Abstract
The iminosugar N-butyldeoxynojirimycin (NB-DNJ), an endoplasmic reticulum α-glucosidase inhibitor, has an antiviral effect against bovine viral diarrhea virus (BVDV). In this report, we investigate the molecular mechanism of this inhibition by studying the folding pathway of BVDV envelope glycoproteins in the presence and absence of NB-DNJ. Our results show that, while the disulfide-dependent folding of E2 glycoprotein occurs rapidly (2.5 min), the folding of E1 occurs slowly (30 min). Both BVDV envelope glycoproteins associate rapidly with calnexin and dissociate with different kinetics. The release of E1 from the interaction with calnexin coincides with the beginning of E1 and E2 association into disulfide-linked heterodimers. In the presence of NB-DNJ, the interaction of E1 and E2 with calnexin is prevented, leading to misfolding of the envelope glycoproteins and inefficient formation of E1-E2 heterodimers. The degree of misfolding and the lack of association of E1 and E2 into disulfide-linked complexes in the presence of NB-DNJ correlate with the dose-dependent antiviral effect observed for this iminosugar.
Bovine viral diarrhea virus (BVDV) is a pestivirus member of the Flaviviridae family, which also comprises the genera Flavivirus and Hepacivirus (24). In the absence of an efficient cell culture system able to support hepatitis C virus (HCV) replication, BVDV has been adopted as a model and surrogate for HCV (1), as both viruses share molecular and virological features. HCV and BVDV are small, enveloped viruses with positive single-stranded RNA genomes of approximately 9,600 and 12,600 nucleotides, respectively. The polypeptide precursor is transcribed from a single large open reading frame and subsequently co- and posttranslationally processed into structural and nonstructural proteins. Most BVDV and HCV proteins are functionally homologous. The envelope glycoproteins E1 and E2 interact either noncovalently (HCV) (7) or through disulfide bonds (BVDV) (26, 28) to form a dimer, which has been proposed as the functional complex present on the surfaces of mature virions.
We have previously shown that endoplasmic reticulum (ER) α-glucosidase inhibitors containing the glucose analogue deoxynojirimycin (DNJ) as the head group have a strong antiviral effect on BVDV (32). Castanospermine (CST), another α-glucosidase inhibitor, was shown to cause misfolding of recombinant HCV E1 and E2 glycoproteins expressed in BHK-21 cells and reduce their association into native dimers (5). Similarly, CST affected the morphogenesis and assembly of Dengue virus (DENV), another member of the Flaviviridae family (6).
The ER α-glucosidases perform the stepwise removal of the three glucose residues on N-linked glycans attached to nascent polypeptides. This removal enables folding intermediates to associate with the lectin-like ER chaperones calnexin and calreticulin, which interact with monoglucosylated glycoproteins and which retain incompletely folded polypeptides and oligomers in the ER (2, 23). However, not all cellular proteins are dependent on the α-glucosidase-mediated folding pathway, and certain cell lines lacking α-glucosidase expression are viable (22). Importantly, the folding of certain viral glycoproteins has been shown to be calnexin dependent (19, 20, 29). Thus, targeting the ER α-glucosidases may potentially be of therapeutic use in treating viral infections, without affecting host cell viability. In addition, since the enzymes are host cell and not virus encoded, emergence of drug-resistant viruses is less likely to occur.
While the folding of recombinant HCV envelope proteins has been thoroughly investigated and its dependence upon the calnexin-mediated pathway has been clearly established (9), nothing is known about the folding of BVDV-encoded glycoproteins. Characterization of the folding pathway of the BVDV glycoproteins is important if we want to use BVDV as a model system for HCV to study drugs which interfere with the assembly and secretion of the envelope proteins. BVDV (National Animal Disease Laboratory [NADL] strain) encodes three envelope glycoproteins, Erns, E1, and E2, which carry eight, two, and four potential N-glycosylation sites, respectively (24). Unlike E1 and E2, Erns lacks a membrane anchor and is secreted from infected cells. In this paper we investigate the folding of the BVDV envelope glycoproteins E1 and E2 and the role played by the ER chaperones calnexin and calreticulin in this process. To this end, we have monitored the kinetics of intra- and intermolecular disulfide bond formation and the association of E1 and E2 glycoproteins into heterodimers. The mechanism of the sensitivity of BVDV to α-glucosidase inhibition was also examined. The usefulness of BVDV as a model system for screening anti-HCV drugs is discussed and compared to that of the HCV system available.
MATERIALS AND METHODS
Cell culture, virus, inhibitors, and enzymes.
Noncytopatic BVDV-free MDBK cells (European Collection of Animal Cell Cultures, Porton Down, United Kingdom) and cytopathic BVDV virus (NADL strain; American Type Culture Collection, Manassas, Va.) were used in this study. MDBK cells were grown in RPMI 1640 medium (GIBCO/BRL) supplemented with 10% BVDV-free fetal calf serum (PAA Laboratories, Teddington, United Kingdom). N-Butyl-DNJ (NB-DNJ) was a gift from Searle/Monsanto. N-Butyldeoxygalactojirimycin (NB-DGJ) was purchased from Boehringer Mannheim. The inhibitors were made up as 200 mM stock solutions in water and filtered before use. Partially purified ER α-glucosidases I and II were kindly provided by T. Butters (Oxford Glycobiology Institute).
Antibodies.
Monoclonal antibodies (MAbs) 158 and 214 raised against BVDV E2 glycoprotein were purchased from the Veterinary Laboratories Agency, Weybridge, United Kingdom. The anticalnexin and anticalreticulin polyclonal antibodies were purchased from Bioquote Limited, York, United Kingdom. The antirabbit and antimouse horseradish peroxidase-conjugated secondary antibodies were from Sigma.
BVDV plaque reduction assay.
MDBK cells were grown to subconfluent monolayers in six-well plates and infected with cytopathic BVDV at a multiplicity of infection (MOI) of 1 PFU/cell for 1 h at 37°C. After the inoculum was removed, the cells were washed with phosphate-buffered saline (PBS) and incubated for 3 days in the presence or absence of inhibitors. The medium containing secreted virus was then removed from the wells, centrifuged at low speed to remove cellular debris, and used to infect fresh monolayers of MDBK cells grown in six-well plates. The resulting plaques were counted after 2 days.
Western blotting.
Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to nitrocellulose membranes using a semidry electroblotter (Millipore) and detected with anti-BVDV E2 antibodies (dilution, 1/1,000) or anticalnexin antibodies (dilution, 1/4,000) followed by antimouse (dilution, 1/2,000) or antirabbit (dilution, 1/10,000) antibodies conjugated to horseradish peroxidase. The proteins were detected using an enhanced-chemiluminescence detection system (Amersham) by following the manufacturer's instructions.
α-Glucosidase digestion.
MDBK cells were infected with BVDV at an MOI of 1 and treated or not treated with 2 mM NB-DNJ. Twenty milligrams of total cellular extract was incubated overnight at 37°C using 5 U (each) of ER α-glucosidases I and II. Samples were analyzed by SDS–10% PAGE and stained for Western blotting with anti-E2 antibodies.
Pulse-labeling and chase.
Subconfluent MDBK cell monolayers grown in 25-cm2 flasks were infected with BVDV at an MOI of 1. After 1 h of incubation at 37°C, the viral inoculum was replaced with medium containing 10% fetal calf serum. Eighteen hours postinfection (p.i.), the monolayers were washed once with PBS and incubated in methionine- and cysteine-free RPMI 1640 medium (ICN Flow, Thame, Oxfordshire, United Kingdom). After 1 h, the cells were pulse-labeled with 100 μCi of [35S]methionine-[35S]cysteine (Tran 35S-label, 1,100 Ci/mmol; ICN Flow) per ml at 37°C for the times indicated in the figures. Following labeling, the isotope-supplemented medium was removed and the cells were washed once with PBS and chased for various times in RPMI 1640 medium containing 10 mM unlabeled methionine. At the time points indicated in the figures, the chase media were discarded and the cells were harvested. For experiments in which the association of BVDV envelope proteins with calnexin or calreticulin was analyzed, the cells were treated with actinomycin D (4 μg/ml) before being labeled. When the effect of the inhibition of ER α-glucosidases I and II was investigated, NB-DNJ was added to the cells 2 h before the pulse at the concentrations indicated in Results and was present throughout the chase period. Cells were then lysed for 1 h on ice in a buffer containing 0.5% Triton X-100, 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, and 2 mM EDTA (Triton-TSE buffer) and a mixture of protease inhibitors (Sigma). In experiments in which the formation of disulfide bonds was monitored, 20 mM iodoacetamide was included in the lysis buffer to alkylate free sulfhydryl groups and avoid unspecific aggregation. When the interaction with calnexin or calreticulin was studied, cell lysis was performed under mild conditions using a CHAPS-HSE buffer (2% CHAPS {3-[(3-chloramidopropyl)-dimethylammonio]-1-propanesulfonate} in 50 mM HEPES [pH 7.5]–200 mM NaCl–2 mM EDTA).
Immunoprecipitation and SDS-PAGE.
Labeled cell lysates were clarified by centrifugation at 12,000 × g for 15 min and precleared with 20 μl of either protein A-Sepharose (when polyclonal antibodies were used) or protein G-Sepharose (when MAbs were used) for 1 h at 4°C. The lysates were then briefly centrifuged, and the supernatants were incubated with either anti-BVDV E2 or antichaperone antibodies (diluted 1:50 or 1:200, respectively) overnight at 4°C. Protein A- or G-Sepharose (30 μl) was then added to the supernatants, and the incubation continued for 1 h at 4°C. The slurry was washed six times with 0.2% Triton X-100 in TSE buffer. The washing buffer was replaced by 0.5% CHAPS in HSE buffer for immunoprecipitation with anticalnexin or -calreticulin antibodies. For coimmunoprecipitation experiments, the lysates were first immunoprecipitated with anticalnexin or -calreticulin antibodies (diluted 1:200); the slurry was then washed twice in 0.5% CHAPS–HSE buffer, and bound proteins were eluted by boiling the samples in 1% SDS. The eluates were diluted 10 times with washing buffer and reprecipitated with anti-E2 antibodies (diluted 1:50). The immunoprecipitated complexes were eluted by boiling the samples for 10 min in SDS-PAGE sample buffer, in the presence (reducing conditions) or absence (nonreducing conditions) of 5% 2-mercaptoethanol. Samples were either quantified by liquid scintillation counting or separated by SDS-PAGE. After electrophoresis, the gels were treated with Amplyfier (Amersham), dried, and exposed at −70°C to Hyperfilm-MP (Amersham). The intensities of the bands on the resulting autoradiograms were measured by scan densitometry.
RESULTS
Folding and processing of BVDV envelope proteins in MDBK cells.
The presence of intermolecular disulfide bridges between structural proteins encoded by pestiviruses has been previously shown for classical swine fever virus (CSFV) and the Osloss strain of BVDV (26, 28). Both CSFV gp55 and BVDV gp53 (E2) form homo- and heterodimers, which are also present in mature virions (26). However, the folding of the BVDV envelope glycoproteins involving the formation of intramolecular disulfide bonds, as well as the kinetics of the subsequent association into heterodimers, has not been investigated.
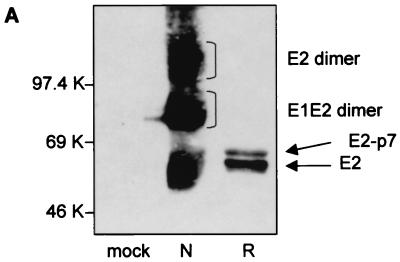
The BVDV strain used in this study is an NADL strain. To determine whether this strain also forms disulfide-linked dimers, lysates of infected MDBK cells were analyzed by SDS-PAGE under both reducing and nonreducing conditions, followed by Western blotting and staining of the membrane with an MAb against the E2 protein (MAb 214). To avoid formation of nonspecific disulfide bonds, free sulfhydryl groups were blocked by the addition of iodoacetamide, an alkylating reagent, before and during lysis. As shown in Fig. 1A (lane N), three broad bands could be detected under nonreducing conditions with apparent molecular masses of 55, 80, and 100 kDa, corresponding to E2 monomers, E1-E2 dimers, and E2-E2 dimers, respectively. Under reducing conditions, only two bands, which migrated slightly more slowly than E2 monomers under nonreducing conditions, were detected (Fig. 1A, lane R). The molecular weights of these bands and their reactivities with MAb 214 are consistent with them being E2 and its uncleaved precursor E2-p7.
FIG. 1.
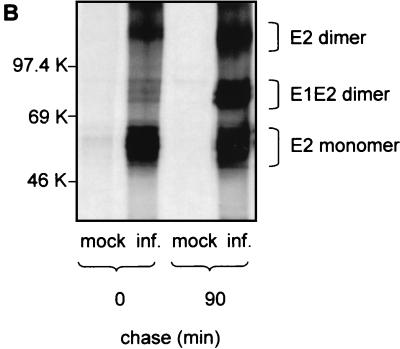
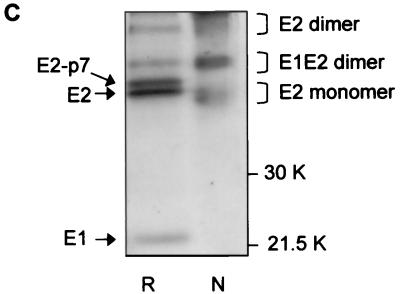
Analysis of BVDV envelope glycoproteins expressed in MBDK cells. (A) MBDK cells were infected with BVDV (NADL strain) at an MOI of 1. Eighteen hours p.i. the cells were lysed and analyzed by SDS–10% PAGE under nonreducing (lane N) and reducing (lane R) conditions, followed by Western blot analysis with MAb 214. (B) MBDK cells were infected with BVDV at an MOI of 1. Eighteen hours p.i. the cells were pulse-labeled with [35S]methionine and [35S]cysteine for 15 min, chased for the times indicated, lysed, and immunoprecipitated with MAb 214. Immunoprecipitated proteins were separated by SDS–10% PAGE under nonreducing conditions and analyzed by autoradiography. (C) Conditions were the same as described for panel B, except that the sample at min 90 of chase was analyzed by SDS–12% PAGE, under both nonreducing and reducing conditions. Mock-infected cells (mock inf.) were included as controls.
To look at the formation of the dimers, a pulse-chase experiment was performed under nonreducing conditions. Immediately after the 15-min pulse, E2 was immunoprecipitated from infected cells mainly in its monomeric form, while 90 min later additional complexes corresponding to E1-E2 and E2-E2 dimers could be detected with MAb 214 (Fig. 1B). When the sample that immunoprecipitated 90 min postpulse was treated with 2-mercaptoethanol prior to SDS-PAGE, the complexes were reduced to E2 or E2-p7 monomers (Fig. 1C). This result shows that E2, as well as E2-p7, is covalently linked into homo- and heterodimers by intermolecular disulfide bonds. An additional band with an apparent molecular mass of 25 kDa appeared after the sample was reduced (Fig. 1C). Anti-BVDV E1 antibodies are currently not available, which makes the precise identification of this coprecipitating band difficult. However, a protein with the same electrophoretic mobility as that expected for glycosylated E1 has also been observed after immunoprecipitation of infected MBDK cells using bovine serum containing polyclonal anti-BVDV antibodies (data not shown), strongly suggesting that E1 is indeed the protein coprecipitating with E2.
The two additional bands which can be seen in lane R (Fig. 1C) comigrating with E1-E2 and E2 dimers are immunoprecipitation-specific contaminants and are not seen in Western blots (e.g., lane R in Fig. 1A).
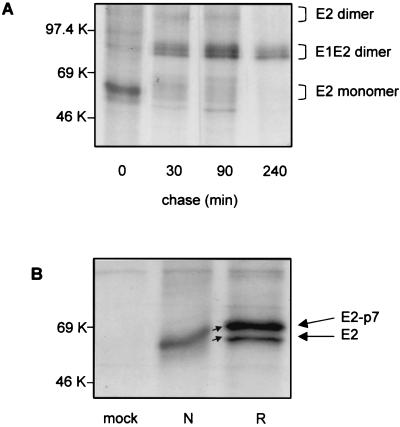
To further characterize the interaction between the two envelope glycoproteins and to determine the kinetics of their association, a pulse-chase experiment of infected cells was performed, followed by immunoprecipitation with MAb 214 and SDS-PAGE under nonreducing conditions (Fig. 2A). The amount of E2 monomers detected immediately after the 15-min pulse slowly decreased at min 30 and 90 of chase and eventually disappeared by min 240, accompanied by a parallel increase in the intensity of the bands corresponding to E1-E2 dimers. E2-E2 dimers were present as diffuse, constant bands between min 30 and 90, and the intensity of their bands decreased by min 240 of the chase, when the major viral products detected in infected cells were E1-E2 heterodimers. This finding suggests that the infected cells produce mainly E1-E2 heterodimers, which have also been shown to be the predominant complex incorporated into the envelopes of mature CSFV virions.
FIG. 2.
Analysis of intra- and intermolecular disulfide bond formation of BVDV envelope glycoproteins. MBDK cells were infected with BVDV at an MOI of 1. Eighteen hours p.i., the cells were either pulse-labeled with [35S]methionine and [35S]cysteine for 15 min and chased for the times indicated (A) or pulse-labeled for 2.5 min and harvested immediately (B). Cell lysates were immunoprecipitated with MAb 214, and proteins bound were analyzed by SDS–10% PAGE under nonreducing conditions (A) and both nonreducing (lane N) and reducing (lane R) conditions (B). Mock-infected cells (mock) were included as controls.
Disulfide bond formation can be determined by monitoring the mobilities of proteins on SDS-PAGE under nonreducing conditions, since proteins when stabilized by disulfide bonds into compact forms migrate faster than their reduced and less compact counterparts (4). With 17 cysteine residues (11 more than are present in E1), the E2 glycoprotein may also form intramolecular disulfide bonds. The upward shift in mobility between reduced and nonreduced E2 monomers (Fig. 1A and C) indicates that this is indeed the case. However, the mobility of E2 monomers does not increase between min 0 and 30 postpulse (Fig. 2A), suggesting that as early as 15 min postsynthesis, E2 has acquired a compact form.
To investigate the formation of E2 intramolecular disulfide bonds, infected cells were pulse-labeled for only 2.5 min. The proteins immunoprecipitated with MAb 214 were then analyzed under nonreducing and reducing conditions on the same gel. As shown in Fig. 2B, both E2 and E2-p7 could be detected and the shift in electrophoretic mobility was already apparent after only 2.5 min of labeling. After this initial fast intramolecular disulfide bond formation, the monomers do not undergo any further shift. Interestingly, no higher-molecular-size precursors containing the E2 polypeptide could be detected in the immunoprecipitated samples, suggesting that intramolecular disulfide bond formation of E2 is rapid and occurs at the same time or shortly after the proteolytic processing of E2-p7-NS2. Also, the appearance of a stable, compact form of E2-p7 during the 2.5 min of labeling suggests that cleavage between E2 and p7 does not depend on E2 having acquired a conformation stabilized by disulfide bonds.
Association of E1 and E2 glycoproteins with calnexin and calreticulin.
Calnexin and calreticulin assist in the folding of various glycoproteins, including viral proteins, with incompletely or incorrectly folded ones being retained in the ER (23). To determine whether BVDV-encoded envelope glycoproteins make use of this folding pathway, a pulse-chase experiment was performed, followed by the sequential immunoprecipitation with anticalnexin and anti-E2 antibodies. Approximately 40% of the total amount of E2 or E2-p7 precipitated by MAb 214 was coprecipitated by anticalnexin antibodies immediately after a 2.5-min pulse (Fig. 3A). Neither E2 nor E2-p7 was found in association with calnexin at min 10 of chase, suggesting that their interaction with calnexin is rapid and that dissociation occurs shortly after translation.
FIG. 3.
Interaction of E1 and E2 BVDV glycoproteins with calnexin. MDBK cells were either infected with BVDV at an MOI of 1 (inf.) or mock infected (mock). (A and C) Eighteen hours p.i., cells were pulse-labeled for 2.5 min with [35S]methionine and [35S]cysteine, chased for the times indicated, and either immunoprecipitated with MAb 214 and polyclonal anticalnexin antibodies (α cal) alone or coimmunoprecipitated with both MAb 214 and α cal, as indicated. The immunoprecipitated proteins were analyzed by SDS–10% (A) or SDS–12% PAGE under reducing conditions and visualized by autoradiography (C, graph). Calnexin-bound E1 was quantified by densitometric analysis (C, graph), with the amount of E1 associated with calnexin at time point 0 equaling 100%. (B) Fractions of the cell lysates were analyzed either before immunoprecipitation (lanes 3 and 4) or following immunoprecipitation with MAb 214 and elution of bound proteins under reducing conditions. The proteins were separated by SDS–10% PAGE and analyzed with a Western blot stained with α cal.
The interaction of E2 with calnexin was confirmed in a reverse experiment in which calnexin was readily detected in Western blots of infected cell lysates immunoprecipitated with anti-E2 antibodies (Fig. 3B).
To analyze the kinetics of E1 interaction with calnexin, an experiment similar to that described in the legend to Fig. 3A was carried out, except that the chase period was extended to 1 h and the lysates were precipitated with either anticalnexin antibodies or anti-E2 antibodies, as a control. As shown in Fig. 3C, after 2.5 min of pulse-labeling, in addition to bands for the E2 and E2-p7 glycoproteins, a 25-kDa band coprecipitated with calnexin. While the former bands disappeared from coprecipitates by min 10 of the chase, the association of the 25-kDa protein with calnexin decreased only slowly throughout the chase period and was no longer detected at 1 h of chase. The identification of the 25-kDa protein as E1 was confirmed by coimmunoprecipitation of the same band with anti-E2 antibodies at 1 h of chase, in the control sample.
When the experiments described above were repeated with anticalreticulin antibodies, none of the BVDV envelope glycoproteins were found to be associated with this chaperone (data not shown).
Calnexin and calreticulin interaction with glycoproteins is based on a lectin-like affinity for monoglucosylated N-linked glycans (14). However, in some cases calnexin has also been reported to associate with proteins through protein-protein interactions (15, 17, 27). To determine whether the interaction between the BVDV envelope proteins and calnexin is mediated by the N-glycan moiety, the experiment described in the legend to Fig. 3A was performed in the presence of the α-glucosidase inhibitor NB-DNJ. Neither E1 nor E2 or E2-p7 interacted with calnexin in the presence of inhibitor (data not shown), indicating the requirement for N-glycan trimming in order for binding to occur.
Folding of BVDV envelope glycoproteins in the presence of NB-DNJ.
Having established that the association of BVDV envelope proteins with calnexin was inhibited in the presence of NB-DNJ, we wanted to determine the effect of this inhibition on the folding of the viral proteins.
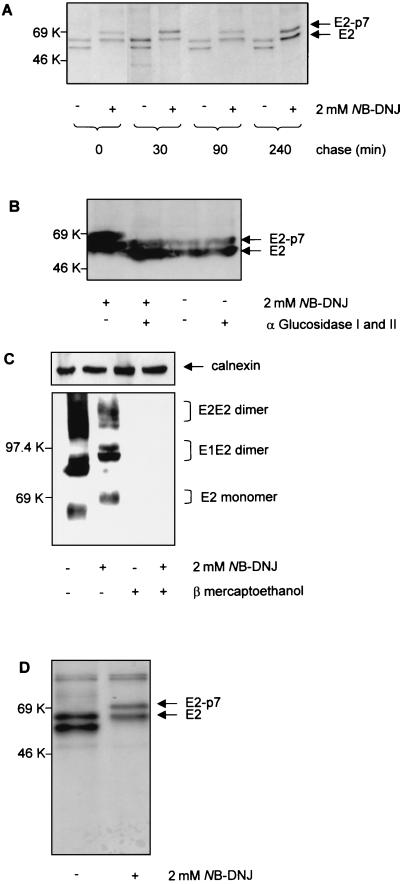
A pulse-chase experiment was performed in the presence or absence of NB-DNJ, followed by immunoprecipitation with anti-E2 antibodies and SDS-PAGE under reducing conditions. The MAb 214 antibody used in this experiment is able to recognize E2 under both nonreducing and reducing conditions, regardless of its folding state (Fig. 1). Two bands corresponding to the E2 and E2-p7 glycoproteins could be detected throughout the chase in both untreated (− lanes) and NB-DNJ-treated (+ lanes) samples (Fig. 4A). No significant difference in the intensities of these bands was observed between untreated and treated samples up to min 240 of chase, indicating that viral protein synthesis is not affected by the presence of the drug. Also, the ratio between E2-p7 and E2 did not change with increasing chase times in either the untreated or NB-DNJ-treated sample, indicating the stable nature of the E2-p7 polypeptide and the lack of a precursor-product relationship between E2-p7 and E2.
FIG. 4.
Biosynthesis and processing of BVDV envelope glycoproteins in the presence of NB-DNJ. MDBK cells were infected with BVDV at an MOI of 1. (A) Eighteen hours p.i., the cells were treated (+) or not treated (−) with 2 mM NB-DNJ. Two hours later, the cells were pulse-labeled with [35S]methionine and [35S]cysteine for 15 min, chased for the times indicated in the continuous presence of the drug, and immunoprecipitated with MAb 214. The proteins were analyzed by SDS–10% PAGE under reducing conditions and visualized by autoradiography. (B) Infected cells were grown for 18 h in the absence (−) or presence (+) of 2 mM NB-DNJ. Cell lysates were analyzed for protein content, and the equivalent of 20 μg of protein was digested (+) or not digested (−) with a mixture of α-glucosidases I and II. The proteins were separated by SDS–10% PAGE under reducing conditions and analyzed with a Western blot stained with MAb 214. (C) Infected cells were grown for 18 h in the absence (−) or presence (+) of 2 mM NB-DNJ. Cell lysates were boiled for 5 min in the absence (−) or presence (+) of 5% β-mercaptoethanol prior to separation by SDS–10% PAGE. The proteins were analyzed with a Western blot stained with MAb 158. Calnexin was detected with an anticalnexin antibody and used as loading control. (D) Conditions were the same as described for panel A, except that infected cells were lysed immediately after the 15 min of pulse and immunoprecipitated with MAb 158.
The mobility of BVDV glycoproteins was reduced in NB-DNJ-treated cells (Fig. 4A), which was most likely due to the inhibition of the trimming of N-linked oligosaccharides. This inhibition was confirmed by subjecting untreated and drug-treated cell lysates to hydrolysis with a mixture of ER α-glucosidases I and II and analyzing the resulting proteins by SDS-PAGE and Western blotting after staining with anti-E2 antibody. The mobilities of the drug-treated viral proteins after ER α-glucosidase digestion were identical to those of the untreated sample (Fig. 4B). This figure shows that glucose trimming is prevented in the presence of NB-DNJ and that the shift in mobility observed is due to the presence of terminal glucose residues which can be removed by treatment with ER α-glucosidases I and II.
To test the impact of α-glucosidase inhibition on the folding of the viral glycoproteins, we used the anti-E2 MAb 158, which recognizes nonreduced E2 (native or heat denatured) but not reduced E2 (Fig. 4C). We therefore concluded that MAb 158 binds to a disulfide bond-dependent epitope and can be used to monitor disulfide bond-dependent protein folding. Untreated and NB-DNJ-treated cells were labeled for 15 min and immunoprecipitated with MAb 158. The precipitated proteins were analyzed by SDS-PAGE under reducing conditions, and the intensities of the bands were measured by scan densitometry. The amount of E2 or E2-p7 glycoprotein immunoprecipitated by MAb 158 in NB-DNJ-treated cells was about 50% of that detected in controls (Fig. 4D), suggesting that a proportion of the E2 proteins was not able to acquire the disulfide-linked epitope recognized by this antibody. This result was confirmed with MAb 158-stained Western blots of infected cell lysates analyzed under nonreducing conditions. Calnexin in the same samples was used as an internal loading control (Fig. 4C).
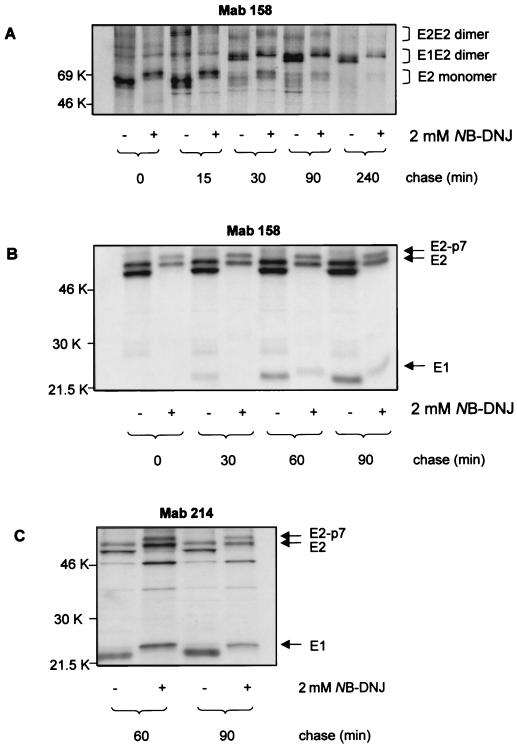
We further investigated the effect of NB-DNJ treatment on the association of E1 and E2 glycoproteins into dimers. A pulse-chase experiment was performed, followed by immunoprecipitation with MAb 158 and SDS-PAGE analysis under both nonreducing and reducing conditions. Under nonreducing conditions, the interaction between E1 and E2 followed the same kinetics in treated and control samples. Up to min 15 of chase, the major form of E2 present was the monomer. E1-E2 complexes started accumulating by min 30 and remained stable up to 240 min of chase. However, the amounts of viral proteins immunoprecipitated in NB-DNJ-treated samples were reduced compared to amounts in controls, an effect which was more evident at the level of the E1-E2 dimers (Fig. 5A). These results were confirmed by repeating the experiment under reducing conditions, which allowed us to also detect the band corresponding to E1. In the presence of NB-DNJ, the amounts of E1 and E2 coprecipitated by MAb 158 were about 45% of those precipitated in the absence of the drug at min 60 and 90 of chase (Fig. 5B), indicating that inhibition of α-glucosidases I and II significantly reduced the formation of native E1-E2 heterodimers. To determine whether misfolded E2 could still associate with E1, duplicate samples at min 60 and 90 of chase were immunoprecipitated with the conformation-independent MAb 214, and proteins bound were analyzed by SDS-PAGE under reducing conditions. The amounts of E1 that coprecipitated with E2 were reduced to 60 and 45% at min 60 and 90 of chase, respectively (Fig. 5C). This result suggests that, in the presence of NB-DNJ, misfolded E2 cannot efficiently associate with E1 into heterodimers.
FIG. 5.
Folding and assembly of E1 and E2 glycoproteins in NB-DNJ-treated cells. (A and B) MDBK cells were infected with BVDV at an MOI of 1. Eighteen hours p.i., the cells were treated (+) or not treated (−) with 2 mM NB-DNJ for 2 h before being pulse-labeled with [35S]methionine and [35S]cysteine for 15 min. At the chase times indicated, the cells were lysed and immunoprecipitated with MAb 158. Proteins bound were separated by SDS–10% PAGE under nonreducing conditions (A) or by SDS–12% PAGE under reducing conditions (B) and visualized by autoradiography. (C) Conditions were the same as those described for panel B, except that the cell lysates were immunoprecipitated with MAb 214.
The antiviral effect of NB-DNJ correlates with misfolding of the E2 glycoprotein.
NB-DNJ has previously been shown to have an impact on the yield of secreted infectious BVDV at 3 days p.i. at a low MOI (0.01 PFU/cell) (32). However, to be able to investigate the impact of α-glucosidase inhibition on the folding of the viral envelope proteins, we needed to increase the MOI in order to achieve a higher level of viral protein expression. We therefore reanalyzed the effect of NB-DNJ on BVDV plaque formation, this time with MDBK cells which were infected at an MOI of 1 (Fig. 6A). NB-DGJ, an iminosugar derivative which does not inhibit the α-glucosidases (18), was included as a control. While NB-DNJ strongly inhibited the cytopathic effect of BVDV on MBDK cells in a dose-dependent manner, NB-DGJ had no impact on plaque formation.
FIG. 6.
BVDV protein folding and infectivity in NB-DNJ-treated cells. (A) MDBK cells were grown to subconfluence in six-well plates and infected with BVDV at an MOI of 1. After 1 h the inoculum was removed and the cells were grown for 24 h in medium containing either NB-DNJ or NB-DGJ at the indicated concentrations (plaque assay). The supernatants containing secreted virus were then used to infect fresh MDBK monolayers, and the plaques were counted after 24 h (yield assay). Results are the percentages of the number of plaques resulting from infection with the inhibitor-free plaque assay supernatant (considered as 100%). (B) MDBK cells were infected with BVDV at an MOI of 1 and grown in the absence or presence of increasing concentrations of NB-DNJ. Eighteen hours p.i., the cells were labeled for 15 min with [35S]methionine and [35S]cysteine and lysed. The amount of radiolabeled protein in the cell lysates was adjusted to 3 × 106 cpm/ml, before immunoprecipitation with MAb 158 and antiactin antibodies (loading control). The amount of immunoprecipitated protein was quantified by liquid scintillation counting and expressed as the percentage of counts per minute determined for the drug-free samples. (C) MDBK cells were infected with BVDV at an MOI of 1 and grown in the absence or presence of NB-DNJ at the concentrations indicated. Eighteen hours p.i., the cells were lysed and the assembly of the viral proteins was analyzed by SDS–10% PAGE under nonreducing conditions followed by Western blotting using MAb 214. Calnexin and actin were detected on the same membrane (loading control) (gels). The band intensities of E2 monomers as well as of E1-E2 and E2-E2 dimers were quantified by densitometric analysis (graph).
We then investigated whether misfolding of E2 in the presence of NB-DNJ correlated with the antiviral effect of the drug. MDBK cells infected at an MOI of 1 were treated with increasing concentrations of NB-DNJ and either labeled for 15 min and immunoprecipitated with MAb 158 followed by quantification of the bound proteins by liquid scintillation counting (Fig. 6B) or directly analyzed by Western blotting using the same antibody (Fig. 6C). Immunoprecipitation of actin in labeled cell lysates or identification of both actin and calnexin by Western blotting was used as an internal loading control. Unlike the amount of actin precipitated with antiactin antibody, the amount of E2 precipitated with MAb 158 decreased with rising inhibitor concentration (Fig. 6B). Western blot analysis of the cell lysates under nonreducing conditions showed that treatment with NB-DNJ resulted in a concentration-dependent reduction in the intensities of the bands corresponding to E2 in both the monomeric and dimeric form (Fig. 6C). As MAb 158 recognizes a disulfide-linked epitope, this decreased signal reflects misfolding. To analyze the amount of E2 present, independent of its folding state, the same Western blot was analyzed using MAb 214 and a clear reduction in the amount of E1-E2 dimers was observed with increasing concentrations of NB-DNJ (data not shown). However, in that experiment, the inefficient association between E1 and E2 did not result in a correlating accumulation of E2 monomers or E2-E2 dimers, suggesting that the misfolded proteins either were targeted for degradation or accumulated in aggregates that were no longer recognized by MAb 214.
DISCUSSION
We used the conformation-dependent and -independent anti-BVDV E2 MAbs available to investigate the disulfide bond-associated folding of BVDV envelope glycoproteins in infected MDBK cells. Our results suggest that, while E2 and E2-p7 acquire a compact configuration stabilized by intramolecular disulfide bonds, either cotranslationally or immediately after translation has been completed, the folding of E1 glycoprotein is rather slow. Both E2 and E2-p7 can be detected as stable polypeptides as early as 2.5 min after pulse-labeling of infected cells, and no further processing at the E2-p7 site occurred within the 4 h of chase. The same unusual lack of a precursor-product relationship between E2-p7 and E2 was reported for HCV (16) and, more recently, for the BVDV CP7 strain (13). Cleavage between E2 and p7 has been reported to be mediated by cellular signal peptidases (10), which cleave the polypeptides at specific sequences, most often immediately after their translocation into the ER. The ability of E2-p7 to acquire very rapidly a conformation stabilized by disulfide bonds in the absence of peptidase peptidase processing indicates that folding and cleavage of this polypeptide may be competing processes. In this hypothetical scenario, a change in conformation may occur during folding of E2-p7, which may lead to the obstruction of the consensus sequence recognized by the host peptidase, preventing subsequent processing.
Interaction of E2 or E2-p7 with calnexin is short, and the kinetics correlate with the formation of intramolecular disulfide bonds. In contrast to E2, E1 remains posttranslationally associated with calnexin for up to 30 min. The release of E1 from calnexin coincides with the beginning of association of E1 and E2 into native, disulfide-linked heterodimers. Since protein assembly into quaternary complexes requires correct folding of the monomers, the folding of E1 appears to be the rate-limiting step for the formation of E1-E2 dimers. The longer association of E1 with calnexin may well play a role in the assembly of the virus, allowing proper interactions between the envelope proteins to occur and promoting formation of functional E1-E2 heterodimers. Although both envelope proteins were found at the same time in calnexin coimmunoprecipitates, no association between E1 and E2 could be detected earlier than 30 min postpulse, suggesting that each monomer interacted independently with calnexin. However, we cannot entirely rule out the possibility that weaker, noncovalent interactions between the two polypeptides may occur before the E1-E2 heterodimer is stabilized by intermolecular disulfide bonds.
In the presence of the α-glucosidase inhibitor NB-DNJ, the association of both BVDV envelope glycoproteins with calnexin was inhibited, indicating that N-glycan trimming is necessary for the interaction to occur. This conclusion is consistent with previously published reports in which calnexin is shown to act solely as a lectin (25, 31). In the absence of the interaction with calnexin, the folding associated with the formation of intramolecular disulfide bonds of E2 is inefficient.
The amount of E2 recognized by a conformation-dependent MAb decreased with increasing concentrations of NB-DNJ and was reduced to approximately 50% of that in controls for the highest concentration used. Since the antibody used is able to recognize a disulfide-dependent epitope, the simplest explanation for this result is that, in the presence of the inhibitor, E2 glycoproteins exist as a mixture of native and misfolded polypeptides rather than as a uniform, partially misfolded population.
E1 was also shown to interact with calnexin, and its folding is therefore expected to be impaired by NB-DNJ treatment. Unfortunately, the lack of anti-E1 antibodies makes a more detailed analysis of the folding state of the polypeptide very difficult. Similarly, potentially heavily N-glycosylated Erns may be a target for the drug, and alterations in Erns folding may interfere with viral infectivity. Misfolding of the BVDV envelope glycoproteins impairs the association of E1 and E2 into heterodimers. A similar result was obtained for the HCV envelope proteins expressed from a vaccinia virus vector in the presence of CST (5). CST has also been shown to cause the misfolding of DENV prM and E envelope proteins and the formation of unstable prME heterodimers (6). In the HCV system, a second constitutive, nonproductive pathway leading to aggregation of misfolded envelope proteins is activated in the presence of α-glucosidase inhibitors. In the DENV system, treatment with α-glucosidase inhibitors leads to a delay of envelope protein assembly. In the recombinant HCV system, calreticulin interacts preferentially with these misfolded aggregates, whereas calnexin preferentially associates with either the monomeric form or the noncovalent complexes (5). In BVDV, neither monomers nor dimers are found to be associated with calreticulin and no high-molecular-weight aggregates can be detected with a panel of four MAbs against E2 (data not shown), indicating that, in a natural pestivirus infection, this nonproductive pathway does not occur.
NB-DNJ treatment of BVDV-infected MDBK cells results in a dose-dependent reduction of the amount of infectious virus. The inhibitor concentration which reduces the number of plaques to 50% compared to the number in untreated controls is about 125 μM, when the cells are infected with an MOI of 1. The antiviral effect correlates with the misfolding of E2 and the inefficient association of E1 and E2 into heterodimers. The E1-E2 dimer is the major component of mature virions and is thought to play a central role during infection. Therefore, any conformational changes in the subunits of the complex may interfere with virus binding to host cell receptors or with other postbinding events, leading to reduced viral infectivity.
BVDV has been suggested as a model for HCV since both viruses share molecular and virological features, such as similar genome organizations, replication strategies, and protein functions (24). In this study we show for the first time that BVDV and HCV have a common dependence on calnexin for the folding of their envelope glycoproteins. BVDV E1 and E2 associate rapidly with calnexin and dissociate at different rates. The disulfide-dependent folding of E2 is fast, while E1 folds rather slowly, as judged by the longer interaction with calnexin. The inhibition of calnexin binding to the envelope proteins by α-glucosidase inhibitors leads to E2 (and probably E1) misfolding and a decrease in the formation of E1-E2 heterodimers.
A difference between the two viruses may reside in the nature of the interaction between E1 and E2 within the dimers: while the BVDV E1-E2 complex is clearly disulfide bonded, both inside the cells and in secreted mature virions (reference 26 and our own observations), the situation for HCV is less clear. Generally, the noncovalently linked E1-E2 heterodimer found in the recombinant HCV system is thought to be the native complex which is subsequently incorporated into mature secreted virions (8, 12). Interestingly, for Newcastle disease virus, it has been reported that the virus produces two forms of the hemagglutinin-neuraminidase dimer, one linked by disulfide bonds and the other one not, and that both forms are transported to the cell surface with identical kinetics. However, only the form containing intermolecular disulfide bonds is incorporated into virions; the noncovalently linked form is not incorporated and presumably degraded (21). For HCV, so far it has not been possible to identify the functional envelope glycoprotein complex in virions.
Treatment with α-glucosidase inhibitors affect the life cycles of other enveloped viruses by inducing misfolding of viral structural proteins. For example, the V1-V2 loop of human immunodeficiency virus gp120, which is involved in virus binding to host cells, has an altered conformation in the presence of NB-DNJ, and this correlates with inhibition of virus entry into the target cells (11). Similarly, recent studies have shown that correct N-glycan trimming is necessary for the secretion of HBV virions and that proper folding and transport of the HBV M and L proteins depend upon the interaction with calnexin (3, 29, 30). The generality of these effects to other viruses remains to be established, for it crucially depends on their envelope glycoproteins using the calnexin-mediated folding pathway.
ACKNOWLEDGMENTS
N.B.-N is supported by a NATO/Royal Society Fellowship. N.Z. is a Royal Society Dorothy Hodgkin Fellow and an EPA Cephalosporin Junior Research Fellow of Linacre College, Oxford, United Kingdom. This work was supported by Synergy Pharmaceuticals and the Oxford Glycobiology Institute Endowment.
REFERENCES
- 1.Baginski S G, Peaver D C, Seipel M, Sun S C C, Benetatos C A, Chunduru S K, Rice C M, Collet M S. Mechanism of action of a pestivirus antiviral compound. Proc Natl Acad Sci USA. 2000;97:7981–7986. doi: 10.1073/pnas.140220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–129. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 3.Block T M, Lu X, Platt F M, Foster G R, Gerlich W H, Blumberg B S, Dwek R A. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc Natl Acad Sci USA. 1994;91:2235–2239. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choukhi A, Ung S, Wychowsky C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courageot M P, Frenkiel M P, Duarte Dos Santos C, Deubel V, Desprès P. α-Glucosidase inhibitors reduce Dengue virus production by affecting the initial steps of virion morphoogenesis in the endoplasmic reticulum. J Virol. 2000;74:564–572. doi: 10.1128/jvi.74.1.564-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deleersnyder V, Pillez A, Wychowsky C, Blight K, Xu J, Hahn Y S, Rice C M, Dubuisson J. Formation of native hepatitis C virus glycoprotein complexes. J Virol. 1997;71:697–704. doi: 10.1128/jvi.71.1.697-704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubuisson J, Hsu H H, Cheung R C, Greenberg H B, Russel D G, Rice C M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol. 1994;68:6147–6160. doi: 10.1128/jvi.68.10.6147-6160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuisson J, Rice C M. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbers K, Tautz N, Becher P, Stoll D, Rümenapf T, Thiel H J. Processing in the pestivirus E2-NS2 region: identification of proteins p7 and E2p7. J Virol. 1996;70:4131–4135. doi: 10.1128/jvi.70.6.4131-4135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer P B, Karlsson G B, Butters T D, Dwek R A, Platt F M. N-Butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with changes in antibody recognition of the V1/V2 region of gp120. J Virol. 1996;70:7143–7152. doi: 10.1128/jvi.70.10.7143-7152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada T, Tautz N, Thiel H J. E2–p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J Virol. 2000;74:9498–9506. doi: 10.1128/jvi.74.20.9498-9506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebert D N, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim P S, Arvan P. Calnexin and BiP act as sequential molecular chaperones during tyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995;128:29–38. doi: 10.1083/jcb.128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C, Lindenbach B D, Pragai B M, McCourt D W, Rice C M. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J Virol. 1994;68:5063–5073. doi: 10.1128/jvi.68.8.5063-5073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo T W, Clarke D M. Prolonged association of temperature sensitive mutants of human P-glycoprotein with calnexin during biogenesis. J Biol Chem. 1994;269:28683–28689. [PubMed] [Google Scholar]
- 18.Lu X, Mehta A, Dwek R, Butters T, Block T. Evidence that N-linked glycosylation is necessary for hepatitis B virus secretion. Virology. 1995;213:660–665. doi: 10.1006/viro.1995.0038. [DOI] [PubMed] [Google Scholar]
- 19.McGinnes L W, Morrison T G. Role of carbohydrate processing and calnexin binding in the folding and activity of the HN protein of Newcastle disease virus. Virus Res. 1998;53:175–185. doi: 10.1016/s0168-1702(97)00144-5. [DOI] [PubMed] [Google Scholar]
- 20.Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro. J Virol. 1998;72:8705–8709. doi: 10.1128/jvi.72.11.8705-8709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison T G, McQuain C, O'Connel K F, McGinnes L W. Mature, cell-associated HN protein of Newcastle disease exists in two forms differentiated by posttranslational modifications. Virus Res. 1990;15:113–133. doi: 10.1016/0168-1702(90)90003-t. [DOI] [PubMed] [Google Scholar]
- 22.Ora A, Helenius A. Calnexin fails to associate with substrate proteins in glucosidase-deficient cell lines. J Biol Chem. 1995;270:26060–26062. doi: 10.1074/jbc.270.44.26060. [DOI] [PubMed] [Google Scholar]
- 23.Peterson J R, Ora A, Nguyen Van P, Helenius A. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoprotein. Mol Biol. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 25.Rodan A R, Simons J F, Trombetta E S, Helenius A. N-linked oligosaccharides are necessary and sufficient for association for glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 1996;15:6921–6930. [PMC free article] [PubMed] [Google Scholar]
- 26.Thiel H J, Stark R, Weiland E, Rümenapf T, Meyers G. Hog cholera virus: molecular composition of virions from a pestivirus. J Virol. 1991;65:4705–4712. doi: 10.1128/jvi.65.9.4705-4712.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Leeuwen J E M, Kears K P. Calnexin associates exclusively with individual CD3d and T cell antigen receptor (TCR), a protein containing incompletely trimmed glycans that are not assembled into multisubunit TCR complexes. J Biol Chem. 1996;271:9660–9665. doi: 10.1074/jbc.271.16.9660. [DOI] [PubMed] [Google Scholar]
- 28.Weiland E, Stark R, Haas B, Rümenapf T, Meyers G, Thiel H J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J Virol. 1990;64:3563–3569. doi: 10.1128/jvi.64.8.3563-3569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werr M, Prange R. Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J Virol. 1998;72:778–782. doi: 10.1128/jvi.72.1.778-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Bruss V, Yen T S B. Formation of intracellular particles by hepatitis B virus large surface protein. J Virol. 1997;71:5487–5494. doi: 10.1128/jvi.71.7.5487-5494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zapun A, Petrescu S M, Rudd P M, Dwek R A, Thomas D Y, Bergeron J J M. Conformation-independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]
- 32.Zitzmann N, Mehta A S, Carrouée S, Butters T D, Platt F M, McCauley J, Blumberg B S, Dwek R A, Block T M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc Natl Acad Sci USA. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]