Abstract
Background
Immunoglobulin A nephropathy (IgAN) is the predominant primary glomerulonephritis globally and remains a subject of active research with a focus on understanding its course and prognosis. Although vascular lesions are associated with IgAN, the current histopathological grading systems do not consider intrarenal vascular lesions when predicting patient prognosis. Therefore, this retrospective study conducted at Kyungpook National University Hospital between October 2016 and December 2021, aimed to elucidate the significance of intrarenal vascular lesions in IgAN by comparing the clinical data of patients with and without such lesions.
Methods
Data of patients with biopsy-confirmed primary IgAN between October 2016 and June 2021 at Kyungpook National University Hospital (Daegu, South Korea) were collected, and their medical records were reviewed. All slides from these 138 cases were independently pathologically reviewed by two nephropathologists (Y. J. K. and M. S. K.) using light microscope. The vascular lesions included in this study were fibrous intimal thickening, arteriolar wall thickening, and arteriolar hyalinosis. All cases were reviewed according to the Oxford Classification of IgA Nephropathy (2016) and Haas classification.
Results
Of the 138 patients, 88 exhibited at least one intrarenal vascular lesion. Patients with arteriolar wall thickening demonstrated a reduced estimated glomerular filtration rate (eGFR), elevated serum creatinine level and urine protein-to-creatinine ratio, an increased proportion of global glomerulosclerosis, and a higher histologic grade of interstitial fibrosis and tubular atrophy at the time of biopsy.
Conclusion
Arteriolar wall thickening in IgAN are associated with reduced eGFR and global glomerulosclerosis. Moreover, reduced eGFR and global glomerulosclerosis are correlated with the progression to end-stage renal disease. Although the direct correlation between vascular lesions and end-stage renal disease is not entirely clear, a marginally significant association (log-rank test, p = 0.06) was observed with arterial wall thickening. This study suggests the potential importance of vascular lesions in the prognosis of IgAN, encouraging further investigation using larger cohort studies to establish a clearer association.
Keywords: Immunoglobulin a nephropathy, IgA nephropathy, IgA, Prognosis, Intrarenal vascular lesion, Glomerular filtration rate, Oxford, Haas
Background
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide [1]. Research on the course and prognosis of the disease is ongoing, and patient prognosis is currently predicted through histopathological grading. The Oxford and Haas classifications are the representative grading systems. The Oxford classification evaluates and grades biopsy specimens based on the following five categories: mesangial hypercellularity (M), segmental sclerosis (S), interstitial fibrosis/tubular atrophy (T), and crescents (C) [2]. These parameters are associated with a reduced estimated glomerular filtration rate (eGFR) or progression to end-stage renal disease. The Haas classification classifies specimens into five grades according to the ratio of glomerular sclerosis to glomerular hypercellularity (classes I to V) [3]. As the proportion of glomeruli with sclerosis or hypercellularity increases, patients tend towards end-stage renal disease. In one study, intrarenal arterial-arteriolar lesions were more frequently associated with IgAN than with non-IgA nephropathy [4]. In this study, the prevalence of intrarenal small artery and arteriolar lesions was 54.6% in patients with IgAN and 26.6% in patients without IgAN (p < 0.01). However, the Oxford and Haas classifications do not consider intrarenal vascular lesions. Therefore, this study aimed to analyze these differences by comparing clinical data between patients with IgAN with intrarenal vascular lesions and patients with IgAN without intrarenal vascular lesions. We ultimately aimed to determine whether vascular lesions can be added to these classification methods.
Methods
Patients
This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital, Daegu, Korea (IRB FILE No. KNUH 2023-12-017, 8 January 2024). This study is a retrospective analysis involving the review of histologic slides and medical records. The IRB determined that obtaining explicit consent from every individual would be impractical and that the study poses minimal risk. Therefore, the IRB waived the requirement for informed consent, provided that sensitive patient information is protected. We assigned case numbers to the records, ensuring the elimination of personal identifiers, including patient identification numbers and Resident Registration Numbers, to maintain participant confidentiality. Data of patients with biopsy-confirmed primary IgAN between October 2016 and June 2021 at Kyungpook National University Hospital (Daegu, South Korea) were collected, and their medical records were reviewed. Patients older than 18 years and those with more than seven glomeruli on biopsy were included. Out of the 166 patients, 32 cases were excluded due to the patient’s age and having fewer than 7 glomeruli. The remaining 138 cases were included in the study. At the time of biopsy, we collected data, including age, sex, past medical history, initial blood pressure, CKD-EPI eGFR [5], serum creatinine, serum blood urea nitrogen (BUN), urine protein-to-creatinine ratio (UPCR), serum albumin, hemoglobin, platelet count, serum uric acid, and total cholesterol levels from electronic medical records. For the survival analysis, we followed the cases with medical records up to November 2023.
Histological evaluation
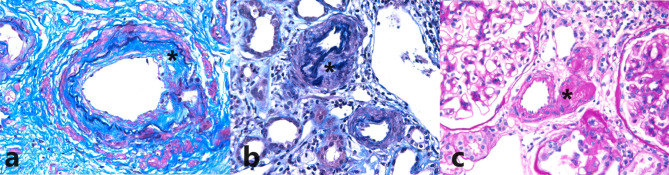
All slides from the 138 cases were independently pathologically reviewed by two nephropathologists (Y. J. K. and M. S. K.) who were blinded to the patient information. Any discrepancies in the pathological diagnosis between the pathologists were resolved through re-evaluation of the cases until consensus. The vascular lesions included in this study were fibrous intimal thickening (Fig. 1a), arteriolar wall thickening (Fig. 1b), and arteriolar hyalinosis (Fig. 1c). No cases with arteriolar fibrinoid necrosis, arteriolar thrombi, subintimal myxoid changes, or arteriolar onion skin changes were observed. Vascular lesions were scored using a two-tier system: no or mild change and moderate to severe change. Moderate to severe changes were considered positive, whereas no or mild changes were considered negative. All cases were reviewed according to the Oxford Classification of IgA Nephropathy 2016 and the Haas classification [6, 7].
Fig. 1.
Representative cases of vascular lesions. (a) Fibrous intimal thickening (modified elastic tissue-Masson trichrome stain, x400). (b) Arteriolar wall thickening (modified elastic tissue-Masson trichrome stain, x400). (c) Arteriolar hyalinosis (periodic acid–Schiff stain, x400)
Outcome
Of the initial 138 patients, 35 were lost after follow-up, leaving 103 patients included in the survival analysis. The median follow-up time was 1442 days (range: 253–2529 days). Survival time was defined as the duration from the date of kidney biopsy until the date of the last follow-up or the occurrence of end-stage renal disease (eGFR < 15 mL/min/1.73 m²) requiring kidney replacement.
Statistical analysis
All analyses were conducted using R version 4.3.2, incorporating the “survival” and “Hmisc” packages from the R Foundation for Statistical Computing (Auckland, New Zealand). A p-value of p < 0.05 was considered statistically significant. Clinicopathological differences between the vascular lesion-positive and vascular lesion-negative groups were assessed using Fisher’s exact test and a two-sample t-test (Tables 1 and 2, and 3). Survival analysis was performed using the log-rank test, Kaplan–Meier method (Fig. 2), and univariate and multivariate Cox proportional hazards models (Tables 4 and 5). The results were expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Table 1.
Clinicopathological characteristics according to arteriolar wall thickening
| Arteriolar wall thickening (+) | Arteriolar wall thickening (-) | p-value | ||||
|---|---|---|---|---|---|---|
| (n = 76) | (n = 62) | |||||
| Clinical Information | ||||||
| Gender (%) | ||||||
| Male | 37(48.7) | 33(53.2) | 0.6 | |||
| Female | 39(51.3) | 29(46.8) | ||||
| Age (mean) | 19–74 (46) | 18–74 (35) | 0.000006* | |||
| Hypertension (%) | 34(44.7) | 16(25.8) | 0.03* | |||
| DM (%) | 5(6.6) | 0(0) | 0.06 | |||
| Systolic BP | 98–210 (128) | 78–172 (124.8) | 0.28 | |||
| mm Hg (mean) | ||||||
| Diastolic BP | 46–120 (76.7) | 46–90 (72.1) | 0.03* | |||
| mm Hg (mean) | ||||||
| eGFR | 9-128 (65.7) | 15–155 (97.2) | 0.00000005* | |||
| mL/min/1.73m2 (mean) | ||||||
| UPCR | 0.09–13.12 (2.17) | 0.05–5.58 (1.07) | 0.0009* | |||
| g/gCr (mean) | ||||||
| BUN | 6-64.1 (20.1) | 5.8–96 (16.6) | 0.08 | |||
| mg/dL (mean) | ||||||
| Scr | 0.5–5.12 (1.4) | 0.49–5.49 (1.0) | 0.004* | |||
| µmol/L (mean) | ||||||
| Uric acid | 3.1–11.4 (6.8) | 3.5–11.3 (6.2) | 0.055 | |||
| µmol/L (mean) | ||||||
| Hemoglobin | 8.4–17.1 (13.1) | 4–19 (13.7) | 0.1 | |||
| g/L (mean) | ||||||
| Platelet count | 24–603 (254.6) | 157–463 (265.6) | 0.44 | |||
| ×109 /L (mean) | ||||||
| Albumin | 1.9–5.1 (4.1) | 2.7–5.4 (4.4) | 0.003* | |||
| g/L (mean) | ||||||
| Total cholesterol | 120–360 (207) | 101–243 (171) | 0.000005* | |||
| mmol/L (mean) | ||||||
| Medications | ||||||
| RAAS blockers (%) | 47(61.8) | 15(24.2) | 0.00001* | |||
| Steroids and other immunosuppressants (%) | 1(1.3) | 1(1.6) | 1 | |||
| Oxford classification score | ||||||
| M0/1(%) | 70(92.1)/6(7.9) | 56(90.3)/6(9.3) | 0.77 | |||
| E0/1(%) | 61(80.3)/15(19.7) | 56(90.3)/6(9.7) | 0.15 | |||
| S0/1(%) | 24(31.6)/52(68.4) | 31(50)/31(50) | 0.036* | |||
| T0/1/2(%) | 35(46.1)/19(25)/22(28.9) | 56(90.3)/4(6.5)/2(3.2) | 0.00000007* | |||
| C0/C1/C2(%) | 51(67.1)/23(30.3)/2(2.6) | 42(67.7)/20(32.3)/0(0) | 0.63 | |||
| Pathological Features | ||||||
| Global glomerulosclerosis % (mean) | 0-96.3 (28.2) | 0-63.6 (9.9) | 0.0000009* | |||
| Haas I/II/III/IV/V (%) | 17(22.4)/16(21.1)/28(36.8)/ | 25(40.3)/14(22.6)/21(33.9)/ | 0.01 | |||
| 7(9.2)/8(10.5) | 2(3.2)/0(0) | |||||
| IFTA 0/1/2/3(%) | 14(18.4)/23(30.3)/18(23.7)/ | 39(62.9)/15(24.2)/5(8.1)/ | 0.0000001* | |||
| 21(27.6) | 3(4.8) | |||||
| I IFTA 0/1/2/3(%) | 17(22.4)/23(30.3)/24(31.6)/ | 41(66.1)/15(24.2)/3(4.8)/ | 0.0000002* | |||
| 12(15.8) | 3(4.8) | |||||
| Ti 0/1/2/3(%) | 18(23.7)/32(42.1)/11(14.5)/ | 42(67.7)/16(25.8)/2(3.2)/ | 0.0000006* | |||
| 15(19.7) | 2(3.2) | |||||
BP, blood pressure; eGFR, estimated glomerular filtration rate; BSA, body surface area; UPCR, urine protein-to-creatinine ratio; BUN, blood urea nitrogen; Scr, serum creatinine; RAAS renin-angiotensin-aldosterone system; IFTA, interstitial fibrosis and tubular atrophy. I IFTA, inflammatory IFTA; Oxford classification: M, mesangial hypercellularity; S, segmental sclerosis; T, interstitial fibrosis/tubular atrophy; C, and crescents.
Table 2.
Clinicopathological characteristics according to fibrous intimal thickening
| Intimal thickening (+) | Intimal thickening (-) | p-value | |
|---|---|---|---|
| (n = 47) | (n = 86) | ||
| Clinical Information | |||
| Gender (%) | |||
| Male | 21(44.7) | 47(54.7) | 0.28 |
| Female | 26(55.3) | 39(45.3) | |
| Age (mean) | 24–74 (49) | 18–73 (37) | 0.000004* |
| Hypertension (%) | 21(44.7) | 27(31.4) | 0.14 |
| DM (%) | 1(2.1) | 3(3.5) | 1 |
| Systolic BP | 100–210 (130.1) | 78–172 (125.2) | 0.11 |
| mm Hg (mean) | |||
| Diastolic BP | 55–110 (77.1) | 46–120 (73.7) | 0.12 |
| mm Hg (mean) | |||
| eGFR | 9-120 (64.6) | 9-141 (88) | 0.0002* |
| mL/min/1.73m2 (mean) | |||
| UPCR | 0.2–8.18 (2.19) | 0.05–9.81 (1.29) | 0.003* |
| g/gCr (mean) | |||
| BUN | 10.3–64.1 (20.4) | 6–96 (17.5) | 0.2 |
| mg/dL (mean) | |||
| Scr | 0.5–4.92 (1.37) | 0.55–5.49 (1.18) | 0.2 |
| µmol/L (mean) | |||
| Uric acid | 4.4–11.4 (7) | 3.1–11.3 (6.3) | 0.06 |
| µmol/L (mean) | |||
| Hemoglobin | 8.7–17.1 (12.9) | 4–19 (13.6) | 0.07 |
| g/L (mean) | |||
| Platelet count | 149–603 (273.2) | 24–464 (250.3) | 0.13 |
| ×109 /L (mean) | |||
| Albumin | 2.1-5 (4) | 2.7–5.4 (4.3) | 0.006* |
| g/L (mean) | |||
| Total cholesterol | 138–360 (207) | 101–328 (182) | 0.003* |
| mmol/L (mean) | |||
| Medications | |||
| RAAS blockers (%) | 24(51.1) | 38(44.2) | 0.47 |
| Steroids and immunosuppressants (%) | 1(2.1) | 1(1.2) | 1 |
| Oxford classification score | |||
| M0/1(%) | 42(89.4)/5(10.6) | 80(93)/6(7) | 0.52 |
| E0/1(%) | 40(85.1)/7(14.9) | 73(84.9)/13(15.1) | 1 |
| S0/1(%) | 16(34)/31(66) | 36(41.9)/50(58.1) | 0.46 |
| T0/1/2(%) | 22(46.8)/11(23.4)/14(29.8) | 67(77.9)/10(11.6)/9(10.5) | 0.001* |
| C0/C1/C2(%) | 29(61.7)/16(34)/2(4.3) | 59(68.6)/27(31.4)/0(0) | 0.17 |
| Pathological Features | |||
| Global glomerulosclerosis, % (mean) | 0-96.3 (26.3) | 0–90 (16.2) | 0.01* |
| Haas I/II/III/IV/V(%) | 10(21.3)/12(25.5)/18(38.3)/ | 30(34.9)/16(18.6)/31(36)/ | 0.47 |
| 3(6.4)/4(8.5) | 5(5.8)/4(4.7) | ||
| IFTA 0/1/2/3(%) | 8(17)/15(31.9)/9(19.1)/ | 44(51.2)/22(25.6)/12(14)/ | 0.0002* |
| 15(31.9) | 8(9.3) | ||
| I IFTA 0/1/2/3(%) | 13(27.7)/16(34)/10(21.3)/ | 43(50)/21(24.4)/15(17.4)/ | 0.066 |
| 8(17) | 7(8.1) | ||
| Ti 0/1/2/3(%) | 13(27.7)/17(36.2)/6(12.7)/11(23.4) | 45(52.3)/30(34.9)/5(5.8)/ | 0.006* |
| 11(23.4) | 6(7) |
BP, blood pressure; eGFR, estimated glomerular filtration rate; BSA, body surface area; UPCR, urine protein-to-creatinine ratio; BUN, blood urea nitrogen; Scr, serum creatinine; RAAS renin-angiotensin-aldosterone system; IFTA, interstitial fibrosis and tubular atrophy. I IFTA, inflammatory IFTA; Oxford classification: M, mesangial hypercellularity; S, segmental sclerosis; T, interstitial fibrosis/tubular atrophy; C, and crescents.
Table 3.
Clinicopathologic characteristics according to vascular lesions
| Vascular Lesions (+) | Vascular Lesions (-) | p-value | |
|---|---|---|---|
| (n = 88) | (n = 50) | ||
| Clinical Information | |||
| Gender (%) | |||
| Male | 42(47.7) | 28(56) | 0.38 |
| Female | 46(52.3) | 22(44) | |
| Age (mean) | 19–74 (46) | 18–73 (32.5) | 0.00000005* |
| Hypertension (%) | 40(45.5) | 10(20) | 0.003* |
| DM (%) | 5(5.7) | 0(0) | 0.16 |
| Systolic BP | 98–210 (127.8) | 78–172 (124.5) | 0.28 |
| mm Hg (mean) | |||
| Diastolic BP | 46–120 (76.4) | 46–90 (71.6) | 0.02* |
| mm Hg (mean) | |||
| eGFR | 9-135 (66.8) | 15–155 (102.8) | 0.000000001* |
| mL/min/1.73m2 (mean) | |||
| UPCR | 0.09–13.12 (2.0) | 0.05–5.58 (1.0) | 0.004* |
| g/gCr (mean) | |||
| BUN | 6–96 (20.7) | 5.8–62.3 (14.8) | 0.005* |
| mg/dL (mean) | |||
| Scr | 0.5–5.12 (1.39) | 0.49–5.49 (1) | 0.01* |
| µmol/L (mean) | |||
| Uric acid | 3.1–11.4 (6.9) | 3.5–11.3 (6) | 0.009* |
| µmol/L (mean) | |||
| Hemoglobin | 8.4–17.1 (13.2) | 4–19 (13.7) | 0.12 |
| g/L (mean) | |||
| Platelet count | 24–603 (257.7) | 157–462 (262.8) | 0.7 |
| ×109 /L (mean) | |||
| Albumin | 1.9–5.1 (4.1) | 2.7–5.4 (4.4) | 0.008* |
| g/L (mean) | |||
| Total cholesterol | 101–360 (202.4) | 102–243 (170.1) | 0.00009* |
| mmol/L (mean) | |||
| Medications | |||
| RAAS blockers (%) | 48(54.5) | 14(28) | 0.004* |
| Steroids and immunosuppressants (%) | 2(2.3) | 0(0) | 0.5 |
| Oxford classification score | |||
| M0/1(%) | 80(90.9)/8(9.1) | 46(92)/4(8) | 1 |
| E0/1(%) | 73(83)/15(17) | 44(88)/6(12) | 0.47 |
| S0/1(%) | 31(35.2)/57(64.8) | 24(48)/26(52) | 0.15 |
| T0/1/2(%) | 44(50)/21(23.9)/23(26.1) | 47(94)/2(4)/1(2) | 0.0000002* |
| C0/C1/C2(%) | 60(68.2)/26(29.5)/2(2.3) | 33(66)/17(34)/0(0) | 0.62 |
| Pathological Features | |||
| Global glomerulosclerosis, % (mean) | 0-96.3 (26.8) | 0-51.9 (8) | 0.000001* |
| Haas I/II/III/IV/V (%) | 21(23.9)/21(23.9)/31(35.2)/ | 21(42)/9(18)/18(36)/ | 0.049 |
| 7(8)/8(9) | 2(4)/0(0) | ||
| IFTA 0/1/2/3(%) | 17(19.3)/29(33)/19(21.6)/ | 36(72)/9(18)/4(8)/ | 0.000000004* |
| 23(26.1) | 1(2) | ||
| I IFTA 0/1/2/3(%) | 22(25)/28(31.8)/24(27.3)/ | 36(72)/10(20)/3(6)/ | 0.0000004* |
| 14(15.9) | 1(2) | ||
| Ti 0/1/2/3(%) | 22(25)/38(43.2)/11(12.5)/ | 38(76)/10(20)/2(4)/ | 0.00000001* |
| 17(19.3) | 0(0) | ||
BP, blood pressure; eGFR, estimated glomerular filtration rate; BSA, body surface area; UPCR, urine protein-to-creatinine ratio; BUN, blood urea nitrogen; Scr, serum creatinine; RAAS renin-angiotensin-aldosterone system; IFTA, interstitial fibrosis and tubular atrophy. I IFTA, inflammatory IFTA; Oxford classification: M, mesangial hypercellularity; S, segmental sclerosis; T, interstitial fibrosis/tubular atrophy; C, and crescents.
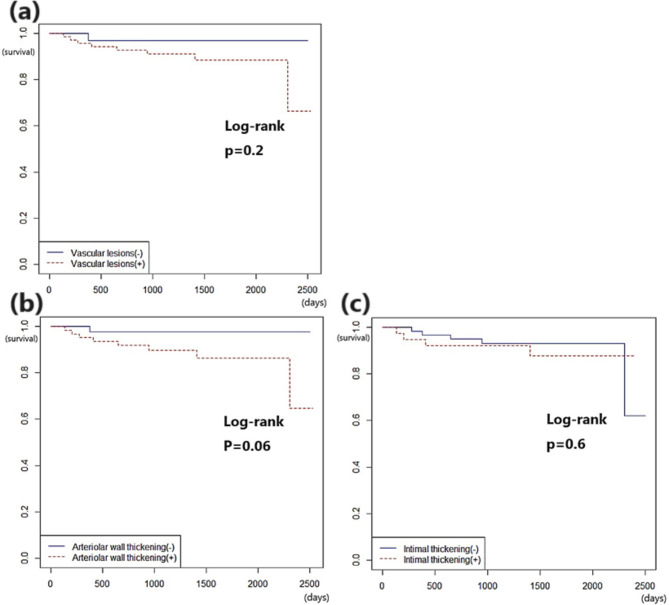
Fig. 2.
Kaplan–Meier curves of Immunoglobulin A nephropathy (IgA) according to vascular lesions. (a) vascular lesions, (b) arteriolar wall thickening, (c) fibrous intimal thickening
Table 4.
Univariate hazards model of survival according to clinicopathological characteristics
| HR (95% CI) | p-value | |
|---|---|---|
| Vascular changes | ||
| Any vascular lesions | 3.89 (0.49–31.09) | 0.2 |
| Arteriolar wall thickening | 5.76 (0.72–46.2) | 0.1 |
| Fibrous intimal thickening | 1.37 (0.37–5.15) | 0.6 |
| Pathological Features | ||
| Global glomerulosclerosis | 1.05 (1.03–1.07) | 0.00006* |
| Clinical Information | ||
| Age | 1.05 (0.99–1.1) | 0.067 |
| eGFR | 0.93 (0.9–0.97) | 0.0004* |
| UPCR | 1.15 (0.96–1.37) | 0.13 |
| BUN | 1.14 (1.08–1.21) | 0.000003* |
| Serum creatinine | 3.264 (2.12–5.03) | 0.00000009* |
| Albumin | 0.37 (0.17–0.81) | 0.013* |
| Duration of hypertension prior to biopsy | 1 (0.99–1.01) | 0.58 |
| Duration of kidney injury prior to biopsy | 0.99 (0.97–1.01) | 0.31 |
HR, hazard ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ratio; BUN, blood urea nitrogen.
Table 5.
Multivariate hazards model of survival according to clinicopathological characteristics
| Multivariate hazard model | |||
|---|---|---|---|
| Arteriolar wall thickening (N = 103) | Co-variable | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value |
| Global glomerulosclerosis | |||
| 1.66 (0.17–16.22) | 0.66 | 1.05 (1.02–1.07) | 0.0006* |
| eGFR | |||
| 1.76 (0.21–14.98) | 0.6 | 0.93 (0.9–0.97) | 0.0007* |
| Hypertension history (+) | |||
| 5.85 (0.71–47.88) | 0.1 | 0.94 (0.24–3.62) | 0.92 |
| Serum creatinine | |||
| 4.28 (0.51–35.85) | 0.18 | 3.28 (2.1–5.13) | 0.0000002* |
| Fibrous intimal thickening(N = 99) | Co-variable | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value |
| Global glomerulosclerosis | |||
| 0.78 (0.19–3.16) | 0.73 | 1.05 (1.03–1.08) | 0.00007* |
| eGFR | |||
| 1.14 (0.28–4.6) | 0.86 | 0.92 (0.87–0.96) | 0.0003* |
| Hypertension history (+) | |||
| 1.34 (0.35–5.06) | 0.67 | 1.23 (0.32–4.67) | 0.76 |
| Serum creatinine | |||
| 9.2 (0.98-86) | 0.052 | 5.39 (2.54–11.43) | 0.00001* |
| Any vascular lesions(N = 103) | Co-variable | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value |
| Global glomerulosclerosis | |||
| 0.8 (0.07–8.88) | 0.86 | 1.05 (1.02–1.08) | 0.0003* |
| eGFR | |||
| 1.1 (0.13–9.14) | 0.93 | 0.93 (0.9–0.97) | 0.0004* |
| Hypertension history (+) | |||
| 3.99 (0.48–33.38) | 0.2 | 0.93 (0.24–3.62) | 0.91 |
| Serum creatinine | |||
| 3.61 (0.42–31.2) | 0.24 | 3.36 (2.15–5.26) | 0.0000001* |
HR, hazard ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
Results
The cohort comprised 70 male and 68 female patients, with a male-to-female ratio of 1:0.97. The mean age was 41.1 years (range, 18–74 years). Hypertension (HTN) was defined as a diastolic blood pressure of 90 mmHg or higher, or a systolic blood pressure of 140 mmHg or higher, measured on at least two occasions. The mean systolic blood pressure was 126.6 mmHg, and 50 patients (36.2%) had a history of hypertension. The distributions across the Haas classification classes I, II, III, IV, and V were 30.4%, 21.7%, 35.5%, 6.5%, and 5.8%, respectively. The mean eGFR 79.8 ml/min/1.73m2 and the mean UPCR was 1.6 g/gCr.
Clinical and pathologic features related to arteriolar wall thickening
Clinical and pathologic features related to arteriolar wall thickening are summarized in Table 1. Old age, a history of hypertension, high diastolic blood pressure, low eGFR, high UPCR, high serum creatinine, low albumin, high total cholesterol, and use of renin-angiotensin-aldosterone system (RAAS) blockers were associated with arteriolar wall thickening. In the Oxford classification, high S scores and T scores were associated with arteriolar wall thickening. Moreover, global glomerulosclerosis, high interstitial fibrosis and tubular atrophy (IFTA) grade, high inflammatory IFTA grade, and high total inflammation score were also associated with arteriolar wall thickening.
Clinical and pathological features related to fibrous intimal thickening
The clinical and pathological features related to fibrous intimal thickening are summarized in Table 2. Five people were excluded because of the absence of available vessels. Similarly, old age, low eGFR, high UPCR, low albumin, and high total cholesterol were related to fibrous intimal thickening. In the Oxford classification, a high T-score was observed. Moreover, global glomerulosclerosis, high IFTA grade, and total inflammation score were related to fibrous intimal thickening.
Clinical and pathological features according to any vascular lesions
The clinical and pathological features according to any vascular lesions are summarized in Table 3. If the specimen exhibited at least one vascular lesion (arteriolar wall thickening, arteriolar hyalinosis, or fibrous intimal thickening), it was categorized as a vascular lesion (+). In the cohort, 88 specimens had vascular lesions and 50 had no vascular lesions. The vascular lesion (+) group was associated with old age, a history of hypertension, high diastolic blood pressure, low eGFR, high UPCR, high BUN, high serum creatinine, high uric acid, low albumin, high total cholesterol, and the use of renin-angiotensin-aldosterone system (RAAS) blockers. Specimens with vascular lesions had a high T-score in the Oxford classification. Moreover, global glomerulosclerosis, high IFTA grade, high inflammatory IFTA grade, and high total inflammation score were associated with vascular lesions.
Vascular lesions and prognosis
Among the 138 enrolled patients, 103 patients had follow-up data (mean follow-up date: 1404.9 days), and during that period, nine patients reached the endpoint (the occurrence of end-stage renal disease, eGFR < 15 mL/min/1.73 m², requiring kidney replacement). The univariate Cox regression analysis showed that global glomerulosclerosis, low eGFR, high BUN, high serum creatinine, and low albumin were associated with eventual progression to end-stage renal disease (Table 4). The vascular lesion was adjusted for several significant covariates in univariate analysis, including global glomerulosclerosis, eGFR, HTN, and serum creatinine, respectively, using multivariate Cox regression analysis. The results are presented in Table 5. The vascular lesion could not predict prognosis after adjustment for clinical covariates. Only fibrous intimal thickening showed marginal significance for prognosis after adjustment for serum creatinine (HR: 9.2; p = 0.052). Global glomerulosclerosis, eGFR and serum creatinine levels were associated with progression to end-stage renal disease after adjustment for any vascular lesions, arteriolar wall thickening, and fibrous intimal thickening. According to the log-rank test, arterial wall thickening group showed a poorer outcome than the negative group with marginal significance (Fig. 2b log-rank test: p = 0.06). However, any vascular lesions did not show a direct relationship with the occurrence of end-stage renal disease (Fig. 2a, Log-rank test: p = 0.2). Fibrous intimal thickening also showed no significant relationship with prognosis. (Fig. 2c, log-rank test: p = 0.6).
Discussion
Vascular lesions are frequently observed in renal biopsy specimens from patients with IgAN. However, the prognostic significance of these lesions remains unclear. Our study indicates that arteriolar wall thickening and fibrous intimal thickening are associated with a reduced eGFR and increased UPCR. These lesions also correlated with the presence of global sclerosis and tubular atrophy, consistent with the findings of other studies [8–11]. Notably, global sclerosis and high serum creatinine levels were observed and eventually lead to patients requiring dialysis, thereby suggesting the potential involvement of vasculopathies in the progression or prognosis of IgAN. Although our study was cross-sectional, the presence of these lesions implies the need for an aggressive treatment approach in affected patients.
The results indicate that vascular lesions are related to hypertension, and decreased eGFR aligns with the findings of other studies [12–15]. While this association was also observed in the original Oxford cohort, it did not exhibit a significant relationship with kidney failure events, and as a result, vascular lesions were not included in the classification criteria. In our study, vascular lesions did not show a significant relationship with the occurrence of end-stage renal disease. Even when analyzed separately for arteriolar wall thickening and fibrous intimal thickening, no significant relationship was found. However, a case-control study by Huang et al. reported a significant relationship between the presence of vascular lesions and the transition to end-stage renal disease or death in patients with IgAN [15]. The discrepancy in the results may be attributed to the relatively small number of cases in our study, which resulted in a limited number of endpoint events. Nonetheless, in our study, arterial wall thickening showed a marginally significant correlation with the endpoint, and we hypothesized that similar results could be obtained with an increased sample size and comprehensive eGFR follow-up data.
The current treatment approach for IgAN aims to decelerate renal damage progression, encompassing blood pressure and proteinuria control, through the use of RAAS blockade. In cases where serum creatinine levels are elevated and biopsy reveals features, such as endocapillary hypercellularity or crescents, or if there is proteinuria in the nephrotic range, immunosuppressors, such as steroids, may be employed [16]. In our study, individuals with vascular lesions had a high prevalence of hypertension. Vascular lesions are associated with hypertension. However, among individuals with vascular lesions, 45.5% were diagnosed with hypertension, leaving a substantial proportion without hypertension. This suggests that although high blood pressure may contribute to vascular lesions, it is also plausible that vascular lesions could lead to secondary hypertension. Further research on the impact of nonhypertensive intrarenal vascular lesions on the disease course could contribute to a more comprehensive understanding. If a robust correlation is established, interventions aimed at slowing the progression of IgAN, based on biopsy findings before the onset of proteinuria or hypertension, may become a viable treatment strategy.
Conclusion
Intrarenal vascular lesions in IgAN are linked to reduced eGFR and global glomerulosclerosis. Moreover, reduced eGFR and global glomerulosclerosis are correlated with the progression to end-stage renal disease. Although the direct correlation between vascular lesions and end-stage renal disease is not entirely clear, a marginally significant association (log-rank test, p = 0.06) was observed with arterial wall thickening. This study suggests the potential importance of vascular lesions in the prognosis of IgAN, encouraging further investigation using large cohort studies to establish a clarified association.
Acknowledgements
None.
Author contributions
Conception and design of study: Yong-Jin Kim, Man-Hoon HanAcquisition of data: Sun-Hee Park, Jeong-Hoon LimAnalysis and/or interpretation of data: Mee-seon KimDrafting the manuscript: Hyeon Tae Yang, Mee-seon KimRevising the manuscript critically for important intellectual content: Man-Hoon Han, Tae In Park, Yoo Na Kang, DongJa Kim.
Funding
None.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by Institutional Review Board (IRB) of Kyungpook National University Hospital, Daegu, Korea (IRB FILE No. KNUH 2023-12-017, 8 January 2024) and has been conducted in full accordance with the Declaration of Helsinki. The study was retrospective therefore the Institutional Review Board (IRB) of Kyungpook National University Hospital, Daegu, Korea (IRB FILE No. KNUH 2023-12-017, 8 January 2024) waived the need for written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rodrigues JC, Haas M, Reich HN, Nephropathy IA. Clin J Am Soc Nephrol. 2017;12:677–86. 10.2215/cjn.07420716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 2017;91:1014–21. 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42. 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Chen X, Xie Y, Yamanaka N, Shi S, Wu D, Liu S, Cai G. Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transpl. 2005;20:719–27. 10.1093/ndt/gfh716. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS. Chronic kidney Disease Prognosis C: comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51. 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29(6):829 – 42. 10.1016/s0272-6386(97)90456-x. PMID: 9186068. [DOI] [PubMed]
- 7.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J, IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–1021. doi: 10.1016/j.kint.2017.02.003. Epub 2017 Mar 22. PMID: 28341274. [DOI] [PubMed]
- 8.Zhang Y, Sun L, Zhou S, Xu Q, Xu Q, Liu D, Liu L, Hu R, Quan S, Xing G. Intrarenal arterial lesions are associated with higher blood pressure, reduced renal function and poorer renal outcomes in patients with IgA nephropathy. Kidney Blood Press Res. 2018;43:639–50. 10.1159/000489290. [DOI] [PubMed] [Google Scholar]
- 9.Chen FF, Yu XJ, Wang H, Zhang X, Tan Y, Qu Z, Wang SX, Yu F, Chen M, Zhao MH. Clinical value of the renal pathologic scoring system in complement-mediated thrombotic microangiopathy. Ren Fail. 2023;45:2161396. 10.1080/0886022x.2022.2161396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Shi S, Wang S, Ren Y, Hou W, Liu L, Lv J, Haas M, Zhang H. Microangiopathic lesions in IgA nephropathy: a cohort study. Am J Kidney Dis. 2019;74:629–39. 10.1053/j.ajkd.2019.03.416. [DOI] [PubMed] [Google Scholar]
- 11.Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, Chen N, Pan X, Fu P, Hu Z, Wang L, Fan Q, Zheng H, Zhang D, Wang Y, Huo Y, Lin H, Chen S, Sun S, Wang Y, Liu Z, Liu D, Ma L, Pan T, Zhang A, Jiang X, Xing C, Sun B, Zhou Q, Tang W, Liu F, Liu Y, Liang S, Xu F, Huang Q, Shen H, Wang J, Shyr Y, Phillips S, Troyanov S, Fogo A, Liu Z-H. A multicenter application and evaluation of the Oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–20. 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts ISD, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R. Perkowska-Ptasinska VALIGA study of the ERA-EDTA Immunonephrology Working Group, Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–36. 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA, Fervenza FC, Cattran DC. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80:310–7. 10.1038/ki.2011.126. [DOI] [PubMed] [Google Scholar]
- 14.Shi SF, Wang SX, Jiang L, Lv J-C, Liu L-J, Chen Y-Q, Zhu S-N, Liu G, Zou W-Z, Zhang H, Wang H-Y. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the Oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–84. 10.2215/cjn.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Hu Y, Chen B, Liang Y, Li D, Qiu W, Zhang J, Chen C. Clinical significance of intrarenal vascular lesions in non-hypertensive patients with IgA nephropathy. J Nephrol. 2023;36:429–40. 10.1007/s40620-022-01511-w. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease. Improving global outcomes (KDIGO) Glomerular Diseases Work Group, KDIGO 2021 Clinical Practice Guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–276. 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.