Abstract
Background
The global effort to cure COVID-19 is still ongoing. Thus, a prospective, block-balanced, open-label, randomized controlled trial was conducted to evaluate how Tenofovir Alafenamide Fumarate affects hospitalized COVID-19 patients’ outcomes.
Methods
The intervention and control groups of 60 hospitalized COVID-19 patients were randomly allocated. Along with normal medication, the intervention group received 25 mg of tenofovir orally daily for seven days. The control group got normal therapy, including remdesivir and corticosteroids. ICU hospitalization duration, laboratory data, fever, dyspnea, arterial blood oxygen saturation with and without an oxygen face mask, mechanical ventilation, and mortality were the outcomes.
Results
Sixty of 236 eligible patients between September 2020 and February 2021 were enrolled. The intervention group had a mean age (±SD) of 61.33 (±13.09) years and the control group 60.03 (±18.03). Sixteen (53.3%) intervention patients and 15 (50.0%) control patients were males. The intervention group had fewer mechanical ventilation and ICU days. Tenofovir Alafenamide Fumarate did not improve fever, dyspnea, oxygen saturation with or without a face mask or nasal cannula, or laboratory data including WBC, ESR, CRP, AST, ALT, AlkP, total and direct bilirubin, in COVID-19 patients.
Conclusion
According to this pilot trial, Tenofovir Alafenamide Fumarate, along with conventional treatment, significantly reduced mechanical ventilation and ICU stay in COVID-19 patients. Further thorough research is necessary to verify this conclusion.
Keywords: Randomized clinical trial, Tenofovir, Remdesivir, Corticosteroids, COVID-19
Introduction
Early in the pandemic, efforts were made to repurpose drugs for prophylaxis against Coronavirus disease (COVID-19) [1]. Diverse antiviral medications, such as those used for influenza, human immunodeficiency virus (HIV), and hepatitis C virus (HCV), have been used in the treatment of individuals with COVID-19. Given the same structural and transcriptional characteristics of COVID-19 and several other viruses, some antiviral medications have undergone testing for their efficacy in treating individuals with COVID-19 [2]. Nevertheless, there is now insufficient evidence to substantiate the use of favipiravir, ivermectin, azithromycin, doxycycline, oseltamivir, lopinavir–ritonavir, hydroxychloroquine, itolizumab, bevacizumab, interferon alfa-2b (IFN-α2b), fluvoxamine, convalescent plasma, or herbal medicines for the management of COVID-19 [3]. For example, randomized studies investigated the use of hydroxychloroquine (HCQ) as a preventive measure before exposure to disease was initiated early on, mostly based on findings from laboratory experiments conducted outside of a living organism while these experiments were of limited scale and yielded inaccurate estimations of the effects [4, 5].
Tenofovir derivatives as FDA-approved medicines were considered potential candidates for repurposing because of their epidemiological data [6, 7], in vitro and in vivo investigations [8, 9], and their significant bioavailability in various tissues [10, 11]. Tenofovir alafenamide fumarate (TAF) is a novel prodrug of tenofovir, which replaces tenofovir disoproxil fumarate (TDF) [12].
TAF acts as an antiviral medication against HIV (RNA virus) and hepatitis B virus (HBV, DNA virus). This drug specifically targets DNA polymerase and prevents virus replication [13, 14], but it can also serve as an oral phosphonoamidate prodrug that inhibits the HIV reverse transcriptase. Therefore, TAF has shown efficacy in managing RNA and DNA viruses [15].
The urgent need to find a medicine that effectively improves the condition of COVID-19 patients, along with the lack of human trials on this drug and inadequate clinical data, led us to carry out a pilot study to investigate the impact of TAF on outcomes of hospitalized COVID-19 patients through a block-balanced, open-label, randomized clinical trial.
Methods
Study population
A prospective, block-balanced, open-label, randomized controlled trial was carried out at Razi Hospital in Ahvaz, Iran. Between September 2020 and February 2021, individuals aged 18 and above, exhibiting a moderate to severe clinical manifestation of COVID-19 infection as confirmed by real-time polymerase chain reaction (RT-PCR), and necessitating hospitalization for mild, moderate, or severe pneumonia, were enrolled in the study. The illness severity was graded based on clinical findings [16]. Moderate individuals were defined according to these criteria: signs of lower respiratory illness during clinical evaluation or imaging and oxygen saturation (SpO2) of 94% or higher when breathing normal air at sea level. Severe ones: blood oxygen saturation (SpO2) levels below 94% while breathing normal air at sea level, a ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2) below 300 mm Hg, a respiratory rate over 30 breaths per minute, or lung infiltrates covering more than 50% of the lung area. The inclusion criteria encompassed individuals who were at least 18 years old, tested positive for SARS-CoV-2 using real-time PCR following the collection of nasopharyngeal and oropharyngeal swab samples, and had pneumonic signs of the virus; the lungs should be visible on CT scans, exhibiting a 4% lower O2 Saturation to a level of 93%. Exclusion criteria comprised individuals who were vaccinated during this period, had a prior diagnosis of renal failure, individuals with documented allergies to any of the medications, those taking medications that interact with tenofovir, pregnant or breastfeeding women, patients requiring mechanical ventilation, individuals who left the hospital or expressed a desire to leave the study at any point during the study, and participants who had previously taken part in other clinical trials. The study protocol and the informed consent form were approved by the ethical committees of Ahvaz-Jundishapur University of Medical Sciences in Ahvaz, Iran, 29/04/2020 (IR.AJUMS.REC.1399.082). The protocol was made available on the website WWW.IRCT.ir with the identification IRCT20200422047168N1. Individuals were admitted to the hospital and granted written permission after being informed, and the study was carried out following the guidelines of the Declaration of Helsinki.
Randomization and masking
A total of 60 patients who met the inclusion criteria were chosen and then split into 10 blocks, with each group consisting of six individuals. Furthermore, with the standard therapy according to the established guidelines of Iran during the trial period (which included the administration of Remdesivir, and corticosteroids), 3 patients in each block were randomly assigned to receive a daily oral dose of 25 mg TAF tablets for seven days. Every participant was provided with suitable supplementary therapy as recommended.
Procedures
The physician evaluated patients daily using a checklist to record the main outcome measures including clinical symptoms such as chills, sore throat, fever, cough, dyspnea, sneezing, sputum production, abdominal pain, loss of appetite, vertigo, weakness, lethargy, headache, nausea, vomiting, diarrhea, constipation, sudden olfactory or gustatory loss, icterus, loss of consciousness, orthopnea, mouth dryness, eye irritation, rhinorrhea, and other symptoms, from the initial state until discharge. In addition, the underlying diseases in patients were recorded and the following measurements were taken: respiratory rate, oxygen saturation with and without supplemental oxygen, complete blood count with differential, erythrocyte sedimentation rate, sodium, potassium, blood urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and creatine phosphokinase were measured. Subsequent laboratory measurements were conducted 48 h later. Furthermore, a chest CT scan was acquired before the operation. Patients had daily evaluations for 7 days to assess disease progression or the emergence of any new symptoms. The study also documented the need for supplementary oxygen, the method of oxygen delivery, the use of invasive mechanical ventilation, and other resultant measures.
Outcomes
The primary outcome, clinical improvement, was defined 7 days after initiating therapy. However, we monitored the occurrence or non-occurrence of fever and dyspnea, percentage of blood oxygen saturation with and without oxygen, duration of hospitalization, and laboratory data [(white blood cell count (WBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), liver function tests (LFT)] every other day. The criteria for clinical improvement consisted of achieving a normal body temperature, absence of dyspnea, an oxygen saturation level higher than 93% in room air, and stability for up to 24 h. Additional outcomes included the need for mechanical ventilation, ICU admission, the duration of ICU stay, and death.
Statistical analysis
The data were analyzed using IBM SPSS Statistics, specifically version 18. The statistical measure of means (±SD) was used to present quantitative data, whilst qualitative variables were represented using frequency and percentage. The T-test was used to examine discrepancies between the intervention and control groups in analyzing quantitative data that followed a normal distribution. Conversely, the Mann-Whitney non-parametric equivalent was utilized to analyze data that exhibited an abnormal distribution. The Chi-Square test (or Fisher’s exact test) was used for the examination of qualitative data. A P-value less than 0.05 was considered to be statistically significant.
Results
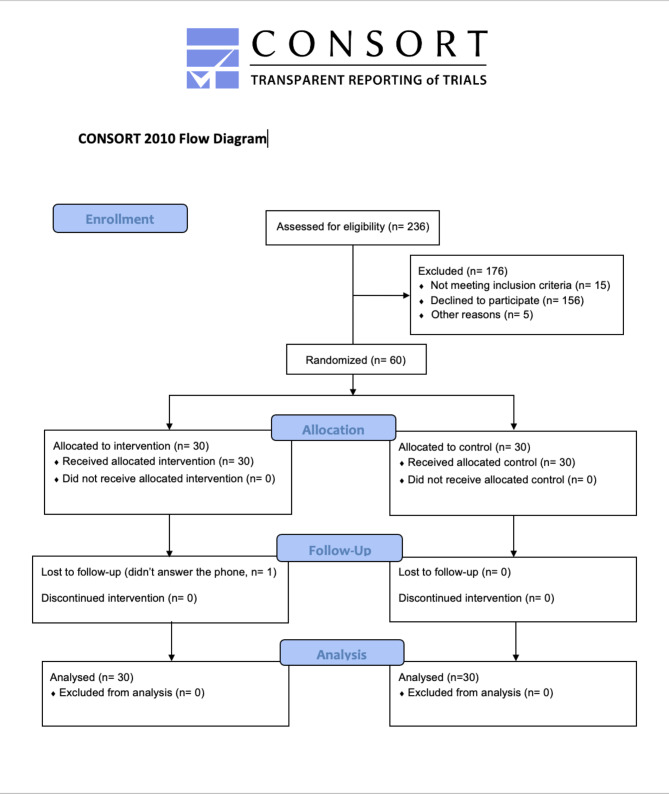
Of the 236 eligible patients screened for enrollment, 60 were randomized into two groups: 30 in the intervention group to receive TAF 25 mg once daily for 7 days, and 30 in the control group (Fig. 1).
Fig. 1.
CONSORT flow diagram. In the study, 236 participants were accessed initially, 176 were excluded and 60 patients were randomized and participated in the trial
Baseline characteristics were well balanced between the two allocation groups. The patients’ ages ranged between 23 and 94 years. The mean (±SD) ages of the intervention and control groups were 61.33 (±13.09) and 60.03 (±18.03) years, respectively (P = 0.105). Sixteen patients (53.3%) in the intervention group and 15 patients (50.0%) in the control group were male (P = 0.246). Diabetes mellitus and Hypertension were the main comorbidities (P = 0.592) (Table 1).
Table 1.
Baseline characteristics of index cases in each study arm
| Individual characteristics | Intervention, n = 30 | Control, n = 30 | P-value |
|---|---|---|---|
| Age, mean (±SD) | 61.33 (±13.09) | 60.03 (±18.03) | 0.105 |
| Male, n (%) | 16 (53.3%) | 15 (50.0%) | 0.292 |
| Coexisting comorbidities | |||
| Respiratory disease, n (%) | 1 (3.3) | 0 (0) | 0.313 |
| Cardiovascular disease, n (%) | 1 (3.3) | 4 (13.3) | 0.161 |
| Hypertension, n (%) | 10 (33.3) | 12 (40) | 0.592 |
| Diabetes mellitus, n (%) | 14 (46.7) | 11 (36.7) | 0.432 |
| Central nervous system disease, n (%) | 0 (0) | 4 (13.3) | 0.038* |
| Cancer history, n (%) | 0 (0) | 0 (0) | 0* |
| Severe flu history, n (%) | 1 (3.3) | 1 (3.3) | 1 |
| Arrhythmia, n (%) | 0 (0) | 1 (3.3) | 0.313 |
| Chronic kidney disease, n (%) | 0 (0) | 0 (0) | 0* |
| Chemotherapy history, n (%) | 0 (0) | 0 (0) | 0* |
| Surgical history, n (%) | 3 (10) | 4 (13.3) | 0.688 |
| Laboratory data | |||
| ALT, mean difference (±SD) | 4.63 (31.69) | 6.82 (23.07) | 0.763 |
| AST, mean difference (±SD) | −10.33 (41.72) | −7.37 (25.2) | 0.744 |
| ALK, mean difference (±SD) | −13.23 (34.86) | −5.44 (32.85) | 0.381 |
| Bilirubin total, mean difference (±SD) | −0.04 (0.43) | −0.05 (0.54) | 0.885 |
| Bilirubin direct, mean difference (±SD) | 0.00 (0.1) | −0.01 (0.11) | 0.559 |
| ESR, mean difference (±SD) | −5.6 (20.66) | 3.46 (29.03) | 0.169 |
| CRP, mean difference (±SD) | −12.26 (55.18) | −4.9 (31.13) | 0.527 |
| WBC×9/L, mean difference (±SD) | 1.798 (11.093) | 1.850 (4.353) | 0.981 |
Data are the frequency of individuals (percentage) and mean (±SD); *p < 0.05
Table 1 also displays the virologic outcomes from day 1st to day 7th of admission. The laboratory test results, such as WBC, ESR, CRP, and LFT, were not significantly different between the two groups upon admission.
The most prevalent signs and symptoms on the first day of admission in both groups were dyspnea (83.3% in the intervention group and 63.3% in the control group) (P = 0.08) (Table 2).
Table 2.
Comparative presentation of key symptoms in intervention and control groups
| Symptoms | Intervention group, n = 30 | Control group, n = 30 | P-value |
|---|---|---|---|
| Dyspnea, n (%) | 25 (83.3) | 19 (63.3) | 0.08 |
| Fever and chills, n (%) | 17 (56.7) | 18 (60) | 0.793 |
| Cough, n (%) | 16 (53.3) | 16 (53.3) | 1.00 |
| Weakness, n (%) | 22 (73.3) | 16 (53.3) | 0.108 |
| Lethargy, n (%) | 17 (56.7) | 16 (5.3) | 0.795 |
| Headache, n (%) | 7 (23.3) | 7 (23.3) | 1.00 |
| Muscular pain, n (%) | 9 (30) | 6 (20) | 0.371 |
| Loss of appetite, n (%) | 7 (23.3) | 11 (36.7) | 0.260 |
| Nausea and vomiting, n (%) | 3 (10) | 6 (20) | 0.278 |
Data are the frequency of individuals (percentage); *p < 0.05
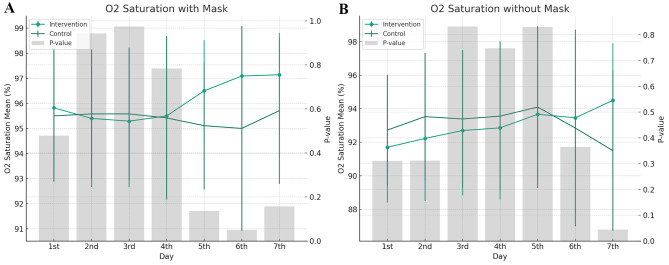
Fever was measured during the days of hospitalization for patients with COVID-19. Although fever reduced more quickly in the intervention group, there was no significant difference between the two groups upon admission. The presence or absence of dyspnea was assessed for 7 days in hospitalized patients, but there was no significant difference between the two groups upon admission (p-value = 0.688). Arterial oxygen saturation, with and without a face mask, was measured. It showed no significant differences between the two groups (p-value = 0.284; p-value = 0.131) (Fig. 2).
Fig. 2.
Graph A illustrates the daily mean oxygen saturation levels for patients with a mask, including the intervention and control groups, over a seven-day period. Error bars represent the standard deviation. The secondary y-axis displays the p-values, with gray bars highlighting the statistical significance of the difference between the two groups each day. Graph B depicts the daily mean oxygen saturation levels for patients without a mask for both the intervention and control groups. Similar to the first graph, error bars show the standard deviation for each group. The p-values are again shown as gray bars along the secondary y-axis, reflecting the statistical significance of the differences between intervention and control groups on each day
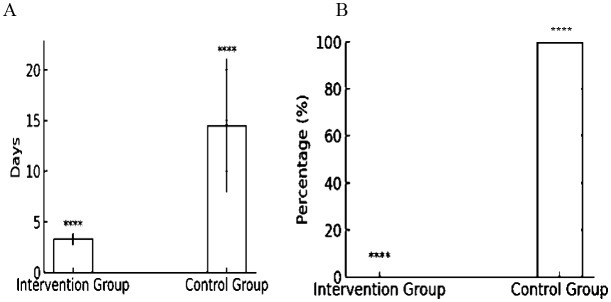
In our study conducted at Razi Hospital, we observed 60 patients in the general ward. Out of these, 7 patients required transfer to the Intensive Care Unit (ICU) during their hospital stay. This group included 4 patients from the control group and 3 from the intervention group. There was a notable difference in the average duration of ICU stays between the two groups. Patients in the intervention group had a mean (±SD) ICU stay of 3.33 (±0.57) days, whereas those in the control group stayed for an average of 14.5 (±6.85) days. This disparity in the length of ICU admission also reflected statistical significance (p-value = 0.04) (Fig. 3—Graph A). Notably, all 4 patients from the control group who were admitted to the ICU experienced severe respiratory distress, evidenced by symptoms such as dyspnea, oxygen saturation falling below 93%, and elevated PCO2 levels, necessitating mechanical ventilation. This occurrence was found to be statistically significant (p-value = 0.038) (Fig. 3—Graph B). No patient died during the study.
Fig. 3.
Outcomes in ICU Interventions and Control Groups. Graph A illustrates the average duration of ICU stays, with the intervention group having a mean stay of 3.3 days and the control group 14.5 days, each with standard deviations of 0.57 and 6.58 days, respectively. Graph B depicts the percentage of patients requiring mechanical ventilation. In the intervention group, none of the patients required mechanical ventilation, while all patients in the control group did, which is also statistically highly significant, as denoted by the four asterisks. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Discussion
This pilot randomized clinical trial demonstrates the potential efficacy of TAF, when taken in conjunction with standard-of-care therapy, in reducing the duration of ICU hospitalization and mitigating the severity of respiratory problems associated with SARS-CoV-2. The results justify exploring the use of this medicine as an alternate approach to reduce the quantity of SARS-CoV-2 virus, including its transmission. These data provide strong justifications to cautiously embrace these findings with hope.
Up to 75% of the theoretically eligible patients were not included in the trial, which is significantly higher compared to another study that tested hydroxychloroquine in the same context when only 11% were eliminated [17]. Our low rate of participation can be attributed to several factors. Firstly, some patients did not meet the required inclusion criteria or they met the exclusion criteria. Additionally, eligible patients chose not to participate in the study due to various reasons. These reasons include the inconvenience of additional visits and testing, concerns about the potential side effects of taking additional drugs, and the lack of media coverage for tenofovir during the recruitment period, in contrast to the attention received by hydroxychloroquine. Furthermore, there was a disproportionate percentage of collaboration among healthcare staff [17, 18]. This might indicate the proximity to the study location, a high level of trust in clinical studies, and a reduced concern about any adverse consequences.
Both TDF and TAF produce the same active chemical, which is tenofovir. In humans, TDF and TAF have distinct pharmacokinetic profiles and diverge in terms of lipid metabolism [19]. We know that lipids play a crucial role in viral propagation, but at this time, there is no evidence of the side effects of these medications on viral lipid metabolism. While, TDF has immunomodulatory effects [20, 21], TAF is a nucleotide reverse transcriptase inhibitor. Closely related to the commonly used reverse-transcriptase inhibitor TDF, TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent [22, 23].
We monitored various parameters of hospitalized COVID-19 patients, including fever progression, dyspnea presence, and arterial oxygen saturation levels, both with and without the use of a face mask. Guan et al. reported fever was identified as the most common manifestation of COVID-19 onset in their study population [24]. However, in the current study, shortness of breath emerged as the most prevalent symptom at the onset.
Various studies have investigated the relationship between antiviral drugs and fever, indicating that the treatment of COVID-19-induced fever with antivirals is achieved through reducing viral load and immunomodulation [25, 26]. Despite a faster reduction in fever in our intervention group, it was not statistically significant, that indicating the use of tenofovir did not significantly affect fever reduction.
COVID-19 triggers dyspnea through several mechanisms, including pulmonary edema and surfactant exposure [27, 28]. Given the antiviral properties of tenofovir, it was hypothesized to lessen dyspnea in the intervention group. Nevertheless, the discrepancy in the degree of dyspnea and blood oxygen saturation levels, with and without an oxygen mask, was not a statistically significant difference between the control and intervention groups.
A noteworthy observation was in the ICU admission and duration. In the ICU-admitted patients, a significant difference was noted in the number of days spent in the ICU between the two groups (p-value = 0.032). Moreover, a significant difference was observed in the intubation rates among patients from the two groups (p-value = 0.029). Notably, of the seven patients hospitalized in ICU (4 from the control group and 3 from the treatment group), and from whom, 4 patients were intubated which belonged to the control group. This suggests that the usage of tenofovir might have contributed to a reduction in the ICU stay duration and the intubation rate.
However, the laboratory test results, encompassing markers such as WBC, ESR, CRP, and LFT, did not show any significant difference between the two groups, suggesting that the intervention did not have a marked effect on these laboratory parameters.
This study has some limitations including: The study incorporated a relatively small sample size, which may not adequately represent the broader population. Additionally, the study did not detail the diversity of the sample population in terms of ethnicity, socio-economic status, and other demographics which could potentially influence the outcomes. The study focused primarily on a select set of outcomes (e.g., ICU admission rate, duration of ICU stay, and certain laboratory parameters). Including a broader array of outcomes, including recovery rates, and quality of life assessments, could provide a more comprehensive view of the intervention’s potential benefits, this study was conducted in a single location, which might limit the generalizability of the findings. Multi-center trials could provide more robust evidence by accounting for variations in clinical practice and patient populations, an open-label design, which might introduce biases as both the researchers and participants know the allocated interventions. A double-blind design could have mitigated potential biases and influences on the results, Although the study identified statistical significance in certain outcomes, it did not thoroughly explore the clinical significance of these findings, which is crucial for understanding the potential real-world benefits of the intervention, Given that the study hinted at positive outcomes in terms of ICU stay and respiratory complications, there might be a potential reporting bias, where positive results are more likely to be reported, and negative or neutral results might be underreported.
This work also has strengths. This clinical study is the first to demonstrate the antiviral properties of TAF, a previously neglected medication that has the potential to be repurposed. TAF has a similar structure to remdesivir and is suitable for treating COVID-19 in outpatient settings. The observed statistically significant findings, despite the limited sample size of 60 participants, suggest a potentially clinically relevant impact of TAF due to its antiviral capabilities. This is further corroborated by previous reports indicating that tenofovir provides protection against COVID-19 [29–31]. The medications under investigation are publicly available, hence enhancing their potential use in low-resource nations that are likewise impacted by COVID-19 but have limited access to vaccines or possible novel antiviral treatments [32]. According to DeJong et al. [33], it is significant to note that even if tenofovir is less effective than other investigational drugs in treating COVID-19, its widespread availability and affordability might still have a significant positive effect on public health. Ultimately, the enduring feeling of using this particular mixture is comforting, even during pregnancy [34].
Conclusion
TAF along with conventional treatment, significantly decreased ICU admission, ICU duration, and intubation in infected COVID-19 inpatients. While the safety profile of TAF within a brief period was favorable, we do not recommend its experimental use in clinical practice based on the findings of this proof-of-concept trial. Nevertheless, this pilot study, which employs a randomized clinical trial design, advances the justification for studies presently enrolling participants to investigate the use of TAF for the prevention and treatment of COVID-19.
Acknowledgements
We thank Ahvaz-Jundishapur University of Medical Sciences for the financial support.
Author contributions
N.Y.P. conceived and designed the study. A.T. and F.A. and N.N. performed the intervention and participated in clinical follow-up of the patients. B.C. analyzed and interpreted the data. Z.S.E. and A.A.S. wrote the main manuscript text and managed the study. All authors approved the final version.
Funding
This work was financially supported by the Ahvaz-Jundishapur University of Medical Sciences (RDC-9903).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and informed consent
This study received ethical approval in 29/04/2020 (IR.AJUMS.REC.1399.082) from the Ahvaz-Jundishapur University of Medical Sciences in Ahvaz, Iran. The protocol was made available on the website WWW.IRCT.ir with the identification IRCT20200422047168N1. Written informed consent was obtained from all the participants, and the study was carried out following the guidelines of the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Footnotes
Zahra Shokati Eshkiki is considered co-corresponding author
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Shokati Eshkiki, Email: zahrashokati@gmail.com.
Ali Akbar Shayesteh, Email: shayeste-a@ajums.ac.ir.
References
- 1.Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust. 2020;213(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rommasi F, Nasiri MJ, Mirsaiedi M. Antiviral drugs proposed for COVID-19: action mechanism and pharmacological data. Eur Rev Med Pharmacol Sci. 2021;25(11):4163–73. [DOI] [PubMed] [Google Scholar]
- 3.Pramesh CS, Babu GR, Basu J, Bhushan I, Booth CM, Chinnaswamy G, et al. Choosing wisely for COVID-19: ten evidence-based recommendations for patients and physicians. Nat Med. 2021;27(8):1324–7. [DOI] [PubMed] [Google Scholar]
- 4.García-Albéniz X, Del Amo J, Polo R, Morales-Asencio JM, Hernán MA. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. Eur J Epidemiol. 2022;37(8):789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naggie S, Milstone A, Castro M, Collins SP, Lakshmi S, Anderson DJ, et al. Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker exposure response and outcomes of Hydroxychloroquine (HERO-HCQ). Int J Infect Dis. 2023;129:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173(7):536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology. 2020;31(6):e49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copertino Jr DC, Casado Lima BC, Duarte RR, Powell TR, Ormsby CE, Wilkin T, et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn. 2022;40(16):7367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanella I, Zizioli D, Castelli F, Quiros-Roldan E. Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-COV-2 infection. Pharmaceuticals. 2021;14(5):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses. 2016;32(10–11):981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):re1124–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Drosu NC, Edelman ER, Housman DE. Tenofovir prodrugs potently inhibit Epstein–Barr virus lytic DNA replication by targeting the viral DNA polymerase. Proc Natl Acad Sci. 2020;117(22):12368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clososki GC, Soldi RA, Silva RMd, Guaratini T, Lopes JN, Pereira PR, et al. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc. 2020;31:1552–6. [Google Scholar]
- 15.Birkus G, Bam RA, Willkom M, Frey CR, Tsai L, Stray KM, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M, Wang Y, Ye J, Da H, Fang S, Chen L. Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19). Ann Transl Med. 2020;8(18). [DOI] [PMC free article] [PubMed]
- 17.Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial. Clin Infect Dis. 2021;73(11):e4073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Inter Med. 2020;173(8):623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorizate M, Kräusslich H-G. Role of lipids in virus replication. Cold Spring Harb Perspect Biol. 2011;3(10):a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melchjorsen J, Risør MW, Søgaard OS, O’Loughlin KL, Chow S, Paludan SR, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57(4):265–75. [DOI] [PubMed] [Google Scholar]
- 21.Zídek Zk, Franková D, Holý A. Activation by 9®[2-(phosphonomethoxy) propyl] adenine of chemokine (RANTES, macrophage inflammatory protein 1α) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1β) production. Antimicrob Agents Chemother. 2001;45(12):3381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg EJ, He G-X, Lee WA. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):1091–8. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz M, Zolopa A, Ruane P, Squires K, Zhong L, Kearney B, et al. editors. GS-7340 demonstrates greater declines in HIV-1 RNA than tenofovir disoproxil fumarate during 14 days of monotherapy in HIV-1 infected subjects. 18th Conference on Retroviruses and Opportunistic Infections; 2011.
- 24.Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382(19):1861–2. [DOI] [PubMed] [Google Scholar]
- 25.Peluso L, Abella BS, Ferrer R, Kucher N, Sunde K, Taccone FS. Fever management in COVID-19 patients. Minerva Anestesiol. 2021;87(1):1–3. [DOI] [PubMed] [Google Scholar]
- 26.Shokati Eshkiki Z, Shahriari A, Seyedtabib M, Torabizadeh M, Assarehzadegan MA, Nashibi R, Khosravi M, Neisi N, Mard SA, Shayesteh AA. Innate and adaptive immunity imbalance with severe COVID-19 pneumonia in children and adults. Front Pediatr. 2021;9:736013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitan RM. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad Emerg Med. 2020;27(8):785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls [internet]. 2022. [PubMed]
- 29.Kow CS, Ramachandram DS, Hasan SS. The use of tenofovir in patients with COVID-19. HIV Med. 2022;23(7):807–8. [DOI] [PubMed] [Google Scholar]
- 30.Mateos-Muñoz B, Buti M, Vázquez IF, Conde MH, Bernal-Monterde V, Díaz-Fontenla F, et al. Tenofovir disoproxil fumarate reduces the severity of COVID-19 in patients with chronic hepatitis B. Dig Dis Sci. 2023:1–7. [DOI] [PMC free article] [PubMed]
- 31.Polo R, García-Albéniz X, Terán C, Morales M, Rial-Crestelo D, Garcinuño MA, et al. Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers. Clin Microbiol Infect. 2023;29(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Painter WP, Sheahan T, Baric R, Holman W, Donovan J, Fang L, et al. Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir. Top Antivir Med. 2021:304–5.
- 33.DeJong C, Spinelli MA, Okochi H, Gandhi M. Tenofovir-based PrEP for COVID-19: an untapped opportunity? AIDS. 2021;35(9):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Diaz S, Bateman BT, Straub L, Zhu Y, Mogun H, Fischer M, et al. Safety of tenofovir disoproxil fumarate for pregnant women facing the coronavirus disease 2019 pandemic. Am J Epidemiol. 2021;190(11):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.