Abstract
Ethylene (C2H4) inhibited H2 evolution by the Mo-containing nitrogenase of Klebsiella pneumoniae. The extent of inhibition depended on the electron flux determined by the ratio of Fe protein (Kp2) to MoFe protein (Kp1) with KiC2H4 = 409 kPa ([Kp2]/[Kp1] = 22:1) and KC2H4i = 88 kPa ([Kp1]/[Kp2] = 21:1) at 23 degrees C at pH 7.4. At [Kp2]/[Kp1] = 1:1, inhibition was minimal with C2H4 (101 kPa). Extrapolation of data obtained when C2H4 was varied from 60 to 290 kPa indicates that at infinite pressure of C2H4 total inhibition of H2 evolution should occur. C2H4 inhibited concomitant S2O4(2-) oxidation to the same extent that it inhibited H2 evolution. Although other inhibitors of total electron flux such as CN- and CH3NC uncouple MgATP hydrolysis from electron transfer, C2H4 did not affect the ATP/2e ratio. Inhibition of H2 evolution by C2H4 was not relieved by CO. C2H4 was reduced to C2H6 at [Kp2]/[Kp1] ratios greater than or equal to 5:1 in a reaction that accounted for no more than 1% of the total electron flux. These data are discussed in terms of the chemistry of alkyne and alkene reduction on transition-metal centres.
Full text
PDF
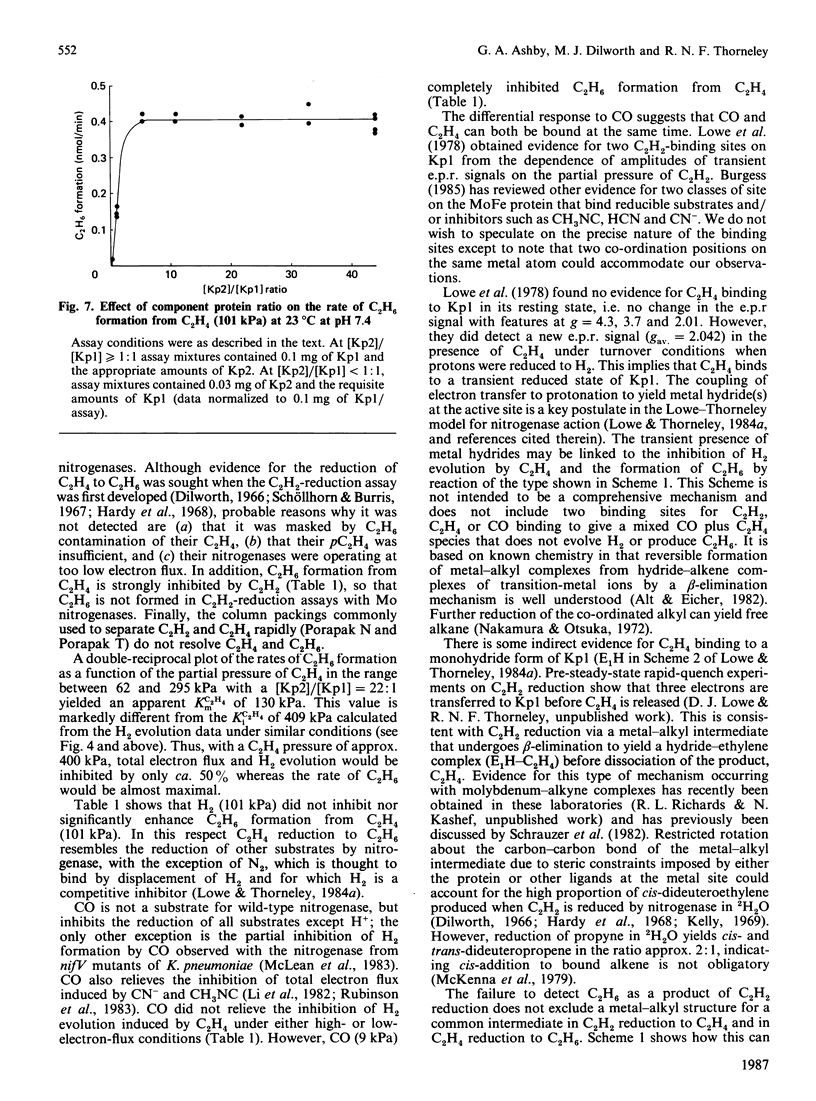
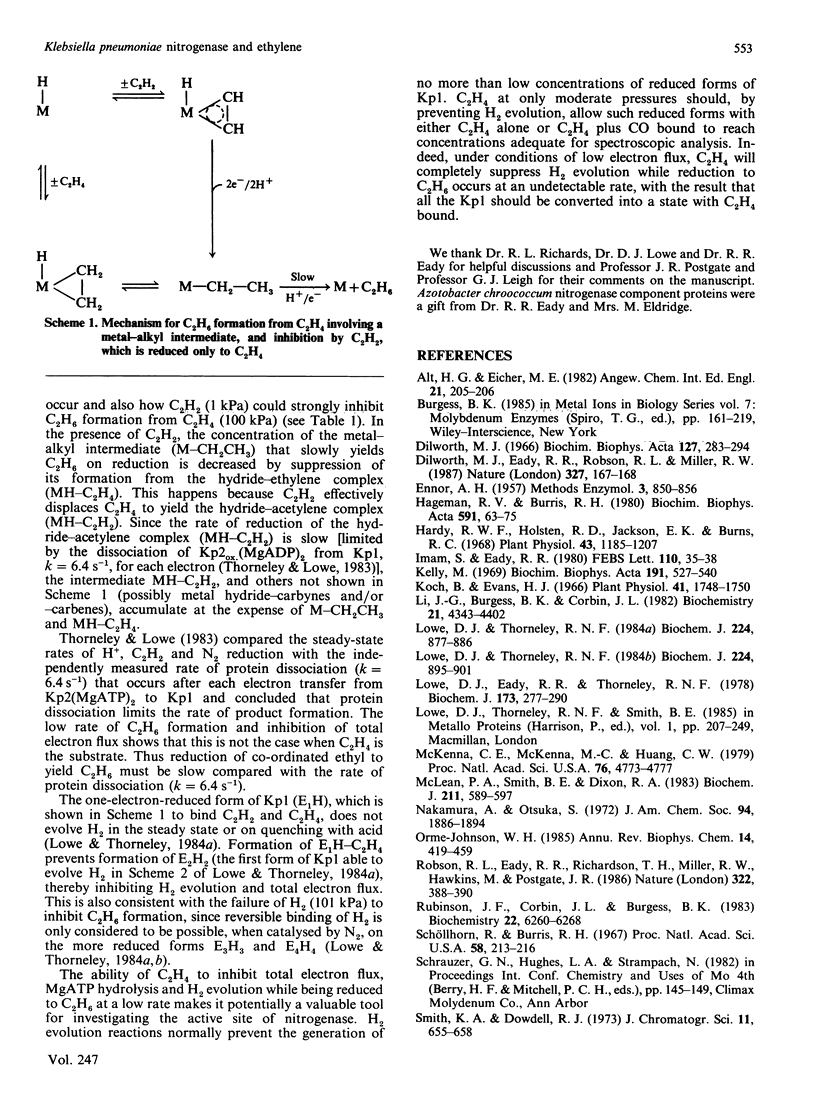

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Electron allocation to alternative substrates of Azotobacter nitrogenase is controlled by the electron flux through dinitrogenase. Biochim Biophys Acta. 1980 Jun 10;591(1):63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S., Eady R. R. Nitrogenase of Klebsiella pneumoniae: reductant-independent ATP hydrolysis and the effect of pH on the efficiency of coupling of ATP hydrolysis to substrate reduction. FEBS Lett. 1980 Jan 28;110(1):35–38. doi: 10.1016/0014-5793(80)80016-0. [DOI] [PubMed] [Google Scholar]
- Kelly M. Comparisons and cross reactions of nitrogenase from Klebsiella pneumoniae, Azotobacter chroococcum and Bacillus polymyxa. Biochim Biophys Acta. 1969;191(3):527–540. doi: 10.1016/0005-2744(69)90346-5. [DOI] [PubMed] [Google Scholar]
- Koch B., Evans H. J. Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol. 1966 Dec;41(10):1748–1750. doi: 10.1104/pp.41.10.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Burgess B. K., Corbin J. L. Nitrogenase reactivity: cyanide as substrate and inhibitor. Biochemistry. 1982 Aug 31;21(18):4393–4402. doi: 10.1021/bi00261a031. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Eady R. R., Thorneley N. F. Electron-paramagnetic-resonance studies on nitrogenase of Klebsiella pneumoniae. Evidence for acetylene- and ethylene-nitrogenase transient complexes. Biochem J. 1978 Jul 1;173(1):277–290. doi: 10.1042/bj1730277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of H2 formation. Biochem J. 1984 Dec 15;224(3):877–886. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. The determination of rate constants required for the simulation of the kinetics of N2 reduction and H2 evolution. Biochem J. 1984 Dec 15;224(3):895–901. doi: 10.1042/bj2240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna C. E., McKenna M. C., Huang C. W. Low stereoselectivity in methylacetylene and cyclopropene reductions by nitrogenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4773–4777. doi: 10.1073/pnas.76.10.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Smith B. E., Dixon R. A. Nitrogenase of Klebsiella pneumoniae nifV mutants. Biochem J. 1983 Jun 1;211(3):589–597. doi: 10.1042/bj2110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Rubinson J. F., Corbin J. L., Burgess B. K. Nitrogenase reactivity: methyl isocyanide as substrate and inhibitor. Biochemistry. 1983 Dec 20;22(26):6260–6268. doi: 10.1021/bi00295a034. [DOI] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Acetylene as a competitive inhibitor of N-2 fixation. Proc Natl Acad Sci U S A. 1967 Jul;58(1):213–216. doi: 10.1073/pnas.58.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of an enzyme-bound intermediate in N2 reduction and of NH3 formation. Biochem J. 1984 Dec 15;224(3):887–894. doi: 10.1042/bj2240887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Simulation of the dependences of H2-evolution rate on component-protein concentration and ratio and sodium dithionite concentration. Biochem J. 1984 Dec 15;224(3):903–909. doi: 10.1042/bj2240903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherland S., Burgess B. K., Stiefel E. I., Newton W. E. Nitrogenase reactivity: effects of component ratio on electron flow and distribution during nitrogen fixation. Biochemistry. 1981 Sep 1;20(18):5132–5140. doi: 10.1021/bi00521a006. [DOI] [PubMed] [Google Scholar]


