Abstract
We have studied the pathways of regulation of cytokine and cell cycle control proteins during infection of human B lymphocytes by Epstein-Barr virus (EBV). Among 30 cytokine RNAs analyzed by the RNase protection assay, tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor, lymphotoxin (LT), and LTβ were found to be regulated within 20 h of EBV infection of primary B cells. Similar results were obtained using the estrogen-regulated EBNA-2 cell line EREB2.5, in which RNAs for LT and TNF-α were induced within 6 h of activation of EBNA-2. Expression of Notch also caused an induction of TNF-α RNA. The induction of TNF-α RNA by EBNA-2 was indirect, and constitutive expression of either LMP-1 or c-myc proteins did not substitute for EBNA-2 in induction of TNF-α RNA. Cyclin D2 is also an indirect target of EBNA-2-mediated transactivation. EBNA-2 was found to activate the cyclin D2 promoter in a transient-transfection assay. A mutant of EBNA-2 that does not bind RBP-Jκ retained some activity in this assay, and activation did not depend on the presence of B-cell-specific factors. Deletion analysis of the cyclin D2 promoter revealed that removal of sequences containing E-box c-myc consensus DNA binding sequences did not reduce EBNA-2-mediated activation of the cyclin D2 promoter in the transient-transfection assay. The results indicate that cytokines are an early target of EBNA-2 and that EBNA-2 can regulate cyclin D2 transcription in EBV-infected cells by mechanisms additional to the c-myc pathway.
Infection of resting human B lymphocytes by Epstein-Barr virus (EBV) induces a cascade of cellular changes which ultimately leads to the generation of continuously proliferating cell lines. Genetic analysis of EBV has implicated several viral genes in the initiation and maintenance of growth of these lymphoblastoid cell lines (LCLs). They include the genes that encode EBNA-1, EBNA-2, EBNA-LP, EBNA-3A, EBNA-3C, and LMP-1 (reviewed in references 12, 13, and 24). These viral immortalization genes are not all expressed simultaneously on infection; EBNA-LP and EBNA-2 are the first to be expressed, followed by the remaining EBNA proteins and then by LMP-1.
In previous studies we investigated the mechanism by which the resting B cells are driven into the cell cycle. Binding of the virus to its main cellular receptor, CD21, not only mediates uptake of the virus but also results in signal transduction (34, 35), leading to activation of NF-κB (39). This preactivation of B cells could be reconstituted by exposure of cells to purified gp340 (a soluble form of the EBV surface glycoprotein that binds to CD21). These gp340-treated B cells were then sufficiently activated to be transiently transfected with expression vectors encoding individual EBV proteins. In this system, transfection of the first two viral genes known to be expressed during infection (EBNA-LP and EBNA-2) resulted in induction of cyclin D2 mRNA (35). We have subsequently confirmed (37) that cyclin D2 was one of the first cell cycle proteins induced following EBV infection and that, in addition, the induction of cyclin D2 was accompanied by a dramatic down regulation of the cyclin inhibitor p27. These changes were evident prior to changes in the phosphorylation status of pocket proteins or induction of the E2F family of transcription factors (37). Comparison of the proportion of p27 that was lost with the fraction of cells found to be infected in those studies had suggested that cytokine function might be important in the early events of EBV infection (37). Although cytokine production has been analyzed extensively in LCLs (reference 32 and references therein), the kinetics of cytokine regulation following infection have not been investigated. We have therefore characterized the expression patterns of 30 cytokine transcripts during EBV infection of primary B cells and identified several that are regulated by EBNA-2 activation in cell lines containing a conditionally active EBNA-2.
We have also investigated how cyclin D2 is induced during EBV infection. Since transfected EBNA-2 and EBNA-LP can induce cyclin D2 in primary B cells, EBNA-2-mediated transactivation plays a critical role. EBNA-2 causes transcriptional activation of both viral and cellular promoters, including LMP-1, LMP-2A, LMP-2B, CD21, CD23, and c-fgr (reviewed in reference 25). More recently EBNA-2 has also been shown to activate the c-myc proto-oncogene (19). EBNA-2 can interact with components of the basal transcription machinery (40–42) but has no intrinsic DNA binding activity. EBNA-2 therefore has to be tethered to EBNA-2-responsive promoters through interactions with several cellular factors which include RBP-Jκ (part of the Notch pathway) (17, 49), PU.1 (20, 26), and ATF/CRE (36). In the case of c-myc, EBNA-2 has been reported to activate the promoter through interactions with CBP and the histone acetyltransferase P/CAF (19). EBNA-2 also interacts with the SWI/SNF chromatin-remodeling complex and targets it to responsive promoters (46, 47).
Recent studies have shown that cyclin D2 is not a direct target of EBNA-2-mediated transactivation (21), but the actual mechanism of regulation is unknown. The cyclin D2 promoter itself has been analyzed previously in response to serum growth factors and contains both positive and negative regulatory regions upstream of the transcription start sites (5). Two recent reports indicate that cyclin D2 transcription can be regulated by c-myc in mouse and human fibroblasts. In both human (8) and murine (2) myc-ER systems, cyclin D2 RNA levels are increased following activation of c-myc. The effect is direct, since cyclin D2 RNA levels increase even in the presence of protein synthesis inhibitors. Further analysis in the mouse system revealed that the mouse cyclin D2 promoter is repressed by binding of Mad/Max complexes to E-box consensus sequences within the promoter. The more distal of the E boxes appears to be important for Mad/Max-mediated repression, and the effect of c-myc involves derepression of the cyclin D2 promoter rather than activation. The mechanism of this derepression does not appear to be simple competition between c-myc and Mad for Max binding but may involve recruitment of histone deacetylase to the promoter by Mad. In primary human B cells, there are also relatively high levels of the USF E-box binding factor, which are down regulated during EBV infection (25). USF antagonizes the growth-inducing activity of c-myc, since reexpression of USF in LCL cells retards proliferation (25).
An attractive hypothesis would therefore be that during EBV infection, EBNA-2 induces c-myc and, concomitant with a reduction in USF activity, c-myc activates the cyclin D2 promoter. Here we provide evidence that transient transfection of EBNA-2 alone in DG75 cells can activate the cyclin D2 promoter and that potential c-myc binding sites within the cyclin D2 promoter are not required for this regulation. These results suggest that EBNA-2 regulates cyclin D2 transcription by mechanisms in addition to those controlled by c-myc but that these are still indirect.
MATERIALS AND METHODS
Purification of B cells, EBV, and virus infections.
Primary B cells from peripheral blood were isolated as described previously (6, 35). Buffy coats were centrifuged over Ficoll-Paque (Pharmacia LKB) gradients, and CD19-positive lymphocytes were immunoselected using pan-B Dynabeads M450 (Dynal). The beads were removed by competition with Detachabeads (Dynal), and the cells were resuspended at 106/ml in RPMI 1640 (Gibco-BRL) supplemented with penicillin, streptomycin, and 15% heat-inactivated fetal calf serum. The cells were incubated for 40 h prior to infection with EBV. The isolated cells were analyzed by flow cytometry for purity and DNA content using fluorescein isothiocyanate-conjugated anti-CD20 and propidium iodide, respectively. Cells were infected with the B95-8 strain of EBV as described previously (18).
Immunofluorescence.
Cytospins of primary B cells were fixed in ice-cold acetone-methanol (1:1) and stored at −20°C. Prior to staining, the cells were rehydrated with phosphate-buffered saline (PBS) for 30 min at room temperature. All antibodies were diluted in a PBS blocking solution containing 2% bovine serum albumin, 5% glycerol, 0.02% sodium azide, and 0.2% Tween −20. Incubations were carried out for 1 h at room temperature in a humidified box. All washes were carried out using 1× PBS for 10 to 15 min with three changes of PBS. Antibodies used and their appropriate dilutions were as follows: mouse monoclonal anti-p27 antibody (G173-524; PharMingen), 1/50; mouse monoclonal anti-EBNA LP antibody (JF186), 1/10; and fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin (Ig) (Dako), 1/40. After staining, the cells were mounted in Citifluor (glycerol-PBS solution) (Citifluor Ltd., London, United Kingdom) and analyzed using confocal microscopy.
Plasmids.
pSG5, pSG5-EBNA-2, and pSG5-EBNA-2 WW323SR were from Diane Hayward. Plasmids expressing mutants of EBNA-2 (FY4SR, IF50SR, SR360VD, YI139SR, Δ344-357, Δ117–147, and Δ58–100) were gifts of Paul Ling. pBS-LMP1 was constructed to make 32P-labeled antisense riboprobes for RNase protection assays (RPA) and contained EBV sequences from positions 169033 to 169423 inserted between the XhoI and BamHI sites of pBluescript II SK (Stratagene). Plasmid pBS 0/1 hcmyc, a gift from R. Eisenman, was linearized with StyI and transcribed with T7 polymerase to make the c-myc RPA probe. Cyclin D2 antisense probes were generated as described previously (35). Plasmid −1384+240 (previously termed −1624-1 [5]) contains the cyclin D2 promoter cloned into pGL2 basic and was donated by Dov Shiffman. We have renumbered the promoter constructs so that they relate more properly to one of the major transcription start sites identified by RPA, which has been defined as +1 (see Fig. 5). Thus, the start codon of cyclin D2 protein now begins at +240. Plasmid −652+240 (previously −892-1) and −204+240 (previously −444-1) were generated from −1384+240 by restriction enzyme digestion. Plasmids −105+240 (previously −345-1), −66+240 (previously −306-1), and +126+240 (previously −114-1) cyclin D2 promoter deletion mutants were also supplied by Dov Shiffman and have been described previously (5).
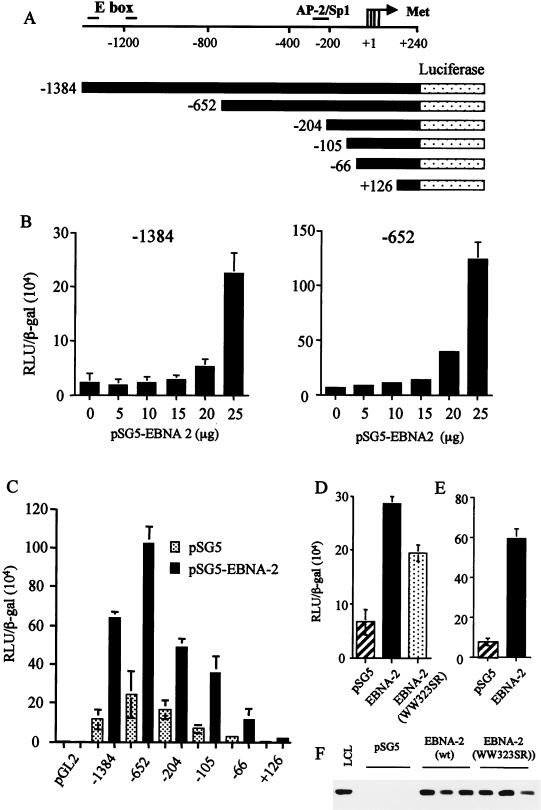
FIG. 5.
(A) Diagram representing regions of the cyclin D2 promoter analyzed for EBNA-2-mediated activation in luciferase reporter assays. Four transcription start sites within the cyclin D2 promoter are indicated by an arrow, and the positions of consensus binding sites for several transcription factors are indicated. E box denotes the c-myc consensus binding sequences, while the AP-2/Sp1 site has been identified previously in DNase 1 footprinting analysis of cycling cells (5). (B) DG75 cells were transiently transfected with −1384 or −652 reporter constructs and various concentrations of an EBNA-2 expression vector. Equivalent amounts of DNA were transfected by adjusting the amount of empty vector pSG5 added. Differences in transfection efficiencies were controlled for by assaying β-galactosidase expressed from cotransfected pCMV-βgal and adjusting relative luciferase units (RLU) accordingly. The values shown are the mean and standard deviation of three transfections. (C) Deletion analysis of the cyclin D2 promoter activated by cotransfection of 20 μg of pSG5-EBNA 2. (D and E) DG75 cells (D) or Jurkat T cells (E) were transiently transfected with −652, pCMV-βgal, and 20 μg of EBNA-2 expression vectors. The activation of −652 induced by wild-type pSG5-EBNA-2 was compared to the activity induced by an equivalent amount of plasmid expressing an RBP-Jκ binding mutant, EBNA-2 WW323SR (D). (F) The expression level of wild-type versus mutant EBNA-2 was compared by Western blot analysis in three separate transfections.
Cell lines.
DG75 (1) is an EBV-negative Burkitt's lymphoma cell line. Jurkat T cells are leukemic lymphoblast cells. LCL-C is an EBV-immortalized LCL generated by infection of peripheral blood B cells with B95-8 virus. The cell lines were maintained in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal calf serum and antibiotics. BL41/P3 Notch-ER cells are stably transfected with a plasmid expressing conditional mouse Notch-IC regulated by estrogen (38). EREB2.5 cells (23) contain a conditional EBNA-2 regulated by estrogen, while SV LMP-1 11c and SV LMP-1 13c are derived from EREB2.5 cells and constitutively express LMP-1. pHEBo1A is a control cell line stably transfected with empty vector pHEBo (48). The cells were maintained in RPMI 1640 without phenol red (Gibco-BRL) and supplemented with 10 to 20% heat-inactivated fetal calf serum, antibiotics, and 1 μM β-estradiol. SV LMP-1 11c, SV LMP-1 13c, and pHEBo1A were maintained in the presence of hygromycin B (75, 75, and 150 μg/ml respectively). For estrogen withdrawal experiments, cells were washed twice in serum-free medium before being resuspended at 5 × 105/ml in RPMI 1640 without β-estradiol. The cells were then incubated for 5 days. Protein synthesis was inhibited by pretreating cells for 2 h with 50 μg of cycloheximide per ml and 100 μM anisomycin (Sigma). 493.6 cells are EREB2.5 cells stably transfected with tetracycline-regulatable c-myc (30). The cells express c-myc constitutively in the absence of tetracycline. To inhibit c-myc expression, tetracycline was added at 1 μg/ml. 493.6 cells were maintained in RPMI 1640 without phenol red and supplemented with 10% heat-inactivated fetal calf serum and antibiotics. β-Estradiol was not required for maintenance of growth.
Immunoblotting and antibiotics.
Radioimmunoprecipitation assay RIPA lysates were prepared and quantitated and immunoblots were performed as described previously (4). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After being blocked with 10% milk powder in PBS, the membranes were probed with the following antibodies: 1/10 dilution of anti-EBNA-LP monoclonal antibody JF 186 (14), 1/500 dilution of anti-EBNA-2 monoclonal antibody PE2 (Dako), 1/10 dilution of anti-LMP-1 monoclonal antibody S12 (27), 1/80 dilution of anti-cyclin D2 (G132-43; PharMingen), 1/1,000 dilution of rabbit polyclonal antibody anti-p27 (C-19; Santa Cruz), and 1/500 dilution of anti-c-Myc monoclonal antibody 9E10 (Santa Cruz). The secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit Ig (Dako) and horseradish peroxidase-conjugated sheep anti-mouse Ig (Amersham). Bound immunocomplexes were detected by enhanced chemiluminescence (Amersham).
RPA.
Total cellular RNA was extracted using RNAzol B (Biogenesis) and quantified by measurement of its absorbance at 260 nm. RPA were performed as recommended by the manufacturers of the RPA II RNase protection assay kit (Ambion). Briefly, 1 μg of linearized plasmid was used to generate 32P-labeled antisense RNA probes. Cellular RNA was hybridized overnight at 45°C with 60,000 cpm of the probe. An equivalent amount of yeast RNA was included in a hybridization reaction as a negative control. Single-strand RNA was digested with an RNase A-RNase T1 mixture for 30 min at 37°C. Protected fragments were precipitated and separated on an acrylamide gel, and the gel was then exposed to an autoradiographic film or analyzed on a PhosphorImager.
Transient-transfection assays.
Exponentially growing cells (107) were electroporated at 250 mV and 960 μF in 0.4-cm cuvettes (Bio-Rad). Each transfection mixture contained 0.5 μg of pCMV-βgal and 1 μg of reporter construct. For EBNA-2 titer determinations, the total amount of DNA transfected was normalized by addition of empty vector pSG5. Following electroporation, cells were resuspended in 10 ml of conditioned medium and incubated for 48 h. Cell pellets were harvested and lysed in 60 μl of luciferase reporter lysis buffer (Promega). A 20-μl volume of lysate was analyzed for luciferase activity, and an additional 20 μl was assayed for β-galactosidase activity using chlorophenol red-β-d-galactopyranoside as a substrate.
RESULTS
Cytokine RNA expression during the early stages of EBV infection.
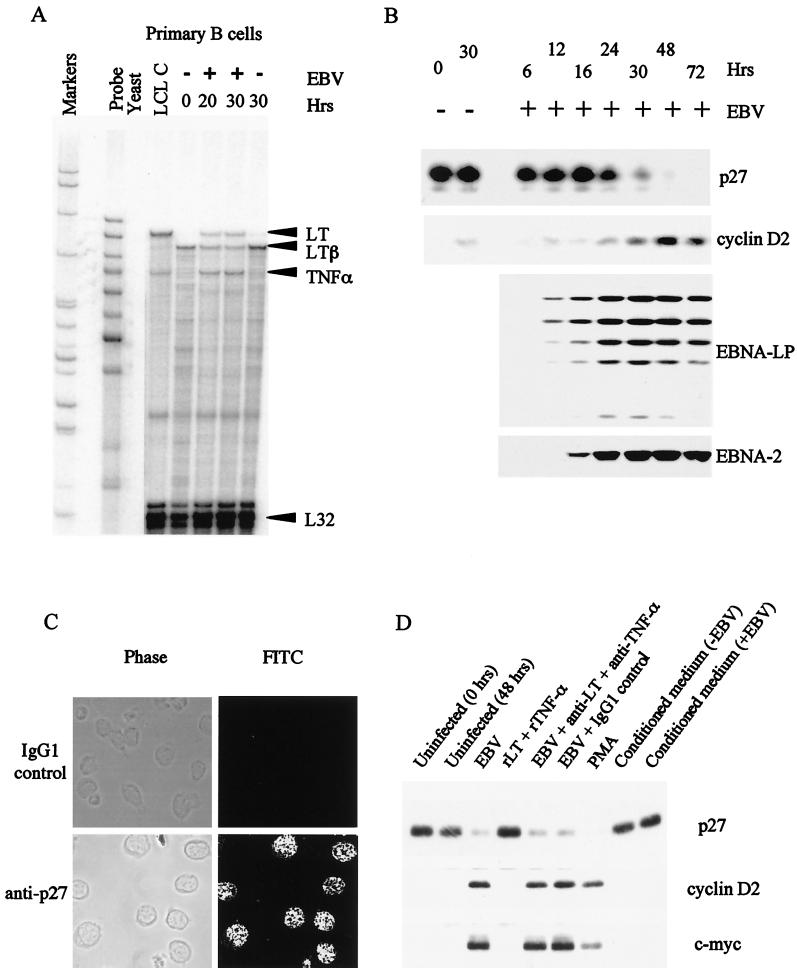
An RPA was used to analyze 30 relevant cytokine transcripts in quiescent B cells or B cells newly infected with the B95-8 strain of EBV. The levels of RNA detected were compared with those present in an established EBV-infected line, LCL-C. A representative gel is shown in Fig. 1A, and, for comparison, a time course of expression of EBNA-2, EBNA-LP, p27, and cyclin D2 is shown in Fig. 1B. The results of several experiments on cytokine expression are summarized in Table 1. Parallel results from peripheral blood mononuclear cells stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin are also shown in Table 1. This mixed population expressed a broader range of cytokines than we were able to detect in purified B cells and so serves as a useful positive control for the assay. The main changes following EBV infection of B cells were in tumor necrosis factor alpha (TNF-α), lymphotoxin (LT), LTβ, and granulocyte colony-stimulating factor (G-CSF) levels. The relatively low-level induction of G-CSF occurred within 20 h and appeared to be transient since G-CSF RNA was not detected in the established LCL. TNF-α and LT were also induced very early after infection, and similar RNA levels of both cytokines were also detected in LCL-C. In contrast, the membrane-bound form of LT (LTβ) decreased in abundance after EBV infection, and its level was reduced further in LCL-C.
FIG. 1.
(A) Autoradiograph of the results of an RPA for cytokine RNAs during the early stages of EBV infection of primary B cells and in an established LCL (LCL C). B cells were purified from PBMCs by positive selection using CD19 Dynabeads. The cells were either uninfected or infected with the B95-8 strain of EBV for 20 or 30 h. Total-cell RNA was isolated, and 10 μg used in a hybridization reaction with antisense riboprobes made using RiboQuant Multiprobe template sets. (B) Western blot analysis of the time course of expression of p27, cyclin D2, EBNA-LP, and EBNA-2 proteins after EBV infection of purified B lymphocytes. (C) Immunofluorescence of resting primary B cells showing uniform p27 staining in all nuclei. FITC, fluorescein isothiocynate. (D) Western blot analysis showing p27, cyclin D2, and c-myc expression in primary B cells. Cells were either left uninfected, treated with PMA (30 ng/ml), or infected with EBV for 48 h. EBV-infected cells treated with 12.5 μg of neutralizing antibodies to LT and TNF-α per ml or the equivalent amount of IgG1 isotype control antibody (25 μg/ml) are indicated. Uninfected primary B cells treated with 10 ng of both human recombinant LT and TNF-α per ml are also shown. Tracks labeled “Conditioned medium” refer to uninfected B cells incubated in medium conditioned for 48 h by uninfected (−EBV) or EBV-infected (+EBV) B cells and subsequently ultracentrifuged to remove contaminating virus particles.
TABLE 1.
Summary of cytokine RPAs
| Cytokine | Amt of cytokine detected ina:
|
||||
|---|---|---|---|---|---|
| PBMC (PMA/ ionomycin) | Primary B cells
|
LCLC | |||
| Resting (0 h) | EBV (20 h) | EBV (30 h) | |||
| IL-1α | + | − | − | − | + |
| IL-1β | +++ | + | + | + | − |
| IL-1Ra | ++ | ± | + | + | − |
| IL-2 | +++ | − | − | − | − |
| IL-3 | + | − | − | − | − |
| IL-4 | − | − | − | − | − |
| IL-5 | − | − | − | − | − |
| IL-6 | + | − | − | − | − |
| IL-7 | − | − | − | − | − |
| IL-9 | − | − | − | − | − |
| IL-10 | − | − | − | − | − |
| IL-12p35 | − | − | − | − | − |
| IL-12p40 | − | − | − | − | − |
| IL-13 | − | − | − | − | − |
| IL-14 | − | − | − | − | − |
| IL-15 | − | − | − | − | − |
| IFN-γ | +++ | − | − | − | − |
| IFN-β | + | ± | − | − | − |
| TNF-α | ++ | − | + | + | + |
| TNF-β (LT) | + | − | + | + | ++ |
| LTβ | + | ++ | + | + | − |
| TGF-β1 | + | + | + | + | + |
| TGF-β2 | + | − | − | − | − |
| TGF-β3 | + | ± | − | − | + |
| M-CSF | − | − | − | − | − |
| G-CSF | − | − | + | + | − |
| GM-CSF | + | − | − | − | − |
| LIF | + | − | − | − | − |
| SCF | − | − | − | − | − |
| hOSM | + | − | − | − | − |
| L32 | +++ | +++ | +++ | +++ | +++ |
| GAPDH | +++ | +++ | +++ | +++ | +++ |
| EBNA-2 | − | − | + | + | + |
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density gradient and were stimulated with PMA (50ng/ml) and ionomycin (500 ng/ml) for 6 h. Purified B cells were isolated by CD19 positive selection and were infected with EBV for 20 or 30 h. Cytokine levels in uninfected cells, EBV-infected cells, and an established LCL line (LCL C) were analyzed using 10 μg of total cellular RNA. The relative amount of cytokine detected is indicated by an arbritary score ranging from +++ (abundant cytokines) to ± or − (weakly expressed or absent cytokines, respectively). EBNA-2 status, determined by Western blotting, is also shown.
Our interest in cytokine induction had been initiated, in part, by our earlier observation that p27 was dramatically down regulated within the first 48 h of EBV infection of primary B cells (Fig. 1B). The ability to overcome G1 arrest induced by p27 in quiescent cells is a crucial step in progression of the cell through the cell cycle. In other systems, p27 levels are controlled primarily by degradation via the ubiquitin/proteasome pathway (29, 33, 44). In cells where S-phase entry of resting cells can be caused by activation of the c-myc oncogene, one consequence of c-myc activity is a loss of p27 from cdk2, resulting in increased cyclin E/cdk2 kinase activity. The p27 is sequestered by cyclin D2/cdk4 complexes presumably formed following c-myc-induced activation of the cyclin D2 gene (2). When we stained infected B cells for production of EBNA-LP, we detected the viral protein in only about 25% of B cells, but Western blot analysis indicated that more p27 was lost than could be accounted for by infection of this proportion of the B-cell population (37). This suggested that a cytokine secreted early in infection might account for the loss of p27. The cytokine analysis presented here shows that several cytokines are induced at early time points following EBV infection. Immunofluorescence studies (Fig. 1C) confirm that p27 in primary B cells is distributed evenly throughout the entire population and support the hypothesis that p27 is lost from a larger proportion of cells than are actually infected with the virus. However, we have so far been unable to make p27 disappear by addition of cytokines to uninfected B cells (Fig. 1D). We have tested whether addition of a combination of recombinant TNF-α and LT to the culture medium of primary B cells could affect p27 levels, but neither cytokine did so (Fig. 1D). Conditioned medium from LCLs or B cells infected for 48 h also had no clear effect on p27 levels (or c-myc or cyclin D2) in primary B cells (Fig. 1D), and antibodies which neutralize TNF-α and LT did not prevent the degradation of p27 caused by EBV (Fig. 1D). The mechanism for the regulation of p27 levels during EBV infection thus remains to be determined, and it is still possible that the B cells which appear to be uninfected are in the process of dying (apoptosis is accompanied by the degradation of p27 [15]) even though cell death was not apparent by trypan blue exclusion or propidium iodide staining and flow cytometry (37).
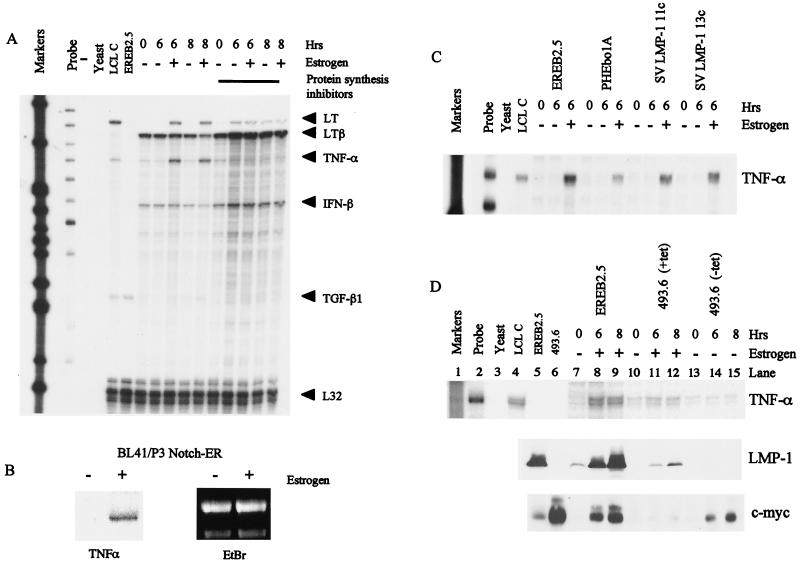
Since the cytokine RNA was induced rapidly after virus infection, we tested whether TNF-α or LT can be induced as a result of EBNA-2 transcriptional activity. We studied their regulation in the EREB2.5 cell line which contains an EBNA-2/estrogen receptor fusion protein. In these cells, the activity of EBNA-2 is dependent on the presence of estrogen in the culture medium. When starved of estrogen, the cells increase expression of the cyclin-dependent kinase inhibitor p27 and downregulate c-myc, cyclin D2, and the viral membrane protein LMP-1 (see below in Fig. 4). The EREB2.5 cells were starved of estrogen, and then RNA was prepared at various times after the addition of estrogen (Fig. 2A). The RNAs for TNF-α and LT were clearly induced by EBNA-2 within 6 h, but the effect was indirect since induction was abolished by prevention of protein synthesis during the reactivation of EBNA-2 (Fig. 2A). EBNA-2 stimulates many of the properties of activated Notch, and activation of a conditional Notch-ER fusion protein from a construct stably transfected in BL41/P3HR1 cells (38) also induced TNF-α RNA expression (Fig. 2B). The LTβ RNA was expressed in estrogen-starved EREB2.5 cells (analogous to uninfected primary B cells), and its level was somewhat reduced in response to EBNA-2 reactivation after 8 h. This effect was also prevented by inhibition of protein synthesis, although this result was partly obscured by a superinduction of the LTβ RNA by the cycloheximide-anisomycin treatment. Such stabilization of certain mRNAs by inhibitors of protein synthesis has been observed in some other situations (9, 16).
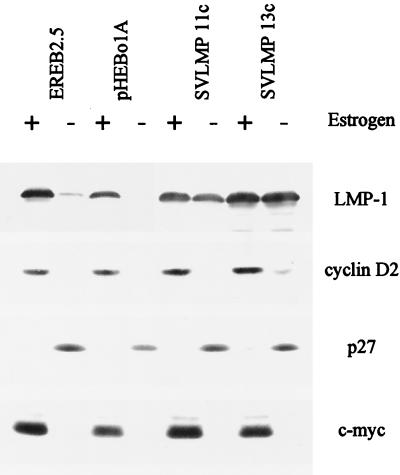
FIG. 4.
Regulation of cyclin D2, c-myc, and p27 in EREB2.5 cells. The levels of cell cycle-related proteins were compared in the parental EREB2.5 cells and cells constitutively expressing LMP-1 from a stably transfected plasmid. Cells were grown in the presence of 1 μM estrogen (+) or washed and starved of estrogen for 5 days (−). RIPA lysates (50 μg of protein) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting for the proteins indicated.
FIG. 2.
(A) RPA for cytokine RNA in the EREB2.5 cell line. Cells were starved of estrogen for 5 days and, where indicated, pretreated with the protein synthesis inhibitors cycloheximide and anisomycin. Samples were either untreated or stimulated with estrogen and harvested 6 or 8 h later. RPAs were performed using 10 μg of total-cell RNA. TGF-β1, transforming growth factor β1. (B) Northern blot analysis showing TNF-α RNA induction following Notch activation in BL41/P3 cells expressing estrogen-regulated murine Notch 1-1C. (C) RPA showing TNF-α levels in the parental EREB2.5 cells, EREB2.5 cells stably transfected with control plasmid pHEbo1A, and two clones constitutively expressing LMP-1 (SVLMP-1 11c and SVLMP-1 13c. (D) RPA for TNF-α RNA in parental EREB2.5 cells and EREB2.5 cells stably transfected with a plasmid expressing tetracycline-regulatable c-myc, i.e., 493.6 cells. 493.6 cells (lane 6) were maintained in RPMI supplemented with 10% fetal calf serum. 493.6 cells in lanes 10 to 15 were treated with tetracycline for 3 days prior to the start of the experiment to repress c-myc expression. They were then either incubated in tetracycline-containing medium and treated with estrogen (lanes 11 and 12) or washed to remove tetracycline (lanes 14 and 15). Cell lysates from the same experiment were also analyzed for LMP-1 and c-myc expression by Western blotting.
Since the induction of cytokine RNA is an indirect effect of EBNA-2 activity, we tested whether LMP-1 (which is induced directly by EBNA-2) could regulate cytokine transcription. LMP-1 is the major EBV-encoded activator of NF-κB in LCL and is relevant because the TNF-α promoter contains NF-κB consensus binding sites and because NF-κB itself is important in activation of the TNF-α promoter in activated macrophages (7). In addition, LMP-1 activates the p38 MAPK pathway and regulates IL-6 and IL-8 production (10). We used EREB2.5 cells that have been stably transfected with plasmids expressing LMP-1. These cells constitutively express LMP-1 even when estrogen has been withdrawn (see below in Fig. 4). We were thus able to determine whether gene regulation occurred as a result of LMP-1 induction by EBNA-2 or a separate function of EBNA-2. Figure 2C shows an RPA for TNF-α RNA. The RNA was present only in cells stimulated with estrogen and not in estrogen-starved cells, regardless of whether LMP-1 was constitutively expressed. In a similar experiment, we used EREB2.5 cells which constitutively express c-myc in the absence of EBNA-2 activity to determine whether c-myc regulates cytokine RNA levels (Fig. 2D). As shown in Fig. 2A, the abundance of TNF-α RNA was increased following addition of estrogen to the culture medium of starved EREB2.5 cells. In 493.6 cells treated with tetracycline, where c-myc is repressed, addition of estrogen to activate EBNA-2 also resulted in an increase in TNF-α RNA, although to a much reduced level compared with increases seen in the parental EREB2.5 cells (Fig. 2D, lanes 11 and 12). The reduced estrogen response in these cells is, we suggest, due to heterogeneity of the population. 493.6 cells overexpress c-myc (Fig. 2D) and do not require estrogen-activated EBNA-2 for their proliferation. Western blots showed that the level of induction of LMP-1 following estrogen addition was lower in 493.6 cells than in EREB2.5 cells, indicating that a proportion of these cells no longer respond to estrogen (Fig. 2D). When treated with tetracycline, c-myc was undetectable and the cells had low levels of TNF-α RNA (Fig. 2D, lane 13). When the cells were washed and resuspended in medium without tetracycline, c-myc was induced but no increase in TNF-α RNA was observed (lanes 14 and 15). We therefore conclude that in these growth-arrested cells, TNF-α RNA is not regulated by LMP-1 or c-myc.
Regulation of cyclin D2 and c-myc expression by EBV.
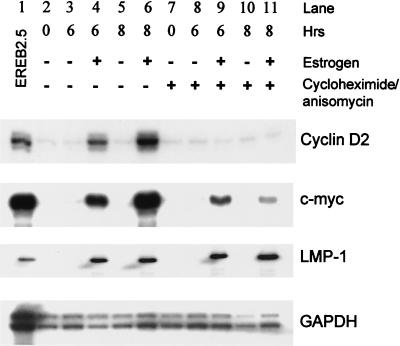
When we started using cyclin D2 up regulation as an early marker for cell cycle entry in response to expression of EBNA-2 and EBNA-LP, it was unclear whether this was a direct effect of these transcription factors on the promoter for cyclin D2 or worked through intermediate steps. Using the EREB2.5 cell line, it was demonstrated that cyclin D2 is an indirect target but c-myc is activated directly by EBNA-2 (21). This and subsequent work in which overexpression of c-myc allowed the selection of cells that no longer required EBNA-2 for proliferation led to the conclusion that the major effect of EBNA-2 on cell proliferation is through c-myc (21, 30). We had independently studied the regulation of cyclin D2 and c-myc in EREB2.5 cells, and it is clear from our data that only a fraction of the normal expression of c-myc is achieved through direct activation of c-myc transcription by EBNA-2 (Fig. 3). When EBNA-2 activity was restored in EREB2.5 cells by addition of estrogen, LMP-1 RNA was induced equally well in the presence or absence of protein synthesis inhibitors. In contrast, c-myc RNA was expressed at substantially higher level without inhibitors. We also found that cyclin D2 was regulated indirectly by EBNA-2 since no cyclin D2 RNA was induced in the presence of protein synthesis inhibitors (Fig. 3). The amount of both EBNA-2 and EBNA-LP protein present within the cells was stable over a 12-h period after treatment with protein synthesis inhibitors (data not shown). The lack of cyclin D2 gene expression in these experiments was therefore not due to insufficient levels of either viral protein. It thus appears that there is both direct and indirect regulation of c-myc by EBNA-2 in these cells.
FIG. 3.
LMP-1 and c-myc RNAs are directly regulated by EBNA-2. EREB2.5 cells were washed and starved of estrogen for 5 days. They were then either left untreated or treated with estrogen for 6 or 8 h as indicated. The protein synthesis inhibitors cycloheximide and anisomycin (lanes 7 to 11) were added for 2 h prior to the start of the experiment. Total cellular RNA was extracted, and 10 μg was used in RPAs for cyclin D2, c-myc, and LMP-1 RNA. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was hybridized with 1 μg of RNA. EREB2.5 cells (lane 1) were maintained continuously in the presence of estrogen.
Because EBNA-2 regulates LMP-1 expression in the EREB2.5 cells and LMP-1 is able to affect transcription factors such as NF-κB and c-jun, we also checked that LMP-1 did not mediate any effects on cyclin D2, c-myc, or p27. Regulation of all three proteins in the EREB2.5 cells under the conditions tested was not affected by the constitutive expression of LMP-1 (Fig. 4).
Since the induction of cyclin D2 by EBNA-2 is indirect, one obvious possibility is that c-myc might be the intermediary factor. Recent studies using EREB2.5 cells that overexpress c-myc have supported this model (30). The human cyclin D2 promoter does contains consensus E-box sequences approximately 1.5 kb upstream of the transcription start sites, although these are much further upstream than in the mouse promoter. We mapped the transcription start sites to four major positions in EBV-immortalized LCLs (Fig. 5A). To map sequences required for regulation of cyclin D2 transcription, we developed a transient-transfection assay in which the human cyclin D2 promoter could be regulated by EBNA-2. We found that a plasmid containing 1,624 bp of the cyclin D2 promoter (−1384) fused to a luciferase reporter gene was activated 9.7-fold over its basal level by EBNA-2 when transfected into DG75 BL cells (Fig. 5B). We tested whether cotransfected EBNA-LP could enhance the activation induced by EBNA-2 alone and found no evidence that the two viral proteins could cooperate in this manner (data not shown). Although the amount of EBNA-2 expression vector required to induce activation was large, it is consistent with the amounts needed in other studies to activate various EBNA-2-responsive promoters (20, 28, 43). Since DG75 cells overexpress c-myc constitutively (because of a chromosomal translocation), this result also suggests that EBNA-2 is able to activate the cyclin D2 promoter in addition to the effect of c-myc.
5′ deletion of the promoter down to −652 removed both E-box consensus sequences, and in this system, the EBNA-2 expression vector activated the promoter by up to 19.6-fold (Fig. 5B). The level of basal activity and activation of −652 was consistently higher than the levels observed with the longest promoter, −1384. Therefore, deletion of potential c-myc binding sites from the promoter did not prevent but may have actually enhanced EBNA-2-mediated activation.
We carried out further deletion analysis of the cyclin D2 promoter to identify elements conferring EBNA-2 responsiveness. Analysis of 5′ deletions of the promoter in the DG75 transient-transfection assay revealed a gradual loss of the basal activity of the promoter, but much of the inducibility by EBNA-2 was maintained down to −66 (Fig. 5C). +126 did not contain the transcription start sites and showed little activity with or without EBNA-2. With this type of analysis, we were not able to assign the sequences involved in EBNA-2 activation more precisely than is shown in Fig. 5. Mutants of EBNA-2 (FY4SR, IF50SR, SR360VD, YI139SR, Δ344-357, Δ117–147, and Δ58–100) were all able to induce the cyclin D2 promoter (data not shown); however, a mutant of EBNA-2 in which two adjacent Trp residues were mutated to Ser and Arg (WW323SR) and which was defective in RBP-Jκ dependent induction of transcription had a partially reduced ability to induce the cyclin D2 promoter construct (Fig. 5D). There was no detectable difference in the expression levels of the two proteins (Fig. 5F). The activation of the cyclin D2 promoter by EBNA-2 was not restricted to DG75 cells since similar results were obtained in the Jurkat T-cell line (Fig. 5E). These data suggested that the effect of EBNA-2 was mediated partly through the RBP-Jκ and partly through other pathways, consistent with its various mechanisms of action at some other promoters. No consensus binding site for RBP-Jκ could be identified within the cyclin D2 promoter sequence, and we therefore conclude that this effect is also likely to be indirect and not mediated by c-myc directly activating the cyclin D2 promoter.
DISCUSSION
In this study we have identified several cytokines that are regulated during the early stage of EBV infection of quiescent, primary B cells. On infection, TNF-α, LT, and G-CSF were the main transcripts detected. The induction occurred within 20 h of infection, consistent with a role for EBNA-2 (the earliest viral transcription factor produced) in their regulation. Using similar methods, other workers have previously analyzed cytokine gene expression in established LCLs and also detected high levels of TNF-α, LT, and transforming growth factor β1 transcripts (32). However, to our knowledge, ours is the first detailed analysis of cytokine expression during the initial stages of immortalization. It was at first surprising that we did not detect RNA for interleukin-6 (IL-6) and IL-10, since they have been reported to be expressed by LCLs, but there is considerable variation between LCL lines in IL-6 and IL-10 expression (32), and the LCL-C which we used as a control may happen to have low expression of these cytokines. In the primary B-cell infections, we assayed RNA prior to the expression of LMP-1, which has been shown to induce IL-6 (10).
Our observations of early cytokine induction are intriguing since both TNF-α and LT have been described as autocrine growth factors for both primary and EBV-immortalized B cells (3, 11, 15, 45). TNF-α, for example, is transcribed rapidly following stimulation of B cells by PMA, via their surface Ig, or after signaling through CD22 or CD40. EBV might benefit from early induction of growth-promoting cytokines during the immortalization process. However, when we treated newly infected B cells or LCLs with recombinant cytokines or neutralizing antibodies, we could find no evidence that TNF-α or LT could enhanced the proliferative response of the B cells (data not shown). Therefore it is unclear whether TNF-α or LT contributes to the immortalization process during the first 72 h of EBV infection. However, transcription of both cytokines is maintained at high levels in the established cell line LCL-C.
Our studies of cytokines had been stimulated partly by earlier observations (37) that more cells seemed to lose p27 expression than could be detected to express EBNA-LP on EBV infection. The data shown here suggest that a cytokine does not mediate this effect since conditioned medium did not cause the effect, but it remains possible that a sequential exposure of the primary B cells to EBV and cytokines is required for the loss of p27. So far, it is clear that in EBV-infected cells p27 is down regulated in response to EBNA-2 activation and that the regulation of p27 is unaffected by LMP-1 expressed constitutively. Recent work suggests that EBNA-3C can inhibit the accumulation of p27 in infected cells (31). Feedback on the Cp promoter by EBNA-2 might be expected to affect EBNA-3C expression, and so it is possible that the accumulation of p27 in estrogen-starved EREB2.5 cells is caused by down regulation of EBNA-3C. This could be tested when antibodies that can recognize the B-type EBNA-3C in EREB2.5 cells become available. The potential role of c-myc activation in p27 regulation was more difficult to assess. The 493.6 cells containing tetracycline-regulated c-myc might be expected to be an ideal tool for this study; however, these cells did not express p27 when growth arrested by tetracycline addition, even though neither EBNA-2 nor c-myc is active in these cells. The reason for their inability to express p27 under growth-arresting conditions is unclear at present.
EREB2.5 cells provided a useful system with which to confirm our findings in primary B cells and to extend the study to investigate the mechanism of cytokine regulation by EBV. We have shown that both LT and TNF-α were induced rapidly after activation of EBNA-2 with estrogen but that the effect was indirect since induction was blocked by protein synthesis inhibitors. We next investigated whether either LMP-1 or c-myc was involved in the regulation of TNF-α. Although LMP-1 is known to induce NF-κB activity, the presence of constitutively expressed LMP-1 in stably transfected EREB2.5 cells was not sufficient to maintain the transcription of TNF-α after withdrawal of estrogen, i.e., in growth-arrested cells. In EREB2.5 cells containing tetracycline-regulated c-myc, induction of c-myc in the absence of EBNA-2 activity did not result in an increase in transcription of the TNF-α gene. These data indicate that EBNA-2 has the ability to regulate cytokine transcription in addition to its effects on LMP-1 and c-myc.
We have also investigated the mechanism by which EBV regulates cyclin D2. Our transient-transfection assay clearly showed that EBNA-2 activates the cotransfected cyclin D2 promoter. This effect was not restricted to a B-cell environment, since similar results were obtained in Jurkat T cells, suggesting that EBNA-2-induced activation of cyclin D2 is unlikely to depend on B-cell-specific factors. Previous work using gp350-primed primary B cells has shown that transient transfection of both EBNA-2 and EBNA-LP expression plasmids is required to induce the transcription of endogenous cyclin D2 (35). Expressed individually, the proteins did not activate the promoter. In contrast, cooperation between EBNA-LP and EBNA-2 was not required to induce activation of the cyclin D2 promoter in our transient-transfection assays. The difference between the two observations might be explained by the fact that the transfection assays are carried out with rapidly cycling cells whereas the primary B cells are quiescent. We speculate that EBNA-LP performs an as yet undefined role in resting cells that is crucial for their transition from G0 to G1 and S phase and for their ability to transcribe the cyclin D2 promoter, perhaps consistent with a recent report that EBNA-LP can bind to the HAX-1 protein (22), which has properties relevant to signal transduction in B cells. In cells that are already in the cell cycle, this specific function of EBNA-LP may no longer be required, and in the EREB2.5 cells, EBNA-LP is present constitutively while EBNA-2 is regulated.
Using the EREB2.5 cell line, we have shown that the regulation of cyclin D2 transcription is indirect since induction of cyclin D2 RNA following estrogen addition was completely blocked by protein synthesis inhibitors. Our results confirm those of Kaiser et al. (21). In these experiments, where the EREB2.5 cells are arrested in G1 by estrogen withdrawal, both EBNA-2 and EBNA-LP are expressed in the cells. Since protein levels were stable during the cycloheximide and anisomycin treatment, neither protein was limiting, indicating that the absence of any cyclin D2 transcription must have been due to the inhibition of synthesis of other unknown factors involved in cyclin D2 transcription. In addition, expression of cyclin D2 was not maintained by constitutive expression of LMP-1 in the absence of EBNA-2 activity. While cyclin D2 is an indirect target of EBNA-2 transcriptional activity, we have also confirmed that c-myc is, at least partially, regulated directly by EBNA-2.
The E-box-dependent repression of the cyclin D2 promoter, which can be overcome by c-myc, appears to involve histone deacetylases (2). The involvement of histone acetylation was implied by the ability of trichostatin A (an inhibitor of histone deacetylase) to activate the mouse cyclin D2 promoter when added to quiescent cells. To test whether the cyclin D2 promoter is repressed by a similar mechanism in our system, we treated primary B cells with trichostatin A and found that treatment does not induce cyclin D2 protein in primary human B cells (data not shown). There may be important differences in cyclin D2 regulation between quiescent human B cells and serum-starved mouse embryo fibroblasts that account for their different susceptibility to trichostatin A treatment. This observation and the data presented in this paper suggest that there are other means by which cyclin D2 is activated, in addition to c-myc-mediated derepression of the promoter.
In agreement with previous studies of the cyclin D2 promoter, deletion of the region containing both E boxes resulted in an increase in the basal activity of the −652 construct compared with the full-length construct. However, deletion of the region containing the E box did not reduce the ability of EBNA-2 to activate the cyclin D2 promoter in our system, also indicating that there are additional mechanisms by which EBNA-2 mediates activation. In our system, analysis of several deletion mutants of the cyclin D2 promoter did not help to define which sequences were important in conferring EBNA-2 responsiveness. This finding and the fact that wild-type EBNA-2 was a better activator of the promoter than an RBP-Jκ binding mutant suggest that activation is likely to be a complex matter involving a number of different cofactors. The production or activity of some of these factors may depend on the ability of EBNA-2 to bind RBP-Jκ.
Overall, our results identify novel aspects of the regulation of TNF-α and cyclin D2 by EBNA-2. Regulation of both genes is indirect, but both are considered to play important roles in the proliferation of B cells induced by EBV.
ACKNOWLEDGMENTS
We thank Diane Hayward, Paul Ling, Graham Packham, and Dov Shifmann for plasmids used in this work and Martin Allday for comments on the manuscript.
REFERENCES
- 1.Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey J M, Cohen M M, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75) Int J Cancer. 1977;19:27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussiotis V A, Nadler L M, Strominger J L, Goldfeld A E. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc Natl Acad Sci USA. 1994;91:7007–7011. doi: 10.1073/pnas.91.15.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brimmell M, Mendiola R, Mangion J, Packham G. BAX frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene. 1998;16:1803–1812. doi: 10.1038/sj.onc.1201704. [DOI] [PubMed] [Google Scholar]
- 5.Brooks A, Shiffman D, Chan C, Brooks E, Milner P. Functional analysis of the human cyclin D2 and D3 promoters. J Biol Chem. 1996;271:9090–9099. doi: 10.1074/jbc.271.15.9090. [DOI] [PubMed] [Google Scholar]
- 6.Cannell E, Farrell P, Sinclair A. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B cells. Oncogene. 1996;13:1413–1421. [PubMed] [Google Scholar]
- 7.Collart M A, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coller H A, Grandori C, Tamayo P, Colbert T, Lander E S, Eisenman R N, Golub T R. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidoff A N, Mendelow B V. Protein synthesis inhibition is not a requisite for puromycin- and cycloheximide-induced c-myc mRNA superinduction. Anticancer Res. 1994;14:1199–1201. [PubMed] [Google Scholar]
- 10.Eliopoulos A G, Gallagher N J, Blake S M, Dawson C W, Young L S. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 11.Estrov Z, Kurzrock R, Pocsik E, Pathak S, Kantarjian H M, Zipf T F, Harris D, Talpaz M, Aggarwal B B. Lymphotoxin is an autocrine growth factor for Epstein-Barr virus-infected B cell lines. J Exp Med. 1993;177:763–774. doi: 10.1084/jem.177.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell P. Epstein-Barr virus immortalizing genes. Trends Microbiol. 1995;3:105–109. doi: 10.1016/s0966-842x(00)88891-5. [DOI] [PubMed] [Google Scholar]
- 13.Farrell P. Signal transduction from the Epstein-Barr virus LMP-1 transforming protein. Trends Microbiol. 1998;6:175–177. doi: 10.1016/s0966-842x(98)01262-1. [DOI] [PubMed] [Google Scholar]
- 14.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons D L, Rowe M, Cope A P, Feldmann M, Brennan F M. Lymphotoxin acts as an autocrine growth factor for Epstein-Barr virus-transformed B cells and differentiated Burkitt lymphoma cell lines. Eur J Immunol. 1994;24:1879–1885. doi: 10.1002/eji.1830240825. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg M E, Hermanowski A L, Ziff E B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986;6:1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkel T, Ling P D, Hayward S D, Peterson M G. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein Jκ. Science. 1994;265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- 18.Hollyoake M, Stuhler A, Farrell P, Gordon J, Sinclair A. The normal cell cycle activation program is exploited during the infection of quiescent B lymphocytes by Epstein-Barr virus. Cancer Res. 1995;55:4784–4787. [PubMed] [Google Scholar]
- 19.Jayachandra S, Low K G, Thlick A E, Yu J, Ling P D, Chang Y, Moore P S. Three unrelated viral transforming proteins (vIRF, EBNA2, and E1A) induce the MYC oncogene through the interferon-responsive PRF element by using different transcription coadaptors. Proc Natl Acad Sci USA. 1999;96:11566–11571. doi: 10.1073/pnas.96.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman S R. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser C, Laux G, Eick D, Jochner N, Bornkamm G W, Kempkes B. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J Virol. 1999;73:4481–4484. doi: 10.1128/jvi.73.5.4481-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi Y, Nakajima K, Igarashi M, Morita T, Tanaka M, Suzuki M, Yokoyama A, Matsuda G, Kato K, Kanamori M, Hirai K. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J Virol. 2000;74:10104–10111. doi: 10.1128/jvi.74.21.10104-10111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart J W, Kremmer E, Delecluse H J, Rottenberger C, Bornkamm G W, Hammerschmidt W. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieff E. Epstein-Barr virus. In: Fields B, Knipe D, Howley P, editors. Fields virology. 3rd ed. Philadelphia, Pa: Raven Press; 1996. pp. 2343–2396. [Google Scholar]
- 25.Kiermaier A, Gawn J, Desbarats L, Saffrich R, Ansorge W, Farrell P, Eilers M, Packham G. DNA binding of USF is required for specific E-box dependent gene activation in vivo. Oncogene. 1999;18:7200–7211. doi: 10.1038/sj.onc.1203166. [DOI] [PubMed] [Google Scholar]
- 26.Laux G, Adam B, Strobl L J, Moreau-Gachelin F. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-Jκ interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 1994;13:5624–5632. doi: 10.1002/j.1460-2075.1994.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann K P, Staunton D, Thorley-Lawson D A. Epstein-Barr virus-encoded protein found in plasma membrane in transformed cells. J Virol. 1985;55:710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsche F, Bell A, Rickinson A. Epstein-Barr virus leader protein (EBNA-LP) enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J Virol. 1997;71:6619–6628. doi: 10.1128/jvi.71.9.6619-6628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagano M, Tam S, Theodoras A, Beer-Romero P, Sal G, Chau V, Yew P, Draetta G, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 30.Pajic A, Spitkovsky D, Christoph B, Kempkes B, Schuhmacher M, Staege M S, Brielmeier M, Ellwart J, Kohlhuber F, Bornkamm G W, Polack A, Eick D. Cell cycle activation by c-myc in a Burkitt lymphoma model cell line. Int J Cancer. 2000;87:787–793. doi: 10.1002/1097-0215(20000915)87:6<787::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Parker G A, Touitou R, Allday M J. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. 2000;19:700–709. doi: 10.1038/sj.onc.1203327. [DOI] [PubMed] [Google Scholar]
- 32.Rochford R, Cannon M J, Sabbe R E, Adusumilli K, Picchio G, Glynn J M, Noonan D J, Mosier D E, Hobbs M V. Common and idiosyncratic patterns of cytokine gene expression by Epstein-Barr virus transformed human B cell lines. Viral Immunol. 1997;10:183–195. doi: 10.1089/vim.1997.10.183. [DOI] [PubMed] [Google Scholar]
- 33.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair A J, Farrell P J. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J Virol. 1995;69:5641–5468. doi: 10.1128/jvi.69.9.5461-5468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjoblom A, Yang W, Palmqvist L, Jansson A, Rymo L. An ATF/CRE element mediates both EBNA2-dependent and EBNA2-independent activation of the Epstein-Barr virus LMP1 gene promoter. J Virol. 1998;72:1365–1376. doi: 10.1128/jvi.72.2.1365-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spender L, Cannell E, Hollyoake M, Wensing B, Gawn J, Brimmell M, Packham G, Farrell P. Control of cell cycle entry and apoptosis in B lymphocytes infected by Epstein-Barr virus. J Virol. 1999;73:4678–4688. doi: 10.1128/jvi.73.6.4678-4688.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strobl L J, Hofelmayr H, Marschall G, Brielmeier M, Bornkamm G W, Zimber-Strobl U. Activated Notch1 modulates gene expression in B cells similarly to Epstein-Barr viral nuclear antigen 2. J Virol. 2000;74:1727–1735. doi: 10.1128/jvi.74.4.1727-1735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugano N, Chen W, Roberts M, Cooper N. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-κB induction. J Exp Med. 1997;186:731–737. doi: 10.1084/jem.186.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong X, Drapkin R, Reinberg D, Kieff E. The 62- and 80-kDa subunits of transcription factor IIH mediate the interaction with Epstein-Barr virus nuclear protein 2. Proc Natl Acad Sci USA. 1995;92:3259–3263. doi: 10.1073/pnas.92.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong X, Drapkin R, Yalamanchili R, Mosialos G, Kieff E. The Epstein-Barr virus nuclear protein 2 acidic domain forms a complex with a novel cellular coactivator that can interact with TFIIE. Mol Cell Biol. 1995;15:4735–4744. doi: 10.1128/mcb.15.9.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong X, Wang F, Thut C J, Kieff E. The Epstein-Barr virus nuclear protein 2 acidic domain can interact with TFIIB, TAF40, and RPA70 but not with TATA-binding protein. J Virol. 1995;69:585–588. doi: 10.1128/jvi.69.1.585-588.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang S F, Wang F, Izumi K M, Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991;65:6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worm M, Ebermayer K, Henz B. Lymphotoxin-alpha is an important autocrine factor for CD40+ interleukin-4-mediated B-cell activation in normal and atopic donors. Immunology. 1998;94:395–402. doi: 10.1046/j.1365-2567.1998.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu D Y, Kalpana G V, Goff S P, Schubach W H. Epstein-Barr virus nuclear protein 2 (EBNA2) binds to a component of the human SNF-SWI complex, hSNF5/Ini1. J Virol. 1996;70:6020–6028. doi: 10.1128/jvi.70.9.6020-6028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu D Y, Krumm A, Schubach W H. Promoter-specific targeting of human SWI-SNF complex by Epstein-Barr virus nuclear protein 2. J Virol. 2000;74:8893–8903. doi: 10.1128/jvi.74.19.8893-8903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimber-Strobl U, Kempkes B, Marschall G, Zeidler R, Van Kooten C, Banchereau J, Bornkamm G, Hammerschmidt W. Epstein-Barr virus latent membrane protein (LMP1) is not sufficient to maintain proliferation of B cells but both it and activated CD40 can prolong their survival. EMBO J. 1996;15:7070–7078. [PMC free article] [PubMed] [Google Scholar]
- 49.Zimber-Strobl U, Kremmer E, Grasser F, Marschall G, Laux G, Bornkamm G W. The Epstein-Barr virus nuclear antigen 2 interacts with an EBNA2 responsive cis-element of the terminal protein 1 gene promoter. EMBO J. 1993;12:167–175. doi: 10.1002/j.1460-2075.1993.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]