Abstract
The COVID-19 pandemic has had profound but incompletely understood adverse effects on youth. To elucidate the role of brain circuits in how adolescents responded to the pandemic’s stressors, we investigated their prepandemic organization as a predictor of mental/emotional health in the first ~15 months of the pandemic. We analyzed resting-state networks from n = 2,641 adolescents [median age (interquartile range) = 144.0 (13.0) months, 47.7% females] in the Adolescent Brain Cognitive Development study, and longitudinal assessments of mental health, stress, sadness, and positive affect, collected every 2 to 3 months from May 2020 to May 2021. Topological resilience and/or network strength predicted overall mental health, stress and sadness (but not positive affect), at multiple time points, but primarily in December 2020 and May 2021. Higher resilience of the salience network predicted better mental health in December 2020 (β = 0.19, 95% CI = [0.06, 0.31], P = 0.01). Lower connectivity of left salience, reward, limbic, and prefrontal cortex and its thalamic, striatal, amygdala connections, predicted higher stress (β = −0.46 to −0.20, CI = [−0.72, −0.07], P < 0.03). Lower bilateral robustness (higher fragility) and/or connectivity of these networks predicted higher sadness in December 2020 and May 2021 (β = −0.514 to −0.19, CI = [−0.81, −0.05], P < 0.04). These findings suggest that the organization of brain circuits may have played a critical role in adolescent stress and mental/emotional health during the pandemic.
Keywords: adolescents, brain circuits, COVID-19, mental health, stress
Introduction
The COVID-19 pandemic has had profound multifaceted adverse impacts on individuals, institutions and societies across the world that may take years to elucidate and recover from. Its effects on mental health are incompletely understood, but likely extensive, especially in children (Singh et al. 2020; Aknin et al. 2022). It is estimated that over 30% of all children in the United States experienced increased anxiety, and ~25% experienced depression. Also, almost 40% of adolescents had worse mental health during the pandemic and almost 50% reported negative emotions, such as fear and sadness (Theberath et al. 2022; Bell et al. 2023; CDC 2020).
As the global mental health crisis continues to grow in the aftermath of the pandemic, there is an urgent need to elucidate protective and risk factors prior to the outbreak that may played an important role in individuals’ responses (including mental health outcomes) to its stressors. This is especially important in developing children in sensitive developmental periods, such as adolescence. During adolescence—a period of heightened neural maturation and extensive biological changes and social development—youth are at higher risk of mental health disorders (Giedd et al. 2008). Developing brain circuits that support mental health undergo profound reorganization and are vulnerable to negative environmental and experiential factors. Social disruption and isolation associated with lockdowns, school closures and limited in-person interactions with peers, and/or a negative family environment may have had profound effects on mental health that are currently poorly understood (Creswell et al. 2021).
Prior studies have reported anxiety and depression, irritability, stress, loneliness, and fear as the most common mental health issues in youth during the pandemic (Nearchou et al. 2020, Garcia de Avila 2020; Duan et al. 2020; Esposito et al. 2021; Hafstad et al. 2021; Panchal et al. 2023; Samji et al. 2022; Theberath et al. 2022; Li et al. 2022). A recent meta-analysis of 80,000 youth showed that the prevalence of depression and anxiety symptoms doubled during the outbreak and further increased in later stages of the pandemic (Racine et al. 2021). Increased media exposure, school closures and disruption to the school day routine, social isolation, parental mental health issues and stress, unsupportive parenting, and family conflict were significant risk factors for mental health issues in youth. In contrast, positive parenting, good parent–youth communication, access to peers, physical activity, sufficient and high-quality sleep, and good nutrition were protective factors (Brown et al. 2020; Fish et al. 2020; Magson et al. 2021; Panchal et al. 2023; Chi et al. 2021; Glynn et al. 2021; Rosen et al. 2021; Bzdok et al. 2022). Furthermore, pre-existing physical conditions, mental health issues, and neurodevelopmental disorders increased the likelihood of pandemic-related mental health and behavioral issues (Ademhan-Tural et al. 2020; Alshahrani et al. 2020; Colizzi et al. 2020; Hawke et al. 2021; Rosenthal et al. 2022; Kleine et al. 2023) and/or amplified existing problems (Masi et al. 2021).
A relatively small number of studies has examined the effects of COVID-19 on youth mental health in the context of the brain. A recent study compared age- and demographics-matched groups of adolescents before and after pandemic-related shutdowns and showed that in youth measured after the shutdowns, mental health issues (including depression, anxiety, and other internalizing problems) were significantly more prevalent than in those measured prior to the pandemic (Gotlib et al. 2023). It also showed that adolescents assessed after the shutdowns had lower bilateral cortical thickness and larger hippocampal and amygdala volume, suggesting that teen brains aged during the pandemic. These findings are in agreement with another study on structural brain development in adolescents, which examined areas of the social brain, showed accelerated thinning of the medial prefrontal cortex and increased hippocampal volume during the pandemic, but overall resilience of the temporoparietal junction to the effects of social restrictions (van Drunen et al. 2023). Furthermore, a study based on a longitudinal adolescent cohort (9 to 15 years at baseline), compared resting-state connectivity before and during the pandemic and showed that youth who had experienced less positive parenting had stronger connections between the subgenual anterior cingulate cortex and basolateral amygdala. Higher connectivity between these regions was associated with increased depressive symptoms during the pandemic (Miller et al. 2021). Finally, a study on adults in Israel measured participants before and after the outbreak and reported increased volume in bilateral amygdala, putamen, and the anterior temporal cortices following the outbreak (Salomon et al. 2021). However, volumetric changes in amygdala decreased over time after the lockdown, suggesting that these changes were likely transient. To date, very few studies have examined the impact of brain structure and function prior to the pandemic on individual responses during the outbreak. One study in adolescents measured amygdala volume and activation during an emotional face processing task prior to the pandemic and found that higher activity in the left amygdala in response to neutral faces compared to fearful ones was associated with increased internalizing problems in the early phases of the pandemic (Weissman et al. 2021).
The historically large longitudinal Adolescent Brain Cognitive Development (ABCD) study (Casey et al. 2018) has also facilitated investigations of relationships between the COVID-19 pandemic and youth mental health in a large sample. Studies have reported significant changes in screen time, sleep duration, and physical activity during the pandemic and their adverse effects on mental and emotional health (Nagata et al. 2022; Kiss et al. 2022, 2023). In addition, area disadvantage, socioeconomic status, parental education, having experienced racism, and family structure were linked to negative mental and physical health outcomes (Marshall et al. 2022; Raney et al. 2022; Yip et al. 2022). Another study showed that attention problems, withdrawal issues, and depression worsened during the pandemic in this cohort (Hamatani et al. 2022). Finally, family financial stress was associated with increased youth depressive symptoms (Argabright et al. 2022), while overall mental health was disproportionately negatively impacted in youth from racial and ethnic minority groups (Xiao et al. 2022).
Independently of the pandemic, mental health problems and disorders have been linked to abnormalities in structural and functional brain circuits (Bassett and Bullmore 2009; Broyd et al. 2009; Lynall et al. 2010; Menon 2011, 2020; Sylvester et al. 2012; Zhang et al. 2016; Yu et al. 2019; Chen et al. 2022; Taylor et al. 2023; Qu et al. 2023), including during development (Roberson-Nay et al. 2006; Krain et al. 2008; Cullen et al. 2009; Roy et al. 2013; Britton et al. 2013; Liu et al. 2015; LeWinn et al. 2014; Holt et al. 2016). However, to date, the majority of studies on mental health in youth during and after the pandemic have not examined characteristics of the brain’s circuitry prior to the pandemic that may have either provided resilience to stressors or may have predisposed youth to mental health problems. As the medical community strives to elucidate the many exogenous and endogenous risk and protective factors that impacted mental health in youth as a result of the pandemic, there is a critical need to investigate the role of developing brain circuits prior to the pandemic on youth responses and mental health outcomes during the outbreak.
To address this significant unmet need, this study investigated whether the prepandemic organization (topological properties) of resting-state brain networks, which represent the backbone of the functional connectome, was a protective or risk factor for mental health and stress during the outbreak. For this purpose, it analyzed fMRI data from adolescents in the ABCD study collected ~9 months before longitudinal assessments of mental/emotional health and stress during the first ~15 months of the pandemic (survey data were collected at seven time points from May 2020 to May 2021). It hypothesized that the organization and resilience of large-scale networks that play a fundamental role in cognitive and mental health predicted youth responses to the pandemic’s stressors. It specifically examined resting-state networks that support emotional processing, attention, and executive function, as well as brain regions that, as a network, support social function. These networks overlap with the underdeveloped (in adolescence) prefrontal cortical network and its subcortical projections. In addition to other topological properties, the study specifically examined topological resilience and fragility of these networks, hypothesizing that the former was a protective factor for mental health, against the effects of social isolation and other stressors during the outbreak, while the latter predisposed youth to higher risks of depression and internalizing behaviors during the lockdowns. Topological properties were investigated as predictors of these outcomes at multiple scales of spatial organization, from the entire connectome to individual brain regions.
Methods
This study was approved by the institutional review board. Publicly available survey, neuroimaging, and other individual data from the ABCD study were analyzed. All data were from release 4.0 and are available through the National Institute of Mental Health Data Archive (NDA).
Participants
Neurotypical adolescents [median age at the time of the fMRI scan = 12.0 years, interquartile range (IQR) = 1.1 years], measured at the 2-year follow-up of the ABCD study were included. In order to study mental health outcomes during the pandemic independently of diagnosed neuropsychiatric and neurodevelopmental disorders, youth with bipolar disorder, schizophrenia, psychotic disorders, autism spectrum disorder, and attention-deficit/hyperactivity disorder were excluded. These disorders have also been associated with aberrant changes in the organization of the connectome (Cherkassky et al. 2006; Monk et al. 2009; Assaf et al. 2010; Müller et al. 2011; Konrad and Eickhoff 2010; Chase and Phillips 2016). A total of 2,641 youth were studied, including 2,174 (82.3% of the cohort) scanned prior to the date when the World Health Organization declared COVID-19 a pandemic (2020 March 11; WHO 2020). Median time from scanning to outbreak was 7 months (IQR = 8 months, maximum = 19 months). To ensure that the interval between the fMRI scan and a survey was shorter than a transition between pubertal stages [e.g. based on the Tanner scale (Marshall and Tanner, 1969, 1970)], a subcohort was identified, which had been scanned at most 9 months prior to a particular survey. This cutoff was selected to minimize potential confounding effects of developmental brain changes in the interval between fMRI scan and survey, independently of the pandemic. Thus, two partially overlapping subcohorts were analyzed: cohort A (primary study cohort): n = 1,414 scanned within 9 months of each survey, and cohort B: n = 2,174 youth scanned prior to the outbreak, irrespective of time of scanning relative to the outbreak. Data from seven surveys were analyzed; thus, from each of the subcohorts, several partially overlapping samples were selected. In cohort A, sample sizes varied from n = 802 at survey 1 to n = 218 at survey 7. In cohort B, sample sizes varied from n = 1,451 in survey 1 (median time from scanning = 9.0 months, IQR = 7 months) to n = 1,135 in survey 7 (median time from scanning = 22.0 months, IQR = 7.0 months). Sample overlap statistics are provided in Tables S1 and S2. Race and ethnicity distributions of n = 2,641 youth in this study reflected those of the overall ABCD cohort, which is predominantly White and non-Hispanic: Over 60% were White [1,655 (62.3%)] vs 944 (35.7%) from a racial minority group, and 2,047 (77.5%) were non-Hispanic. About 25% of participants were in early puberty (n = 658, 24.9%) and ~40% in mid puberty (n = 1,031; 39.0%), and slept on average 8 to 9 h per day. Finally, more than half of primary caregivers had at least a bachelor’s degree (1,452; 55.0%). Detailed participant demographic and other data statistics are provided in Table 1.
Table 1.
Demographic information for n = 2,641 participants who were scanned prior to 2020 March 1 or ≤9 months prior to at least one of the surveys. The “other” race category included participants from smaller groups (Alaska Native, American Indian, Asian Indian, Chinese, Guamanian, Hawaiian, Japanese, Korean, Native Samoan, other Pacific Islander, other Asian, Filipino, Vietnamese), those who selected “other race,” and those who selected more than one racial group.
| n = 2,641 | ||
|---|---|---|
| Age (months) | Median (IQR) | 144 (13) |
| Range | [127, 166] | |
| Sex | Female | 1,260 (47.71%) |
| Male | 1,381 (52.29%) | |
| Race | White | 1,655 (62.67%) |
| Black | 549 (20.79%) | |
| Asian | 172 (6.51%) | |
| Other | 223 (8.44%) | |
| Missing | 42 (1.59%) | |
| Ethnicity | Hispanic | 569 (21.54%) |
| Non-Hispanic | 2,047 (77.51%) | |
| Missing | 25 (0.95%) | |
| BMI | Median (IQR) | 19.43 (5.59) |
| Missing | 12 (0.45%) | |
| Sleep length (h) | Median (IQR) | 8 to 9 (2) |
| Missing | 1 (0.04%) | |
| Pubertal stage | Prepuberty | 318 (12.04%) |
| Early puberty | 658 (24.91%) | |
| Mid puberty | 1,031 (39.04%) | |
| Later pubertal stage | 512 (19.39%) | |
| Missing | 122 (4.62%) | |
| Family income | <5,000 | 39 (1.48%) |
| 5,000 to 24,999 | 149 (5.64%) | |
| 25,000 to 49,999 | 290 (10.98%) | |
| 50,000 to 99,999 | 686 (25.97%) | |
| 100,000 to 199,999 | 872 (33.02%) | |
| ≥200,000 | 418 (15.83%) | |
| Missing | 187 (7.08%) | |
| Primary caregiver education | Advanced degree (Master’s professional (MD, JD, etc.) and doctoral degrees) | 696 (26.35%) |
| Bachelor’s degree | 756 (28.63%) | |
| Associate degree | 358 (13.56%) | |
| Some college | 405 (15.34%) | |
| High school/GED | 251 (9.50%) | |
| Did not graduate high school | 154 (5.83%) | |
| Missing | 21 (0.79%) |
COVID-19-specific surveys
All ABCD participants were invited to complete a series of brief surveys on their overall mental health, emotional responses, stress, and coping during the pandemic. Data from seven Rapid Response Research (RRR) surveys were collected in May, June, August, October, and December of 2020, as well as March and May 2021. Inter-survey intervals, and median time from fMRI scan to each survey (in the range 3.0 to 7.0 months) are shown in Fig. 1. Surveys were sent to participants electronically. About 40% to 50% of eligible participants in the ABCD cohort provided responses (varying from ~6,000 in May and June 2020 surveys to ~4,800 in May 2021). Although surveys were administered to both youth and primary caregivers, this study focused on youth assessments. Surveys asked participants to provide information on social activities, parent interactions, mental health, stress, and overall well-being, and daily routine changes such as sleep and use of electronic devices. The outcomes investigated in this study were overall mental health, stress, sadness, and positive affect. Not all surveys included the same response variables, although all included one or two questions on stress. Four surveys included a question on overall mental health, and four surveys asked participants to report on their sadness. Availability of responses at each survey are summarized in Table S3. Questions from the survey included: (i) “How do you think your mental health (emotional well-being) is in the past week compared to normal?” [measured in a scale 1 (much worse) to 5 (much better)] and (ii) “COVID-19 presents a lot of uncertainty about the future. In the past 7 days, including today, how stressful have you found this uncertainty to be?” [measured in a scale 1 (not at all/very slightly) to 5 (extremely)]. Both responses were standardized and were analyzed as z-scores. In addition, a measure of sadness was provided by the ABCD study as a t-score estimated from the National Institutes of Health Toolbox Emotion Battery (Sadness survey). Higher scores indicated more frequent negative mood and negative views of self and negative social cognition (Salsman et al. 2013). Raw sum of responses from questions on positive affect (from the NIH Toolbox Positive Affect survey) from the RRR survey were standardized as z-scores. Responses to questions from the 4-item Perceived Stress Scale were reverse-coded from the original (for easier interpretation) and then summed and standardized as a z-score. Higher scores indicated more frequent perceived stress (Cohen et al. 1983). In statistical analyses, the five measures of interest (overall mental health, stress associated with the pandemic uncertainty, perceived stress, sadness, and positive affect) were investigated as independent outcomes. All analyses included adjustments for number of months between COVID-19 pandemic onset and date of response to each survey, as well as number of months between MRI scan and each survey.
Fig. 1.
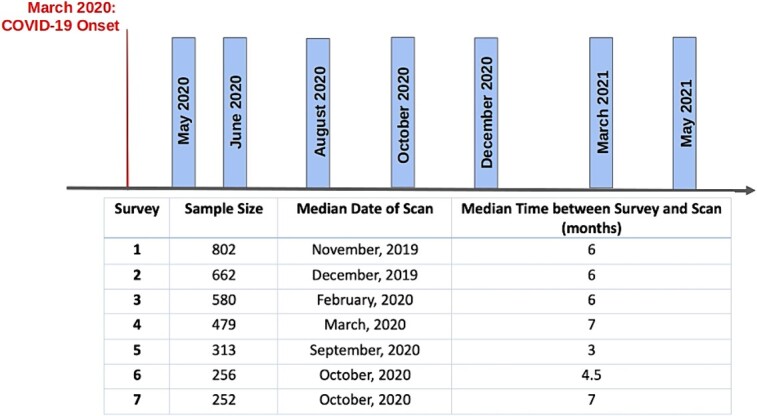
COVID survey timing, sample sizes (out of the primary cohort of n = 1414 youth), date of fMRI scan in survey-specific cohorts, and median time between survey and fMRI scan.
COVID-related mental health, emotions, and stress as additional adjustments
In cohort A, given the inclusion criterion of fMRI scans being within 9 months from a particular COVID survey, some participants were scanned during the pandemic, particularly in cohorts associated with surveys 5 to 7. To account for neuromodulatory effects of the pandemic’s stressors on brain circuits, in models for outcomes measured in surveys 2 to 7, prior survey measurements of that outcome were included as additional adjustments. For example, models examining topological properties as predictors of sadness in survey 7 included an average of reported sadness in surveys 1, 3, and 5 (i.e. all prior surveys in which this outcome was measured) as an additional adjustment. Participants in each of the survey-specific cohorts had some missing data in prior surveys, with ~ 23% to 35% missing the immediate prior survey assessing a particular outcome (for example, mental health in survey 5 missing for the cohort analyzed in survey 7), and higher percentages as a function of longer intervals between surveys. These data were assumed to be missing at random and were imputed using the k-nearest neighbor approach.
fMRI analysis and estimation of topological properties
fMRI preprocessing
Resting-state fMRI (rs-fMRI), collected at 21 sites of the ABCD study (using 3.0T Siemens, GE Medical Systems, or Phillips Medical Systems scanners) were analyzed. Each participant had up to 4 rs-fMRI runs, each 5-min long. Scanning details are provided in Hagler et al. (2019). A common sampling rate of 0.8 samples/s was used across scanners, and signal amplitudes were normalized as part of necessary data harmonization, to account for scanner-related measurement differences. Prior to public release, minimal initial preprocessing of structural MRI (sMRI) and fMRI was performed by the ABCD study’s dedicated Data Analysis, Informatics & Resources Center (DAIRC), which included corrections for head motion, B0 distortions, and distortions associated with gradient nonlinearities (Hagler et al. 2019). Minimally processed fMRI data were further processed using the Next Generation Neural Data Analysis (NGNDA) platform. This level of processing included coregistration of the rs-fMRI to structural MRI, normalization to common MNI space, motion correction via regression, frame removal based on excessive motion, interpolation to reduce artifacts, and filtering in the range 0.01 to 0.25 Hz. Further, the cortical Schaefer-1000, subcortical Melbourne, and cerebellar Diedrichsen atlases were used to downsample voxel-level fMRI time series to parcel-level time-series [averaging voxels within each parcel defined by these atlases (Schaefer et al. 2018; Tian et al. 2020; Diedrichsen et al. 2009)]. Parcel signals were further denoised to suppress additional artifacts (for example, cardiorespiratory and non-biological artifacts) that were unrelated to blood-oxygen-level-dependent (BOLD) activity in the brain (Brooks et al. 2021). Following this extensive preprocessing, fMRI runs with more than 10% of frames censored for motion (based on displacement > 0.3 mm) were excluded from subsequent analyses. For each participant, their best-quality fMRI run was analyzed and had a consistently low percent of frames censored for motion (typically <1%).
Estimation of fMRI properties
Peak cross-correlation between pairs of fMRI time series was estimated as a measure of resting-state connectivity. Resulting matrices were further processed to eliminate weak and/or spurious correlations. A cohort-wide, conservative statistical threshold was estimated (Brooks et al. 2021). Correlation statistics across brains were bootstrapped to estimate multiple thresholds [median, 75th percentile, moderate outlier (= median + 1.5*IQR), and extreme outlier (= median + 3*IQR)]. From these, the moderate outlier was chosen as the most appropriate threshold, under the assumption that brain networks at rest are sparsely connected. An alternative percolation-based method was also used, resulting in a threshold that was almost identical to the 75th percentile, and was thus not sufficiently conservative. Following thresholding, the resulting weighted adjacency matrices (and their binary versions) were used in subsequent estimation of topological properties.
Topological properties of multiple networks were estimated, including previously identified large-scale resting-state networks (Yeo et al. 2011), the reward network (Haber and Knutson 2010), the social network—a set of distributed brain regions that together support social function (Blakemore 2008), and the prefrontal cortex and its projections (fronto-thalamic, fronto-amygdala, and fronto-striatal circuits and their interconnections). These properties included brain-wide and network-specific efficiency, median connectivity (within network and out-of-network), network and regional (local) clustering, modularity, topological stability (Restrepo et al. 2007), fragility, and robustness. Fragility was estimated based on a perturbation approach, as the inverse of the stability radius, which is defined as the smallest perturbation Δ to the adjacency matrix A that renders the underlying dynamic system unstable (Pasqualetti et al. 2020). To ensure stability of the original system (and thus all the eigenvalues of A to be negative), each adjacency matrix was first normalized as: 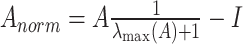 (Karrer et al. 2020). Natural connectivity (the average of the adjacency matrix eigenvalues) was estimated as a measure of robustness (Wu et al. 2009). All topological metrics were calculated using algorithms implemented in the Brain Connectivity Toolbox (Rubinov and Sporns 2010) and the NGNDA platform.
(Karrer et al. 2020). Natural connectivity (the average of the adjacency matrix eigenvalues) was estimated as a measure of robustness (Wu et al. 2009). All topological metrics were calculated using algorithms implemented in the Brain Connectivity Toolbox (Rubinov and Sporns 2010) and the NGNDA platform.
Additional variables
To account for sampling differences across sites, all analyses were adjusted using propensity scores provided by the ABCD (American Community Survey [ACS] Post Stratification Weights Instrument). Demographic variables included age, sex, family income, ethnicity (Hispanic vs non-Hispanic) and race. The latter was dichotomized as White vs non-White in statistical analyses, given that the sample was primarily White and there was insufficient statistical power for granular analyses of racial minority groups. Beyond demographics, body mass index (BMI) was calculated from available height and weight measurements and was then standardized as a z-score stratified by sex. Adjustments for pubertal stage were also included in models. Although cohort A had MRI/fMRI scans within 9 months from a particular survey, cohort B had scans at any time (within the 2-year follow-up period) before the outbreak, so including adjustments for pubertal stage were important for this cohort. Information on the ABCD study site at which each participant was scanned was also available (information was extracted from the ABCD Longitudinal Tracking Instrument).
fMRI/scanning variables
Statistical analyses were adjusted for two fMRI scan parameters: (i) time of acquisition (in hours), which was extracted from MRI QC Raw report and was rounded to the nearest hour at which scanning session began. Prior work has shown that resting-state topological parameters, including in the ABCD cohort, may be impacted by the timing of data acquisition (Vaisvilaite et al. 2022; Hu et al. 2023), and (ii) percent of frames censored for motion in the analyzed fMRI run.
Mental health variables
Information on common mental health and behavioral issues in youth, particularly anxiety (24.3% of the sample had anxiety) and depression (7.1% had depression), and internalizing [median (IQR) score = 46 (15)] externalizing behaviors [median (IQR) score = 41 (15)] was also extracted. Anxiety and depression were represented by binary variables derived from questions on the ABCD Parent Diagnostic Interview for DSM-5 (KSADS-5). Any symptom or diagnosis of anxiety/depression was coded as 1 (and 0 otherwise). Internalizing and externalizing t-scores were extracted from the ABCD Parent Child Behavior Checklist (CBCL). Information on history of trauma (yes = 1, no = 0) was extracted from the Parent Diagnostic Interview (34.4% of the sample reported history of any trauma).
Social environmental variables
Prior research, including studies based on the ABCD cohort, has shown that parent engagement served as a protective factor for youth mental health during the pandemic (Hamatani et al. 2022). Here, it was estimated as a standardized mean of four questions from the Youth ABCD Covid-19 Questionnaire. The resulting z-score was included in analyses to account for confounding effects of parent engagement in the participants’ daily routine. A binary (yes = 1, no = 0) variable for the response to the question “does our child have a best friend” was extracted from ABCD Longitudinal Parent Diagnostic Interview, and a binary (very true/often true = 1, otherwise = 0) variable for the response to the question on whether the child “would rather be alone than with others” was extracted from the CBCL.
Statistical analyses
Linear mixed-effects models were developed and included a random intercept and slope for each of the 21 ABCD sites where participants had been measured, to account for potential differences in youth survey outcomes resulting from differential policies and measures to contain the virus spread in corresponding states. Brain-wide, network-specific, and regional resting-state topological network parameters were the predictors of interest and COVID survey outcomes the dependent variables. Separate sets models were developed for each outcome.
The primary set of models were adjusted for sex, age, race, ethnicity, family income, BMI z-score [prior work on the baseline ABCD cohort has reported significant associations between BMI and topological brain properties (Brooks et al. 2023)], prior survey assessments of the outcome of interest, scan parameters, parent engagement, time between the pandemic onset and each covid survey, and time between the fMRI scan and each covid survey.
Additional sets of models were also developed and included: (i) pre-pandemic anxiety and depression individually and in combination, and similarly for pre-pandemic internalizing and externalizing behaviors; (ii) history of trauma; and (iii) variables related to peer relations and social connectedness prior to the pandemic. An additional adjustment for behavioral inhibition (a score from the Behavioral Inhibition/Behavioral Approach Systems Scales instrument) was included in a separate set of models.
Model validation
Leave-one-out cross-validation was used to evaluate the models and assess their predictive power. The leave-one-out approach was repeated for each observation in the sample in each of the surveys, for the primary set of models and additional sets that adjusted for anxiety and depression in combination, and internalizing and externalizing behaviors in combination. Mean squared error (MSE) was calculated at each repetition, and the median MSE across iterations was used to assess the quality of the predictors. Across analyses, P-values were adjusted for the false discovery rate (FDR), using established approaches (Benjamini and Hochberg 1995). The software Matlab (release R2023a, Mathworks, Inc) was used in all neuroimaging data analyses and statistical modeling.
Results
Across both cohorts and multiple surveys, properties of multiple networks (but most frequently those of the salience network) were significant topological predictors of overall mental health, stress, and/or sadness. In general, higher connectivity and robustness (and/or, to a lesser extent, efficiency) of these networks predicted lower stress, sadness, and better mental health, whereas higher fragility (and, to some extent, modularity) was associated with higher sadness and/or stress.
Results based on primary cohort with rs-fMRI collected within 9 months of COVID-19 surveys
Topological predictors of overall mental health, emotional responses, and stress were identified at two time points (December 2020 and May 2021) across multiple networks shown in Fig. 2.
Fig. 2.
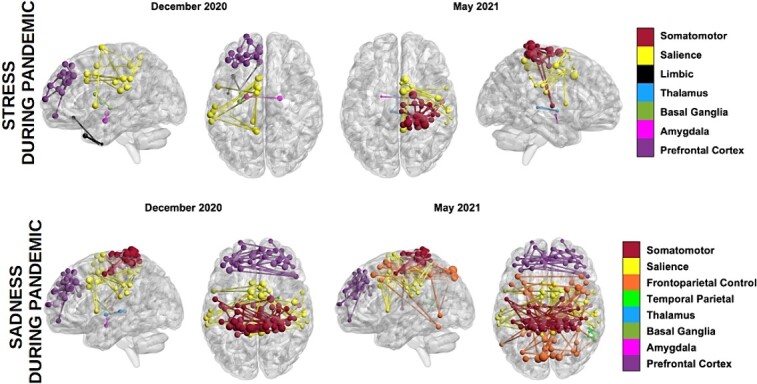
Networks with topological properties predictive of stress and sadness during the pandemic in December 2020 and May 2021.
Higher robustness of the left salience network predicted better mental health reported in the December 2020 survey (P < 0.05, β = 0.19, CI = [0.06, 0.31]), whereas lower median connectivity of the right amygdalo-thalamic circuit and limbic network predicted higher perceived stress (P < 0.05, β = −0.17 to −0.13, CI = [−0.26, −0.04]). MSE values were in the range 0.17 to 0.26. Lower median connectivity within the left somatomotor network and between the network and the rest of the brain, lower connectivity of the right amygdalo-thalamic circuit, and lower median connectivity between right basal ganglia and the rest of the brain predicted higher sadness in December 2020 (P < 0.02, β = −0.20 to −0.19, CI = [−0.32, −0.07]). MSE values were in the range 0.44 to 0.53 (with the lowest MSE associated with connectivity of the right amygdalo-thalamic circuit). Lower median connectivity between the left limbic network and the rest of the brain and similarly for the right amygdalo-thalamic circuit (and connectivity within the circuit) predicted higher sadness reported in the May 2021 survey (P < 0.05, β = −0.24 to −0.17, CI = [−0.40, −0.06]). MSE values were in the range 0.34 to 0.39 (with the lowest MSE associated with out-of-network connectivity of the right amygdalo-thalamic circuit). These results were based on models unadjusted for prepandemic mental health and behavioral issues. Model statistics are provided in Table S4. At both the December 2020 and May 2021 assessments, average contributions of stress and sadness measured in prior surveys (i.e. adjustments for cumulative effects of the pandemic on these outcomes) were significant across models.
Results based on models adjusted for prepandemic mental health problems
In analyses that accounted for history of anxiety and depression, both parameters were significant contributors in some but not all models, particularly those with sadness as the outcome in the December 2020 and May 2021 surveys. Their inclusion did not alter the significance of any of the previously identified predictions of overall mental health and sadness, but eliminated the prediction of perceived stress by the connectivity of the left amygdalo-thalamic circuit and left limbic network. MSE values were in the range 0.17 to 0.52 (with the lowest MSE associated with robustness of the left salience network). Detailed model statistics are provided in Table 2.
Table 2.
Statistics of mixed-effects models with combined adjustments for prior anxiety and depression symptoms/diagnosis (one set of models) and combined internalizing and externalizing behaviors (another) set of models. All reported P-values have been corrected for false discovery. *NS: nonsignificant; CI: confidence interval.
| Network | Property | Statistic | Value | Property | Statistic | Value |
|---|---|---|---|---|---|---|
| Adjustment for prior anxiety and depression (together) | ||||||
| DECEMBER 2020 SURVEY (# 5) | ||||||
| Outcome: OVERALL MENTAL HEALTH | ||||||
| Left hemisphere | Right hemisphere | |||||
| Salience | Robustness | Beta | 0.185 | NS* | ||
| 95th % CI | [0.056, 0.314] | |||||
| P-value | 0.012 | |||||
| Outcome: SADNESS | ||||||
| Left hemisphere | Right hemisphere | |||||
| Somatomotor | Efficiency, global clustering, median conn. (in,out), stability | Beta | −0.227 to −0.135 |
Median conn. (out) | Beta | −0.196 |
| 95th % CI | [−0.342, −0.012] | 95th % CI | [−0.319, −0.074] | |||
| P-value | 0.001 to 0.050 | P-value | 0.015 | |||
| Basal ganglia | NS | Median conn. (out) | Beta | −0.195 | ||
| 95th % CI | [−0.313, −0.077] | |||||
| P-value | 0.010 | |||||
| Amygdalo-thalamic | Median conn. (in) | Beta | −0.178 | |||
| 95th % C | [−0.291, −0.065] | |||||
| P-value | 0.017 | |||||
| Adjustment for prior externalizing and internalizing behavior scores (together) | ||||||
| DECEMBER 2020 SURVEY (# 5) | ||||||
| Outcome: OVERALL MENTAL HEALTH | ||||||
| Left hemisphere | Right hemisphere | |||||
| Somatomotor |
Median conn (out),
robustness |
Beta | 0.279 to 0.362 |
Median conn (out),
robustness |
Beta | 0.215 to 0.333 |
| 95th % CI | [0.067, 0.119] | 95th % CI | [0.016, 0.564] | |||
| P-value | 0.007 to 0.014 | P-value | 0.009 to 0.039 | |||
| Outcome: PERCEIVED STRESS | ||||||
| Salience ventral attention | Median conn. (in, out) | Beta | −0.322 to −0.303 | NS | ||
| 95th % CI | [−0.565, −0.080] | |||||
| P-value | 0.016 | |||||
| Reward | Median conn. (in, out) | Beta | −0.428 to −0.374 | NS | ||
| 95th % CI | [−0.692, −0.150] | |||||
| P-value | 0.008 | |||||
| Limbic | Median conn. (out) | Beta | −0.317 | NS | ||
| 95th % CI | [−0.512, −0.122] | |||||
| P-value | 0.007 | |||||
| Basal ganglia | Median conn. (out) | Beta | −0.377 | NS | ||
| 95th % CI | [−0.610, −0.145] | |||||
| P-value | 0.007 | |||||
| Prefrontal cortex |
Median conn. (in)
median Conn. (out) |
Beta | −0.461 to −0.326 | |||
| 95th % CI | [−0.720, −0.149] | |||||
| P-value | 0.003 | |||||
| Fronto- thalamic circuit | Median conn. (in,out) | Beta | −0.398 to −0.324 | NS | ||
| 95th % CI | [−0.669, −0.126] | |||||
| P-value | 0.028 | |||||
| Fronto- amygdala circuit | Median conn. (in,out) | Beta | −0.432 to −0.354 |
NS | ||
| 95th % C | [−0.685, −0.178] | |||||
| P-value | 0.003 to 0.005 | |||||
| Fronto-striatal circuit | Median conn. (in,out) | Beta | −0.379 to −0.340 | NS | ||
| 95th % CI | [−0.639, −0.116] | |||||
| P-value | 0.020 | |||||
|
Fronto-basal ganglia
circuit |
Median conn. (in,out) | Beta | −0.384 to −0.339 | NS | ||
| 95th % CI | [−0.643, −0.116] | |||||
| P-value | 0.017 | |||||
| Amygdalo- thalamic circuit | Median conn. (out) | Beta | −0.204 | NS | ||
| 95th % Cl | [−0.342, −0.067] | |||||
| P-value | 0.022 | |||||
| Fronto- striato- thalamic circuit | Median conn. (in,out) | Beta | −0.363 to −0.317 | NS | ||
| 95th % CI | [−0.635, −0.091] | |||||
| P-value | 0.021 | |||||
| Fronto-basal ganglia- amygdala circuit | Median conn. (in, out) | Beta | −0.363 to −0.322 | NS | ||
| 95th % CI | [−0.621, −0.106] | |||||
| P-value | 0.023 | |||||
| Outcome: SADNESS | ||||||
| Reward | NS | Robustness | Beta | −0.357 | ||
| 95th % CI | [−0.581, −0.133] | |||||
| P-value | 0.009 | |||||
| Salience | Median conn. (out) | Beta | −0.517 | Median conn. (out) | Beta | −0.249 |
| 95th % CI | [−0.814, −0.221] | 95th % CI | [−0.419, −0.078] | |||
| P-value | 0.007 | P-value | 0.039 | |||
| Somatomotor | NS | Median conn. (in), median conn. (out), robustness | Beta | −0.346 to −0.258 | ||
| 95th % CI | [0.616, −0.042] | |||||
| P-value | 0.033 | |||||
| Prefrontal cortex | NS | Robustness | Beta | −0.397 | ||
| 95th % CI | [−0.641, −0.154] | |||||
| P-value | 0.014 | |||||
| Basal ganglia | Median conn. (out) | Beta | −0.431 | Median conn. (out) | Beta | −0.372 |
| 95th % CI | [−0.730, −0.132] | 95th % CI | [−0.558, −0.185] | |||
| P-value | 0.013 | P-value | 0.001 | |||
| Fronto- thalamic circuit | Robustness | Beta | −0.276 | Robustness | Beta | −0.379 |
| 95th % CI | [−0.465, −0.087] | 95th % CI | [−0.597, −0.160] | |||
| P-value | 0.015 | P-value | 0.007 | |||
| Fronto- amygdala circuit | Robustness | Beta | −0.276 | Robustness | Beta | −0.385 |
| 95th % CI | [−0.466, −0.086] | 95th % CI | [−0.612, −0.159] | |||
| P-value | 0.027 | P-value | 0.009 | |||
| Fronto-striatal circuit | Robustness | Beta | −0.286 | Robustness | Beta | −0.405 |
| 95th % CI | [−0.476, −0.095] | 95th % CI | [−0.635, −0.174] | |||
| P-value | 0.022 | P-value | 0.006 | |||
|
Frontal- striatal-
thalamic circuit |
Robustness | Beta | −0.294 | Robustness | Beta | −0.409 |
| 95th % CI | [−0.484, −0.104] | 95th % CI | [−0.640, −0.177] | |||
| P-value | 0.012 | P-value | 0.006 | |||
| Frontal-basal ganglia- amygdala circuit | Robustness | Beta | −0.291 | Robustness | Beta | −0.404 |
| 95th % CI | [−0.483, −0.099] | 95th % CI | [−0.635, −0.172] | |||
| P-value | 0.018 | P-value | 0.007 | |||
|
Amygdalo-
thalamic circuit |
Median conn. (in,out) | Beta | −0.377 to −0.309 | Median conn. (out) | Beta | −0.331 |
| 95th % CI | [−0.675, −0.084] | 95th % CI | [−0.497, −0.165] | |||
| P-value | 0.023 to 0.025 | P-value | 0.001 | |||
| Other networks/circuits | ||||||
| Thalamus | Median conn. (out) | Beta | −0.299 | |||
| 95th % CI | [−0.470, −0.128] | |||||
| P-value | 0.007 | |||||
| May 2021 Survey (# 7) | ||||||
| Outcome: SADNESS | ||||||
| Left hemisphere | Right hemisphere | |||||
| Somatomotor |
Efficiency,
global clustering, robustness |
Beta | −0.354 to −0.218 | Robustness | Beta | −0.239 |
| 95th % CI | [−0.581, −0.105] | 95th % CI | [−0.359, −0.120] | |||
| P-value | 0.018 to 0.022 | P-value | 0.007 | |||
| Modularity | Beta | 0.181 |
Modularity,
fragility |
Beta | 0.187 to 0.259 | |
| 95th % CI | [0.071, 0.291] | 95th % CI | [0.125, 0.388] | |||
| P-value | 0.022 | P-value | 0.003 to 0.007 | |||
| Salience/Ventral attention | Robustness | Beta | −0.269 | |||
| 95th % CI | [−0.452, −0.086] | |||||
| P-value | 0.046 | |||||
| Fragility | Beta | 0.153 | Fragility | Beta | 0.213 | |
| 95th % CI | [0.073, 0.234] | 95th % CI | [0.145, 0.281] | |||
| P-value | 0.009 | P-value | 0.011 | |||
| Frontoparietal control | Efficiency, stability, robustness | Beta | −0.703 to −0.205 | NS | ||
| 95th % CI | [−1.169, −0.058] | |||||
| P-value | 0.027 to 0.029 | |||||
| Modularity | Beta | 0.302 | Fragility | Beta | 0.267 | |
| 95th % CI | [0.102, 0.501] | 95th % CI | [0.153, 0.381] | |||
| P-value | 0.027 | P-value | 0.010 | |||
|
Temporo-
parietal |
NS |
Global clustering,
robustness |
Beta | −0.214 to −0.190 | ||
| 95th % CI | [−0.331, −0.051] | |||||
| P-value | 0.034 to 0.042 | |||||
| Modularity | Beta | 0.232 | ||||
| 95th % CI | [0.079, 0.385] | |||||
| P-value | 0.040 | |||||
| Prefrontal cortex | NS | Robustness | Beta | −0.252 | ||
| Fragility | Beta | 0.294 | 95th % CI | [−0.401, −0.102] | ||
| 95th % CI | [0.129, 0.459] | P-value | 0.049 | |||
| P-value | 0.039 | |||||
| Outcome: PANDEMIC UNCERTAINTY-RELATED STRESS | ||||||
| Left hemisphere | Right hemisphere | |||||
| Somatomotor | NS | Fragility | Beta | 0.435 | ||
| 95th % CI | [0.236, 0.634] | |||||
| P-value | 0.014 | |||||
| Salience | NS | Fragility | Beta | 0.436 | ||
| 95th % CI | [0.183, 0.688] | |||||
| P-value | 0.045 | |||||
|
Amygdalo- thalamic
circuit |
NS | Median conn. (in) | Beta | −0.742 | ||
| 95th % CI | [−0.868, −0.616] | |||||
| P-value | <0.001 | |||||
When models were adjusted for prior externalizing and internalizing behaviors, additional networks and their topological properties were identified as predictors of overall mental health, perceived stress, sadness, and stress related to the pandemic’s uncertainty, primarily in the December 2020 survey but also the May 2021 survey. Specifically, higher strength and robustness of connections between the somatomotor network and the rest of the brain predicted better overall mental health in December 2020. Lower median connectivity within and between the left salience network and the rest of the brain and similarly for the reward, prefrontal, fronto-thalamic, fronto-amygdala, fronto-striatal (and similarly for the larger fronto-basal ganglia), amygdalo-thalamic, fronto-striatal-thalamic, and fronto-basal ganglia-amygdala circuits predicted higher perceived stress in December 2020 (P < 0.03, β = −0.46 to −0.20, CI = [−0.69, −0.07]). Lower strength of connections between the left basal ganglia and the resting of the brain, and similarly for the left limbic network, also predicted higher stress (P < 0.01, β = −0.38 to −0.32, CI = [−0.61, −0.12]). MSE values were in the range 0.26 to 0.35 (with the lowest MSE corresponding to connectivity of the left prefrontal cortex). Lower topological robustness of these networks was also predictive of higher stress, but when models were adjusted for cumulative pandemic-related stress prior to the December 2020 survey, this prediction was no longer significant (P > 0.05). Overall, properties of the same networks/circuits were predictive of higher sadness in the same survey. Lower topological resilience of bilateral networks was the most frequent predictor of increased sadness, with the exception of the prefrontal cortex and reward networks for which the prediction was lateralized to the right hemisphere (P < 0.04, β = −0.40 to −0.32, CI = [−0.61, −0.12]). In addition, weaker connections between the salience network (bilaterally) and the rest of the brain, and similarly for bilateral basal ganglia, the amygdalo-thalamic circuit and the thalamus, were also predictors of higher sadness (P < 0.04, β = −0.52 to −0.30, CI = [−0.81, −0.08]). MSE values were in the range 0.40 to 0.56 (with the lowest MSE corresponding to robustness of the right reward network). Model statistics are summarized in Table 2.
Additional secondary analyses with adjustments for prepandemic social factors
To investigate the impact of social relations and/or connectedness prior to the pandemic on links between network topology and survey-based outcomes, further analyses were conducted, first adjusting models for whether the child had a best friend. Findings were consistently similar to those based on the primary models, and this covariate was nonsignificant in the models. Furthermore, including an adjustment for whether the child preferred to be alone also did not change findings, and this covariate was nonsignificant in models as well. Finally, another set of models also adjusted for whether the child had experienced a traumatic event (before the pandemic). Additional negative associations were estimated between sadness and left somatomotor network connectivity in December 2020 (P < 0.04, β = −0.20 to −0.15, CI = [−0.33, −0.03]). At the regional (node) level, not-consistent topological predictors of any outcome were identified. Finally, no significant predictors of positive affect were identified at any spatial level of investigation (P > 0.05).
Lower topological network resilience as consistent predictor of stress and sadness during the pandemic
Topological robustness, fragility, efficiency, and modularity were frequent predictors of sadness and, to a lesser extent, stress related to the pandemic’s uncertainty in May 2021. Lower bilateral robustness and higher segregation (modularity) of the somatomotor network and higher fragility (in the right hemisphere) predicted higher sadness. Lower efficiency and robustness and higher modularity of left and higher fragility of right frontoparietal control, lower robustness of right temporoparietal, right prefrontal, and right salience, and higher fragility of left prefrontal and salience networks also predicted higher sadness (P < 0.05, β = −0.70 to −0.19, CI = [−1.169, 0.05] for efficiency, robustness, stability, and global clustering, β = 0.14 to 0.29, CI = [0.05, 0.50] for fragility and modularity). Finally, fragility of right somatomotor and salient networks and lower connectivity of the amygdalo-thalamic circuit predicted higher pandemic uncertainty-related stress in May 2021 (P < 0.05, β = 0.43 to 0.44, CI = [0.18, 0.69] for fragility, β = −0.74, CI = [−0.87, −0.62] for connectivity). MSE values were in the range 0.12 to 0.46 (with the lowest MSE corresponding to robustness of the salience network). Model statistics are summarized in Table 2. Predicted sadness in December 2020 and May 2021 as a function of topological robustness of multiple networks is shown in Fig. 3.
Fig. 3.
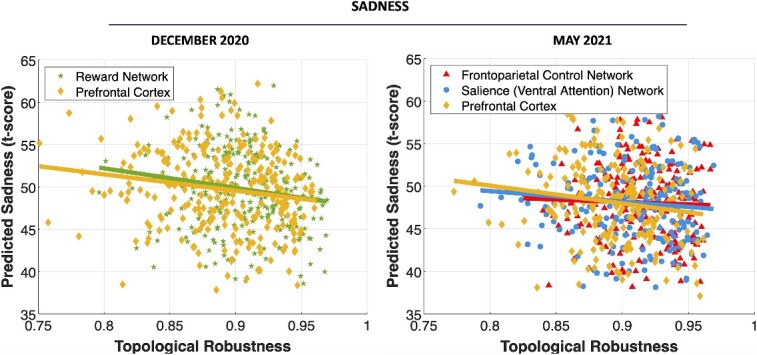
Predicted sadness in both December 2020 and May 2021, as a function of topological robustness of the reward and prefrontal networks (in December 2020), and frontoparietal control, prefrontal, and salience networks (in May 2021).
Results based on youth with rs-fMRI data collected prior to the outbreak
In cohort B, higher connectivity in the dorsal attention network was associated with lower stress in June 2020 (P ≤ 0.03, β = −0.09 to −0.07, CI = [−0.14, −0.02]), and higher connectivity in the bilateral salience and right reward networks was associated with better mental health in May 2021 (P < 0.05, β = 0.07 to 0.10, CI = [0.01, 0.167]. In addition, properties of smaller networks, specifically amygdala and basal ganglia, were also predictors of sadness and stress in August and October 2020. Detailed model statistics are provided in Table S5. Finally, at the regional level, higher centrality and local clustering of the left dorsal attention network also predicted lower pandemic uncertainty-related stress, in models with and without adjustments for prior internalizing and externalizing problems (P < 0.05).
Discussion
This study investigated the role of the prepandemic topological organization of brain networks in adolescent mental health, stress, and emotional responses during the pandemic. There are broadly three main findings. First, topological network properties were predictive of stress and sadness, and, to a lesser extent, overall mental health, at multiple time points, but primarily December 2020 and May 2021. December is associated with a holiday period during which many families often come together to celebrate. As a result of the pandemic’s restrictions (and in some families, illness and loss of life or financial instability), such celebrations were scaled down or even impossible in 2020. Thus, youth with weaker brain connections and/or less resilient networks may have been at higher risk for negative emotions, such as sadness, worse overall mental health, and higher stress during the holiday period. May 2021 was toward the end of the first school year entirely spent during the pandemic and was also ~15 months since the outbreak. In addition to the cumulative burden of the pandemic, in several states, cities, and communities, some restrictions and requirements for social isolation remained in place, although COVID-19 vaccines were administered widely by then. Thus, youth with more fragile brain circuits may have been more predisposed to negative emotions and higher stress as a result of the pandemic’s prolonged burden on their everyday life.
The second main finding is that connection strength (connectivity) and topological resilience (robustness) were the two main topological properties that consistently predicted overall mental health, sadness, and stress. Stronger connections and more resilient networks were significant predictors of better mental health, lower stress, and lower sadness. In contrast, weaker connections and less resilient and more fragile networks predicted higher sadness add stress.
The third main finding is that several large networks and smaller circuits were consistently associated with these outcomes. In December 2020, stress was consistently predicted by connection strength of multiple networks/circuits in the left hemisphere, whereas sadness was predicted primarily by robustness of multiple bilateral networks. In May 2021, bilateral fragility and/or robustness were the primary predictors of stress and sadness. Prior work has shown the stress may modulate functional hemispheric asymmetry, which may, in part, explain the lateralized prediction of stress by connection strength of distributed circuits in the left hemisphere (Ocklenburg et al. 2016). Of note is that a number of predictions of stress by connection strength/and resilience of circuits in the right hemisphere were estimated, but were eliminated when models were adjusted for cumulative effects of stress prior to the December 2020 and May 2021 surveys, respectively. This suggests that while prepandemic strength of network connections in the left hemisphere predicted stress, robustness of networks in the right hemisphere may have been modulated by stress during the pandemic (and thus covaried with it).
Topological properties of specific resting-state networks/circuits, most consistently the salience network, were identified as consistent predictors. Specifically, lower connection strength and lower resilience (and in some cases, higher fragility) of salience network were consistent predictors of mental health, stress, and sadness in December 2020 and sadness and stress in May 2021. A number of prior studies have shown that the salience network plays a central role across domains (Seeley 2019), including emotion, reward, and pain processing and regulation (Uddin 2014; Menon 2015; Rosen et al. 2018), but may also be impacted by social isolation (Jankowski et al. 2018), including during the pandemic (Bzdok and Dunbar 2022). It has also been implicated in mental health issues, including suicide ideation (Ho et al. 2021) and anxiety, specifically in adolescents (Geng et al. 2016). In addition, connection strength of the dorsal attention network predicted higher stress in June 2020 (~3 months since the outbreak). These results are in agreement with prior work (Thomason et al. 2011; Seeley 2019; Schimmelpfennig et al. 2023), and suggest that strength and robustness of the salience network prior to the pandemic may have been a protective factor for mental health, stress, and sadness, whereas its fragility and weaker connections were risk factors that increased vulnerability to the pandemic’s adverse effects. The involvement of the dorsal attention in stress has also been consistently reported in prior studies (Soares et al. 2013; Sousa 2016).
Connection strength and/or resilience of several circuits involving the amygdala, including amygdalo-thalamic, fronto-amygdala, fronto-basal ganglia-amygdala, and the limbic network was also predictive of stress in August 2020, December 2020, and May 2021 and similarly for sadness (though only in December 2020 and May 2021). Extensive prior work has linked the amygdala to emotional processing, stress and their interactions (LeDoux 1992; Phelps 2006; Ressler 2010; Gallagher and Ciba 1996; Puccetti et al. 2021). Overall, studies have reported higher amygdala activation in response to negative stimuli and emotions during related tasks. Our findings are based on resting-state circuits between the amygdala and other regions and reflect the organization of these networks independently of any emotion processing task. Prior work has reported anticorrelated task and resting-state brain networks (Fox et al. 2005; Uddin et al. 2009), which could, in part, explain the prediction of sadness and stress by hypoconnectivity. In addition, recent studies focusing on the COVID-19 pandemic have reported that strength of connections between the dorsomedial prefrontal cortex and amygdala prior to the pandemic was inversely correlated with stress during the pandemic (Zhou et al. 2023). The fronto-amygdala circuit also plays a central role in emotional regulation and attenuation of negative affect (Banks et al. 2007), and the amygdalo-thalamic circuit may also have a similar role (Pessoa 2017). Our findings suggest that weaker and less robust (or more fragile) connections between the amygdala and distributed brain regions may reflect emotion dysregulation prior to the pandemic, a risk factor that may have predisposed youth to higher stress and sadness during the pandemic.
Connection strength and/or resilience of the thalamic network and its connections with the prefrontal cortex, amygdala, and the striatum were also predictors of stress and sadness. The involvement of the thalamus in emotion regulation has been reported as early as the work of Cannon and Bard (and the Cannon–Bard [thalamic] theory of emotions; Cannon 1929; Bard 1934; Simic et al. 2021). Prior research on a network model for emotional processing has included the thalamus as an important element, in addition to the amygdala, the striatum, and frontal cortex (Pessoa 2017). Our findings are aligned with this model as well. Furthermore, the thalamus may also play the role of an emotional brain “hub,” in that it integrates signals from other regions involved in emotional processing (Venkatraman et al. 2017).
Another important finding is that topological properties of the prefrontal cortex, and its cortical and sub-cortical projections were predictors of stress and sadness. The prefrontal cortex is a particularly vulnerable region in adolescence, a period characterized by profound neuroanatomical changes, heightened myelination, decreased synaptic density, neural connection pruning, and selective connection strengthening, all part of a broader process of circuit rewiring and topological optimization (Rakic et al. 1994, Arain et al. 2013; Spear 2013; Larsen and Luna 2018). Thus, the underdeveloped prefrontal cortex is vulnerable to stressors and environmental risk factors, which may adversely impact its wiring (Casey et al. 2008a; Tottenham and Galvan 2016). Lower robustness of this region prior to the pandemic, possibly the result of negative impacts of environmental and experiential stressors, may have increased the likelihood of higher stress and decreased control and regulation of negative emotions during the pandemic. In addition, robustness and/or strength of circuits connecting frontal (including prefrontal) cortical regions, with the amygdala, striatum, and/or thalamus was also predictive of higher stress and/or sadness. A number of studies have linked these circuits to emotion and stress regulation (Cardinal et al. 2002; Hare et al. 2005; Banks et al. 2007; Furman et al. 2011; Gabbay et al. 2013; Tottenham and Galvan 2016). In addition, stress has been shown to weaken connections within frontal regions and their projections to other brain regions (Arnsten 2009). Thus, in some youth, prepandemic stress may have contributed to weaker and less resilient circuits involving the prefrontal cortex, which may have predisposed youth to experience higher stress and dysregulation of emotions, such as sadness, during the pandemic.
Finally, properties of the basal ganglia, and specifically the striatum (which changes substantially in adolescence), individually or as part of part of multiple circuits predicted stress and sadness in October and December 2020. These structures and have been linked to mental health and well-being, as well as emotion processing and regulation in youth (Ring and Serra-Mestres 2002; Killgore and Yurgelun-Todd 2007; Del-Piero et al. 2016; Boyes et al. 2022). They are also part of a larger developing network that includes the prefrontal cortex, striatum, and amygdala and is vulnerable to stress in adolescence (Casey et al. 2008a; Tottenham and Galvan 2016; Lago et al. 2017). In addition, sadness has been specifically associated with reduced activity in this network, as well as the striatum individually (Levesque et al. 2003; Gabbay et al. 2013; Arias et al. 2020).
Despite a number of strengths, including a relatively large cohort with fMRI data and longitudinal survey data during the first ~15 months of the pandemic (which captured related changes in mental health, emotions, and stress in adolescents) and advanced analytics to robustly quantify the organization of the developing connectome and individual brain networks and circuits, this study also had some limitations. Each survey was associated with a sample that partially overlapped with others, i.e. not all participants had data across all surveys, and the neuroimaging samples also partially overlapped. In addition, surveys only assessed mental and emotional health and stress at a relatively high level, and not all surveys assessed all outcomes of interests. By design, they were not meant to be exhaustive and comprehensive assessments of mental health. Instead, they included sufficient questions to assess youth behaviors, emotions, and responses to pandemic-related restrictions and disruptions of everyday life. It is, therefore, possible that additional associations between brain networks and mental health would have been identified if more granular information was available. Furthermore, some participants were scanned during the pandemic, and thus, the latter’s impact on the brain, particularly at later survey assessments, needed to be accounted for in analyses. For each outcome of interest, the “history” of that outcome, i.e. related cumulative responses in previous surveys, were incorporated in analyses. Other potentially confounding factors were not included, in part because of limits in statistical power. However, all analyses were adjusted for prepandemic mental health and behavioral issues that may have increased the risk of negative responses to the pandemic. Furthermore, since the ABCD cohort is geographically diverse, and COVID measures adopted by the state, region, and/or community where each participant lived were unknown, it was impossible to adequately account for this information. Thus, only random effects for site were included in models, to account for potential broad differences. Finally, as all retrospective investigations, this study and collected data were limited by scientific decisions made by the ABCD consortium. Nevertheless, extensive participant data were available, and survey data captured a sufficiently broad range of youth responses to the pandemic’s stressors.
Despite some limitations, this study makes a significant contribution toward highly incomplete understanding of the brain’s role in youth responses during the COVID-19 pandemic. In a relatively large cohort of over 2,600 youth, this first-of-its-kind investigation has examined the role of the prepandemic adolescent brain wiring as a protective or risk factor for mental and emotion health and stress during the COVID-19 pandemic. It has identified two aspects of network organization, connection strength and topological resilience, of multiple developing networks as the primary predictors of overall mental health, sadness, and stress. It has also identified a common set of structures and circuits that predicted these outcomes. These include salience, limbic, reward, and dorsal attention networks and an interconnected set of structures comprised of the prefrontal cortex, amygdala, striatum, and thalamus. These are rapidly maturating and thus vulnerable structures that may have been modulated by stressors prior to the pandemic and predisposed youth to amplified effects of the pandemic on their mental and emotional health and stress responses. Our findings suggest the prepandemic organization of specific neural circuits in adolescents may have played a critical role in youth responses to the pandemic. The developing brain is highly plastic, and its circuits continue to reorganize until they attain their optimal configuration in young adulthood. Thus, it also has a high capacity for recovery. As efforts to combat the rapidly rising mental health crisis in youth and reverse the pandemic’s effects on it are underway, identified circuits in this study could be specifically targeted by new interventions, to improve short- and longer-term mental health outcomes in youth.
Author contributions
Linfeng Hu (Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing—original draft, Writing—review & editing) and Catherine Stamoulis (Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review & editing).
Funding
This work was supported by the National Science Foundation (awards 1940096, 2116707, 2207733).
Conflict of interest statement: None declared.
Supplementary Material
Contributor Information
Linfeng Hu, Department of Pediatrics, Division of Adolescent and Young Adult Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Department of Biostatistics, Harvard T.H. Chan School of Public Health, 77 Huntington Ave, Boston, MA 02115, United States.
Catherine Stamoulis, Department of Pediatrics, Division of Adolescent and Young Adult Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Department of Pediatrics, Harvard Medical School, 25 Shattuck St, Boston, MA 02115, United States.
References
- Ademhan-Tural D, Emiralioglu N, Tural-Hesapcioglu S, Karahan S, Ozsezen B, Sunman B, Nayir-Buyuksahin H, Yalcin E, Dogru D, Ozcelik U, et al. Psychiatric and general health effects of COVID-19 pandemic on children with chronic lung disease and parents' coping styles. Pediatr Pulmonol. 2020:55(12):3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aknin LB, De-Neve JE, Dunn EW, Fancourt DE, Goldberg E, Helliwell JF, Jones SP, Karam E, Layard R, Lyubomirsky S, et al. Mental health during the first year of the COVID-19 pandemic: a review and recommendations for moving forward. Perspect Psychol Sci. 2022:17(4):915–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshahrani M, Elyamany G, Sedick Q, Ibrahim W, Mohamed A, Othman M, Al-Thibani N, Alsuhaibani O, Al-Amro M, Gharawi A, et al. The impact of COVID-19 pandemic in children with cancer: a report from Saudi Arabia. Health Serv Insights. 2020:13:1178632920984161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013:9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argabright ST, Tran KT, Visoki E, DiDomenico GE, Moore TM, Barzilay R. COVID-19-related financial strain and adolescent mental health. Lancet Reg Health. 2022:16:100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias JA, Williams C, Raghvani R, Aghajani M, Baez S, Belzung C, Booij L, Busatto G, Chiarella J, Fu CH, et al. The neuroscience of sadness: a multidisciplinary synthesis and collaborative review. Neurosci Biobehav Rev. 2020:111:199–228. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009:10(6):410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010:53(1):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007:2(4):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard P. On emotional expression after decortication with some remarks on certain theoretical views: part I. Psychol Rev. 1934:41:309–329. [Google Scholar]
- Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009:22(4):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell IH, Nicholas J, Broomhall A, Bailey E, Bendall S, Boland A, Robinson J, Adams S, McGorry P, Thompson A. The impact of COVID-19 on youth mental health: a mixed methods survey. Psychiatry Res. 2023:321:115082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995:57(1):289–300. [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008:9(4):267–277. [DOI] [PubMed] [Google Scholar]
- Boyes A, McLoughlin LT, Anderson H, Schwenn P, Shan Z, Gatt JM, Lagopoulos J, Hermens DF. Basal ganglia correlates of wellbeing in early adolescence. Brain Res. 2022:1774:147710, 1–9. [DOI] [PubMed] [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, et al. Response to learned threat: an fMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013:170(10):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Parks SM, Stamoulis C. Widespread positive direct and indirect effects of regular physical activity on the developing functional connectome in early adolescence. Cereb Cortex. 2021:31(10):4840–4852. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Smith C, Stamoulis C. Excess BMI in early adolescence adversely impacts maturating functional circuits supporting high-level cognition and their structural correlates. Int J Obes. 2023:47(7):590–605. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009:33(3):279–296. [DOI] [PubMed] [Google Scholar]
- Brown SM, Doom JR, Lechuga-Peña S, Watamura SE, Koppels T. Stress and parenting during the global COVID-19 pandemic. Child Abuse Negl. 2020:110:104699, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Dunbar RIM. Social isolation and the brain in the pandemic era. Nat Hum Behav. 2022:6:1333–1343. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929:9:399–431. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002:26(3):321–352. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008a:1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018:32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . Understanding the pandemic’s impact on children and teens; 2020. https://www.cdc.gov/nssp/partners/Understanding-the-impact.html.
- Chase HW, Phillips ML. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016:1(3):288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tam A, Kebets V, Orban C, Ooi LQ, Asplund CL, Marek S, Dosenbach NU, Eickhoff SB, Bzdok D, et al. Shared and unique brain network features predict cognitive, personality, and mental health scores in the ABCD study. Nat Commun. 2022:13(1):2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006:17(16):1687–1690. [DOI] [PubMed] [Google Scholar]
- Chi X, Liang K, Chen ST, Huang Q, Huang L, Yu Q, Hossain MM, Yeung A. Mental health problems among Chinese adolescents during the COVID-19: the importance of nutrition and physical activity. Int J Clin Health Psychol. 2021:21(3):100218, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:24(4):385–396. [PubMed] [Google Scholar]
- Colizzi M, Sironi E, Antonini F, Ciceri ML, Bovo C, Zoccante L. Psychosocial and behavioral impact of COVID-19 in autism spectrum disorder: an online parent survey. Brain Sci. 2020:10(6):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell C, Shum A, Pearcey S, Skripkauskaite S, Patalay P, Waite P. Young people's mental health during the COVID-19 pandemic. Lancet Child Adolesc Health. 2021:5(8):535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009:460(3):227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Piero LB, Saxbe DE, Margolin G. Basic emotion processing and the adolescent brain: task demands, analytic approaches, and trajectories of changes. Dev Cogn Neurosci. 2016:19:174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009:46(1):39–46. [DOI] [PubMed] [Google Scholar]
- Duan L, Shao X, Wang Y, Huang Y, Miao J, Yang X, Zhu G. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020:275:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Giannitto N, Squarcia A, Neglia C, Argentiero A, Minichetti P, Cotugno N, Principi N. Development of psychological problems among adolescents during school closures because of the COVID-19 lockdown phase in Italy: a cross-sectional survey. Front Pediatr. 2021:8:628072, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JN, McInroy LB, Paceley MS, Williams ND, Henderson S, Levine DS, Edsall RN. “I'm kinda stuck at home with unsupportive parents right now”: LGBTQ youths' experiences with COVID-19 and the importance of online support. J Adolesc Health. 2020:67(3):450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van-Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005:102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011:1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013:52(6):628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Ciba AA. The amygdala and emotion. Cur Op Neurobiol. 1996:6(2):221–227. [DOI] [PubMed] [Google Scholar]
- Garcia-de-Avila MA, Hamamoto-Filho PT, Jacob FL, Alcantara LR, Berghammer M, Jenholt-Nolbris M, Olaya-Contreras P, Nilsson S. Children's anxiety and factors related to the COVID-19 pandemic: an exploratory study using the Children's anxiety questionnaire and the numerical rating scale. Int J Environ Res Public Health. 2020:17(16):5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Li X, Chen J, Li X, Gu R. Decreased intra- and inter-salience network functional connectivity is related to trait anxiety in adolescents. Front Behav Neurosci. 2016:9:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Keshavan M, Paus T. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008:9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Luby JL, Baram TZ, Sandman CA. A predictable home environment may protect child mental health during the COVID-19 pandemic. Neurobiology of stress. 2021:14:100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Miller JG, Borchers LR, Coury SM, Costello LA, Garcia JM, Ho TC. Effects of the COVID-19 pandemic on mental health and brain maturation in adolescents: implications for Analyzing longitudinal data. Biological Psychiatry Global Open Science. 2023:3(4):912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010:35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstad GS, Sætren SS, Wentzel-Larsen T, Augusti EM. Adolescents' symptoms of anxiety and depression before and during the Covid-19 outbreak - a prospective population-based study of teenagers in Norway. Lancet Reg Health Eur. 2021:5:100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, et al. Image processing and analysis methods for the adolescent brain cognitive development study. NeuroImage. 2019:202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani S, Hiraoka D, Makita K, Tomoda A, Mizuno Y. Longitudinal impact of COVID-19 pandemic on mental health of children in the ABCD study cohort. Sci Rep. 2022:12(1):19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005:57(6):624–632. [DOI] [PubMed] [Google Scholar]
- Hawke LD, Monga S, Korczak D, Hayes E, Relihan J, Darnay K, Cleverley K, Lunsky Y, Szatmari P, Henderson J. Impacts of the COVID-19 pandemic on youth mental health among youth with physical health challenges. Early Interv Psychiatry. 2021:15(5):1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Walker JC, Teresi GI, Kulla A, Kirshenbaum JS, Gifuni AJ, Singh MK, Gotlib IH. Default mode and salience network alterations in suicidal and non-suicidal self-injurious thoughts and behaviors in adolescents with depression. Transl Psychiatry. 2021:11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RJ, Graham JM, Whitaker KJ, Hagan CC, Ooi C, Wilkinson PO, Van-Nieuwenhuizen AO, Lennox BR, Sahakian BJ, Goodyer IM, et al. Functional MRI of emotional memory in adolescent depression. Dev Cogn Neurosci. 2016:19:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Katz EN, Stamoulis C. Modulatory effects of fMRI acquisition time of day, week and year on adolescent functional connectomes across spatial scales: implications for inference. NeuroImage. 2023:284:120459. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Batres J, Scott H, Smyda G, Pfeifer JH, Quevedo K. Feeling left out: depressed adolescents may atypically recruit emotional salience and regulation networks during social exclusion. Soc Cogn Affect Neurosci. 2018:13(8):863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer TM, Kim JZ, Stiso J, Kahn AE, Pasqualetti F, Habel U, Bassett DS. A practical guide to methodological considerations in the controllability of structural brain networks. J Neural Eng. 2020:17(2):026031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Neural correlates of emotional intelligence in adolescent children. Cogn Affect Behav Neurosci. 2007:7:140–151. [DOI] [PubMed] [Google Scholar]
- Kiss O, Alzueta E, Yuksel D, Pohl KM, De-Zambotti M, Műller-Oehring EM, Prouty D, Durley I, PelhamWE III, CJ MC, et al. The pandemic's toll on young adolescents: prevention and intervention targets to preserve their mental health. J Adolesc Health. 2022:70(3):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss O, Nagata JM, De-Zambotti M, Dick AS, Marshall AT, Sowell ER, Van-Rinsveld A, Guillaume M, PelhamWE III, Gonzalez MR, et al. Effects of the COVID-19 pandemic on screen time and sleep in early adolescents. Health Psychol. 2023:42(12):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine R, Galimov A, Hanewinkel R, Unger J, Sussman S, Hansen J. Impact of the COVID-19 pandemic on young people with and without pre-existing mental health problems. Sci Rep. 2023:13(1):6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010:31(6):904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, Milham MP. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008:63(6):563–568. [DOI] [PubMed] [Google Scholar]
- Lago T, Davis A, Grillon C, Ernst M. Striatum on the anxiety map: small detours into adolescence. Brain Res. 2017:1654(Pt B):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev. 2018:94:179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Yale J Biol Med. 1992, pp. 339–351.
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003:53:502–510. [DOI] [PubMed] [Google Scholar]
- LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, Simmons AN, Yang TT. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014:53(8):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Beames JR, Newby JM, Maston K, Christensen H, Werner-Seidler A. The impact of COVID-19 on the lives and mental health of Australian adolescents. Eur Child Adolesc Psychiatry. 2022:31(9):1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Yin DZ, Cheng WH, Fan MX, You MN, Men WW, Zang LL, Shi DH, Zhang F. Abnormal functional connectivity of the amygdala-based network in resting-state fMRI in adolescents with generalized anxiety disorder. Med Sci Monit. 2015:21:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010:30(28):9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magson NR, Freeman JY, Rapee RM, Richardson CE, Oar EL, Fardouly J. Risk and protective factors for prospective changes in adolescent mental health during the COVID-19 pandemic. J Youth Adolesc. 2021:50:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, Hackman DA, Baker FC, Breslin FJ, Brown SA, Dick AS, Gonzalez MR, Guillaume M, Kiss O, Lisdahl KM, et al. Resilience to COVID-19: socioeconomic disadvantage associated with positive caregiver–youth communication and youth preventative actions. Front Public Health. 2022:10:734308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969:44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970:45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Mendoza-Diaz A, Tully L, Azim SI, Woolfenden S, Efron D, Eapen V. Impact of the COVID-19 pandemic on the well-being of children with neurodevelopmental disabilities and their parents. J Paediatr Child Health. 2021:57(5):631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011:15(10):483–506. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale functional brain organization. Brain Mapping. 2015:2:449–459. [Google Scholar]
- Menon V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry. 2020:19(3):309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Ho TC, Kirshenbaum JS, Chahal R, Gifuni AJ, Gotlib IH. Testing a developmental model of positive parenting, amygdala–subgenual anterior cingulate cortex connectivity, and depressive symptoms in adolescents before and during the COVID-19 pandemic. Biological Psychiatry Global Open Science. 2021:1(4):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009:47(2):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011:21(10):2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata JM, Cortez CA, Dooley EE, Iyer P, Ganson KT, Bibbins-Domingo K, Baker FC, Gabriel KP. 10. Screen time and moderate-to-vigorous intensity physical activity among adolescents during the COVID-19 pandemic: findings from the adolescent brain cognitive development study. J Adolesc Health. 2022:70(4):S6. [Google Scholar]
- National Institute of Mental Health Data Archive (NDA) . https://nda.nih.gov/. [DOI] [PubMed]
- Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int J Environ Res Public Health. 2020:17(22):8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Next-Generation Neural Data Analysis (NGNDA) : https://github.com/cstamoulis1/Next-Generation-Neural-Data-Analysis-NGNDA.
- Ocklenburg S, Korte SM, Peterburs J, Wolf OT, Güntürkün O. Stress and laterality - the comparative perspective. Physiol Behav. 2016:164:321–329. [DOI] [PubMed] [Google Scholar]
- Panchal U, Salazar-de-Pablo G, Franco M, Moreno C, Parellada M, Arango C, Fusar-Poli P. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023:32(7):1151–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti F, Zhao S, Favaretto C, Zampieri S. Fragility limits performance in complex networks. Sci Rep. 2020:10(1):1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa LA. Network model of the emotional brain. Trends Cogn Sci. 2017:21(5):357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006:57:27–53. [DOI] [PubMed] [Google Scholar]
- Puccetti NA, Schaefer SM, van ReekumCM, Ong AD, Almeida DM, Ryff CD, Davidson RJ, Heller AS. Linking amygdala persistence to real-world emotional experience and psychological well-being. J Neurosci. 2021:41(16):3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu YL, Chen J, Tam A, Ooi LQ, Dhamala E, Cocuzza C, Lawhead C, Yeo BT, Holmes AJ. Distinct brain network features predict internalizing and externalizing traits in children and adults. bioRxiv. 2023:541490. [Google Scholar]
- Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021:175(11):1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–43. [DOI] [PubMed] [Google Scholar]
- Raney JH, Testa A, Jackson DB, Ganson KT, Nagata JM. Associations between adverse childhood experiences, adolescent screen time and physical activity during the COVID-19 pandemic. Acad Pediatr. 2022:22(8):1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry. 2010:67(12):1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo JG, Ott E, Hunt BR. Approximating the largest eigenvalue of network adjacency matrices. Phys Rev E. 2007:76(5):056119. [DOI] [PubMed] [Google Scholar]
- Ring H, Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry. 2002:72:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol Psychiatry. 2006:60(9):966–973. [DOI] [PubMed] [Google Scholar]
- Rosen ML, Rodman AM, Kasparek SW, Mayes M, Freeman MM, Lengua LJ, Meltzoff AN, McLaughlin KA. Promoting youth mental health during the COVID-19 pandemic: a longitudinal study. PLoS One. 2021:16(8):e0255294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Sheridan MA, Sambrook KA, Dennison MJ, Jenness JL, Askren MK, Meltzoff AN, McLaughlin KA. Salience network response to changes in emotional expressions of others is heightened during early adolescence: relevance for social functioning. Dev Sci. 2018:21(3):e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E, Franklin-Gillette S, Jung HJ, Nelson A, Evans SW, Power TJ, Yerys BE, Dever BV, Reckner E, DuPaul GJ. Impact of COVID-19 on Youth With ADHD: Predictors and Moderators of Response to Pandemic Restrictions on Daily Life. J Atten Disord. 2022:26(9):1223–1234. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013:52(3):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010:52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- Salomon T, Cohen A, Barazany D, Ben-Zvi G, Botvinik-Nezer R, Gera R, Oren S, Roll D, Rozic G, Saliy A, et al. Brain volumetric changes in the general population following the COVID-19 outbreak and lockdown. NeuroImage. 2021:239:118311. [DOI] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Kupst MJ, Kelly MA, Bode RK, Choi SW, et al. Emotion assessment using the NIH toolbox. Neurology. 2013:80(11 Suppl 3):S76–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H, Wu J, Ladak A, Vossen C, Stewart E, Dove N, Long D, Snell G. Review: mental health impacts of the COVID-19 pandemic on children and youth - a systematic review. Child Adolesc Mental Health. 2022:27(2):173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BT. Local-global Parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018:28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci. 2019:39(50):9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Tkalčić M, Vukić V, Mulc D, Španić E, Šagud M, Olucha-Bordonau FE, Vukšić M, R-Hof P. Understanding emotions: origins and roles of the amygdala. Biomol Ther. 2021:11(6):823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Roy D, Sinha K, Parveen S, Sharma G, Joshi G. Impact of COVID-19 and lockdown on mental health of children and adolescents: a narrative review with recommendations. Psychiatry Res. 2020:293:113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress impact on resting state brain networks. PLoS One. 2013:8(6):e66500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N. The dynamics of the stress neuromatrix. Mol Psychiatry. 2016:21:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013:52:S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012:35(9):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Lin C, Talmasov D, Ferguson MA, Schaper FL, Jiang J, Goodkind M, Grafman J, Etkin A, Siddiqi SH, et al. A transdiagnostic network for psychiatric illness derived from atrophy and lesions. Nat Hum Behav. 2023:7(3):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberath M, Bauer D, Chen W, Salinas M, Mohabbat AB, Yang J, Chon TY, Bauer BA, Wahner-Roedler DL. Effects of COVID-19 pandemic on mental health of children and adolescents: a systematic review of survey studies. SAGE Open Med. 2022:10:20503121221086712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiatry. 2011:52(10):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelpfennig J, Topczewski J, Zajkowski W, Jankowiak-Siuda K. The role of the salience network in cognitive and affective deficits. Front Hum Neurosci. 2023:17:1133367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci. 2020:23(11):1421–1432. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galvan A. Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev. 2016:70:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2014:16:55–61. [DOI] [PubMed] [Google Scholar]
- Uddin L, Clare-Kelly A, Biswal B, Xavier-Castellanos F, Milham M. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009:30(2):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvilaite L, Hushagen V, Grønli J, Specht K. Time-of-day effects in resting-state functional magnetic resonance imaging: changes in effective connectivity and blood oxygenation level dependent signal. Brain Connect. 2022:12(6):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen L, Toenders YJ, Wierenga LM, Crone EA. Effects of COVID-19 pandemic on structural brain development in early adolescence. Sci Rep. 2023:13:5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman A, Enlow BL, Immondino-Young MH. The brainstem in emotion: a review. Front Neuroanat. 2017:11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Rodman AM, Rosen ML, Kasparek S, Mayes M, Sheridan MA, Lengua LJ, Meltzoff AN, McLaughlin KA. Contributions of emotion regulation and brain structure and function to adolescent internalizing problems and stress vulnerability during the COVID-19 pandemic: a longitudinal study. Biol Psychiatry Glob Open Sci. 2021:4:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) Director-General . WHO Director-General’s opening remarks at the media briefing on COVID-19; 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- Wu J, Barahona M, Tan Y, Deng H. Robustness of regular graphs based on natural connectivity. ArXiv preprint. 2009:0912(2144):1–18. [Google Scholar]
- Xiao Y, Yip P, Pathak J, Mann JJ. Association of Social Determinants of health and vaccinations with child mental health during the COVID-19 pandemic in the US. JAMA Psychiatry. 2022:79(6):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011:106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Jordan A, Kohler RJ, Holmes A, Bzdok D. Multivariate, transgenerational associations of the COVID-19 pandemic across minoritized and marginalized communities. JAMA Psychiatry. 2022:79(4):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Linn KA, Shinohara RT, Oathes DJ, Cook PA, Duprat R, Moore TM, Oquendo MA, Phillips ML, McInnis M, et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci U S A. 2019:116(17):8582–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cheng W, Liu Z, Zhang K, Lei X, Yao Y, Becker B, Liu Y, Kendrick KM, Lu G, et al. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain. 2016:139(8):2307–2321. [DOI] [PubMed] [Google Scholar]
- Zhou Y, He Y, Jin Y, Zeidman P, Gao L, Rong B, Huang H, Feng Y, Cui J, Zhang S, et al. Amygdala connectivity related to subsequent stress responses during the COVID-19 outbreak. Front Psych. 2023:14:999934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


