel disease and TIMI III flow: a long lesion in the mid to distal left anterior descending artery (LAD) with significant caliber reduction, a short distal circumflex-marginal bifurcation lesion (LCX), and a long lesion in the distal right coronary artery (RCA) including the posterior descending artery (Fig. 2, Video 2). Intracoronary nitrate derivatives were administered to eliminate any vasospasm effect. The angiographic appearance remained unchanged in all three vessels. Given the atypical aspect of the distal multivessel disease that could be related to coronary multivessel dissection rather than simple atherosclerotic involvement in a hemodynamically stable patient with TIMI III flow, the initial therapeutic decision was to prioritize medical treatment, including dual antiplatelet therapy, beta-blockers, renin–angiotensin system blockers, and statins, and to complete further investigations to look for signs of inflammatory involvement in the context of systemic or vascular disease, followed by planning for subsequent angiographic control. Coronary imaging was considered but not carried out due to a lack of resources. Initial work-up for possible connective tissue disorder was performed, with erythrocyte sedimentation rate at 25 mm at the end of the first hour, negative antinuclear cytoplasmic antibody (C and P), anticardiolipin antibody, rheumatoid factor, and hepatitis and HIV serologies were also negative. However, elevated homocysteine levels were found 23.32 μmol/l (normal limits 4.3–11.4 μmol/l). Whole-body computed tomography angiography was performed, revealing no evidence of aneurysm or dissection in medium- or large-sized arteries, and no connective tissue disorder was identified. The patient was discharged under medical treatment. Two months later, faced with the progressive worsening of her angina, she was managed at another hospital where she underwent a left internal mammary artery (LIMA) to distal left anterior descending artery (LAD) bypass grafting. Intraoperative findings indicated the presence of an infiltrative inflammatory coronary artery disease without calcification, with pericoronary inflammation. Upon opening the anterior interventricular artery, there was a thickening of the wall with a tendency to dissect. A sample from the distal part of the LIMA was taken for histopathological study.
Fig. 2.
Coronary angiogram findings: an abrupt change in arterial caliber in peripheral segments (blue arrowheads), persisting after intracoronary nitrate derivatives, suggesting type 2 spontaneous coronary artery dissection
The diagnosis of inflammatory vasculitis was strongly considered, with a high suspicion of PAN. Therefore, a test for antineutrophil cytoplasmic antibodies was performed, which came back negative, in line with the diagnosis of PAN.
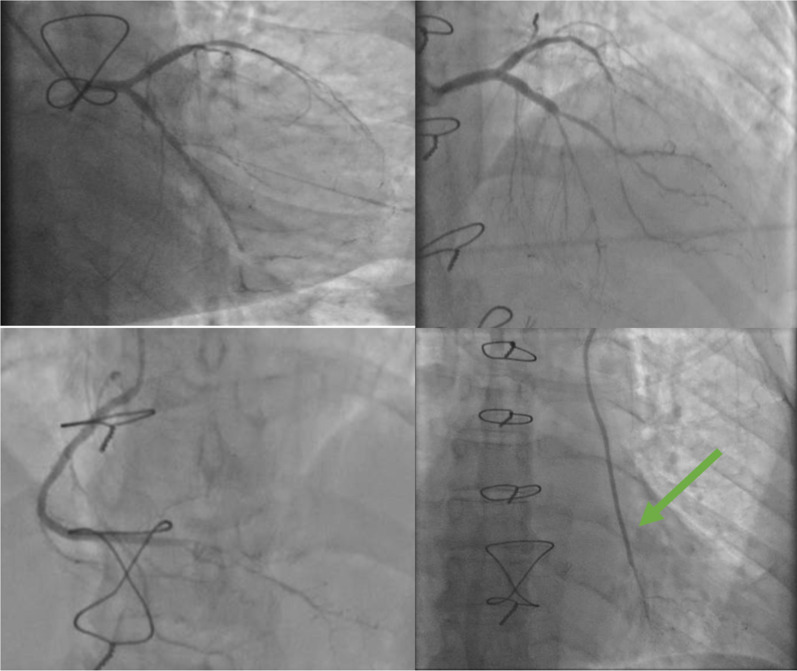
The follow-up coronary angiography of the native coronary network showed worsening of the long-segment involvement of the LAD with a significant reduction in caliber to the distal end, as well as worsening involvement of the middle LCX and the distal RCA. The LIMA graft was functional with infiltration of its distal part upstream of the anastomosis with a small infiltrated distal coronary network of the LAD (Fig. 3, Video 3).
Fig. 3.
The follow-up coronary angiogram findings: worsening of the long-segment involvement of the left anterior descending artery with a significant reduction in caliber to the distal end, as well as worsening involvement of the middle circumflex-marginal bifurcation lesion and the distal right anterior descending artery. The left internal mammary artery graft (green arrow) was functional with infiltration of its distal part
The diagnosis of PAN was confirmed by the histopathological study of the surgical specimen, showing a vascular wall with an inflammatory infiltrate rich in neutrophils. (Fig. 4). The patient was started on corticosteroid therapy alongside cyclophosphamide with 3 months follow-up showing clinical improvement of her angina, biological inflammatory syndrome and improvement of her left ventricular systolic function to 57% upon echocardiographic control (Video 4).
Fig. 4.
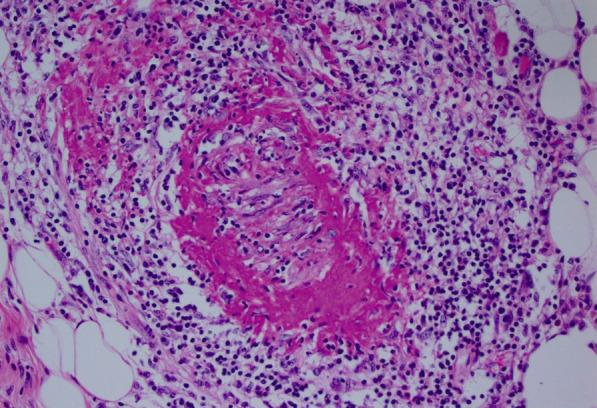
Anatomopathological findings: vascular wall of medium caliber, site of fibrinoid necrosis in the wall with the presence of an inflammatory infiltrate rich in neutrophil polymorphs (Hematoxylin and Eosin (HE) ×200)
Discussion
SCAD is a frequently overlooked cause of ACS that poses significant risks of morbidity and mortality, particularly in young women. It is estimated that SCAD contributes to up to 35% of ACS cases in women under 50 years old [1]. This highlights the importance of considering SCAD in differential diagnoses for young women presenting with ACS. However, several large cohort studies have demonstrated that SCAD can affect women who are postmenopausal, as evidenced by our case [6]. Understanding its angiographic patterns is crucial for accurate diagnosis. SCAD can be classified into three types based on angiographic appearance [7]: type 1 SCAD: this pathognomonic form involves contrast dye staining of the arterial wall with multiple radiolucent lumens, making it the most recognizable on angiography. Type 2 SCAD: characterized by diffuse stenosis of varying severity, and it can be easily missed due to its subtle presentation. It often shows an abrupt change in arterial caliber from normal diameter to diffuse narrowing. Type 3 SCAD: this form is the most challenging to differentiate from atherosclerosis. Accurate diagnosis requires a high index of suspicion and often necessitates the use of intracoronary imaging techniques.
In our case, despite not using intracoronary imaging techniques, we identified features consistent with type 2 SCAD, including an abrupt change in arterial caliber in peripheral segments, which were confirmed intraoperatively. The LAD artery is most commonly involved in SCAD, followed by the RCA and the LCX. The pathophysiology of nonatherosclerotic SCAD is not fully understood. Proposed mechanisms include: intimal tear and vasa vasorum bleeding or inflammation [8]. Histological studies have identified inflammatory reactions, such as eosinophilic infiltrates in the adventitia, suggesting periarteritis. This may weaken the medial–adventitial layer, predisposing the artery to dissection. It remains uncertain whether this inflammatory response is causative or reactive. This hypothesis may explain the development and the extent of SCAD in the context of PAN. In a 2021 study published in the Canadian Journal of Cardiology, Lai et al. [9] reported that only 13.1% of patients with PAN had coronary artery lesions, and 5% experienced MI. This contrasts with necropsy findings, which frequently revealed coronary arteritis and MI pathology in 62% of patients with PAN [10]. The disparity observed between clinical observations and necropsy findings suggests that coronary arteritis could be a significant complication and a primary cause of mortality in patients with PAN. Within PAN, coronary issues can manifest in various forms such as stenosis, occlusion, aneurysm, or dissection, potentially leading to conditions such as congestive heart failure, hypertension, pericarditis, and arrhythmias. Nevertheless, our case is distinguished by the patient presenting with triple vessel SCAD as the initial manifestation of PAN. This underscores the uncommon and unconventional aspect of our case, given its distinct clinical presentation. Because of the relative rarity of this disease and because of the potentially severe adverse effects related to treatment, the diagnosis should be confirmed by biopsy whenever possible. In our specific case, the diagnosis was confirmed by biopsy. For the majority of individuals with SCAD, conservative treatment is typically favored once the diagnosis is confirmed, contingent upon the patient’s clinical stability. However, determining the optimal management remains uncertain, particularly among patients where it is secondary to vasculitis, owing to limited clinical experience. Because of heightened vascular fragility resulting from vascular inflammation, if the patient’s condition permits, surgical intervention should be conducted during the inactive phase of their disease. In this regard, Canpolat et al. [11] documented a case involving a 28-year-old female diagnosed with PAN who experienced ACS linked to right coronary SCAD. The patient underwent stent placement, resulting in a favorable outcome. An intriguing observation from this case report is that, despite the patient being on PAN therapy comprising prednisone and cyclophosphamide, she nonetheless presented with coronary involvement. Our patient’s outcome was hopefully favorable, as she became asymptomatic and her left ventricular ejection fraction improved significantly one year later (Video 4). However, it remains unclear whether this improvement was due to the Coronary Artery Bypass Grafting (CABG) or to the optimized heart failure treatment and vasculitis therapy.
Conclusion
The coexistence of SCAD and PAN, both individually rare conditions, presents significant diagnostic and therapeutic challenges. The patient’s clinical course underscores the importance of considering vasculitic causes in SCAD, particularly in atypical presentations. Further research and accumulation of case studies are essential to better understand and manage such rare and challenging clinical scenarios.
Supplementary Information
Supplementary Material 1: Video 1: TTE findings at her admission.
Supplementary Material 2: Video 2: Coronary angiogram findings at her admission.
Supplementary Material 3: Video 3: The follow-up coronary angiogram findings.
Supplementary Material 4: Video 4: The follow-up TTE findings.
Acknowledgements
None.
Research registration
Not applicable.
Provenance and peer review
Not commissioned.
Author contributions
NL: study concept, data collection, data analysis, and writing the paper. RF: study concept, data collection, data analysis, and writing the paper. SC: data collection and data analysis. ON: data collection and data analysis. MS: data collection and data analysis. OK: data collection and data analysis. IA: data collection and data analysis. JZ: supervision and data validation. MC: supervision and data validation. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(8):961–84. [DOI] [PubMed] [Google Scholar]
- 2.Mahr A, Guillevin L, Poissonnet M, Aymé S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51(1):92–9. [DOI] [PubMed] [Google Scholar]
- 3.Polyarteritis nodosa presenting as acute myocardial infarction with coronary dissection. https://pubmed.ncbi.nlm.nih.gov/9676806/. Accessed 26 May 2024. [DOI] [PubMed]
- 4.Lak H, Rehman KA, Jaber WA, Cho L, Parker J, Siraj B, et al. Three broken vessels in a peripartum patient: a rare case report of spontaneous triple vessel coronary artery dissection. Eur Heart J Case Rep. 2020;4(5):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35. [DOI] [PubMed] [Google Scholar]
- 6.Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40(15):1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adlam D, Tweet MS, Gulati R, Kotecha D, Rao P, Moss AJ, et al. Spontaneous coronary artery dissection: pitfalls of angiographic diagnosis and an approach to ambiguous cases. JACC Cardiovasc Interv. 2021;14(16):1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spontaneous coronary artery dissection-UpToDate. https://www.uptodate.com/contents/spontaneous-coronary-artery-dissection?search=SCAD&source=search_result&selectedTitle=1%7E14&usage_type=default&display_rank=1#H3405782249. Accessed 30 May 2024.
- 9.Lai J, Zhao L, Zhong H, Zhou J, Guo X, Xu D, et al. Characteristics and outcomes of coronary artery involvement in Polyarteritis nodosa. Can J Cardiol. 2021;37(6):895–903. [DOI] [PubMed] [Google Scholar]
- 10.Cassling RS, Lortz JB, Olson DR, Hubbard TF, McManus BM. Fatal vasculitis (Periarteritis nodosa) of the coronary arteries: angiographic ambiguities and absence of aneurysms at autopsy. J Am Coll Cardiol. 1985;6(3):707–14. [DOI] [PubMed] [Google Scholar]
- 11.Canpolat U, Dural M, Atalar E. Acute inferior myocardial infarction in a young female patient with Polyarteritis nodosa. Herz. 2012;37(4):461–4. 10.1007/s00059-011-3567-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Video 1: TTE findings at her admission.
Supplementary Material 2: Video 2: Coronary angiogram findings at her admission.
Supplementary Material 3: Video 3: The follow-up coronary angiogram findings.
Supplementary Material 4: Video 4: The follow-up TTE findings.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.