Abstract
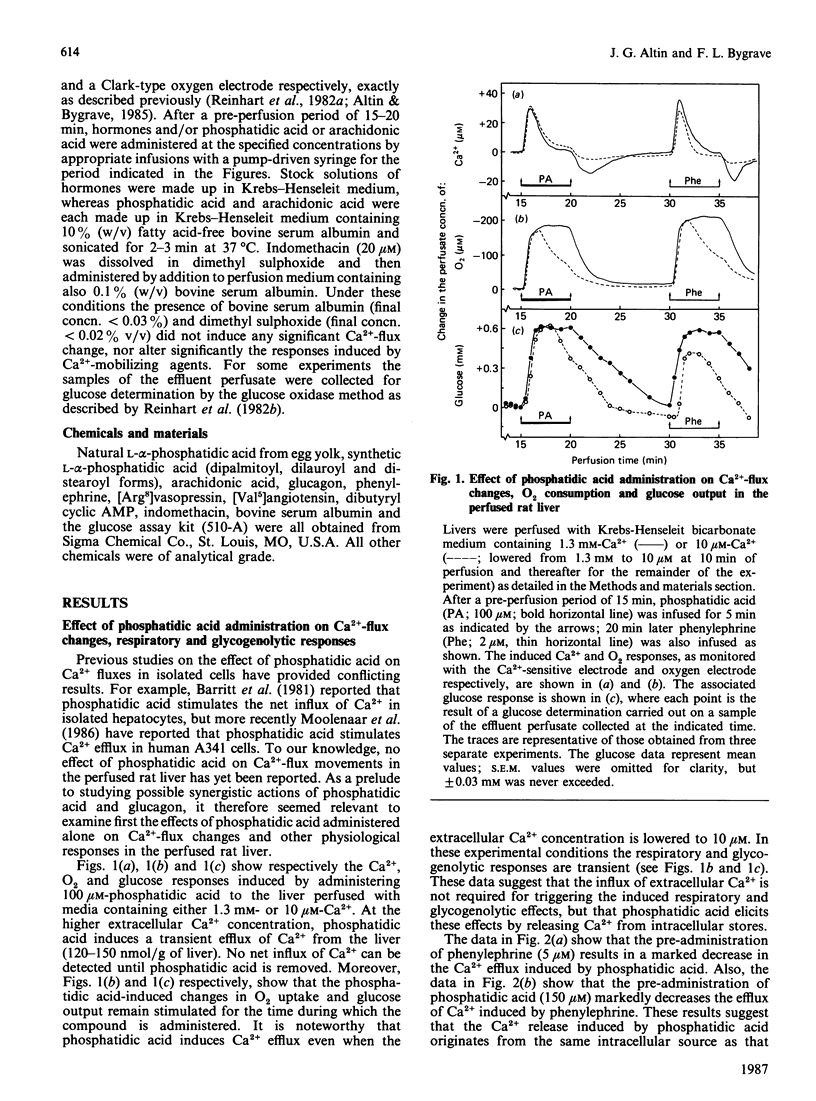
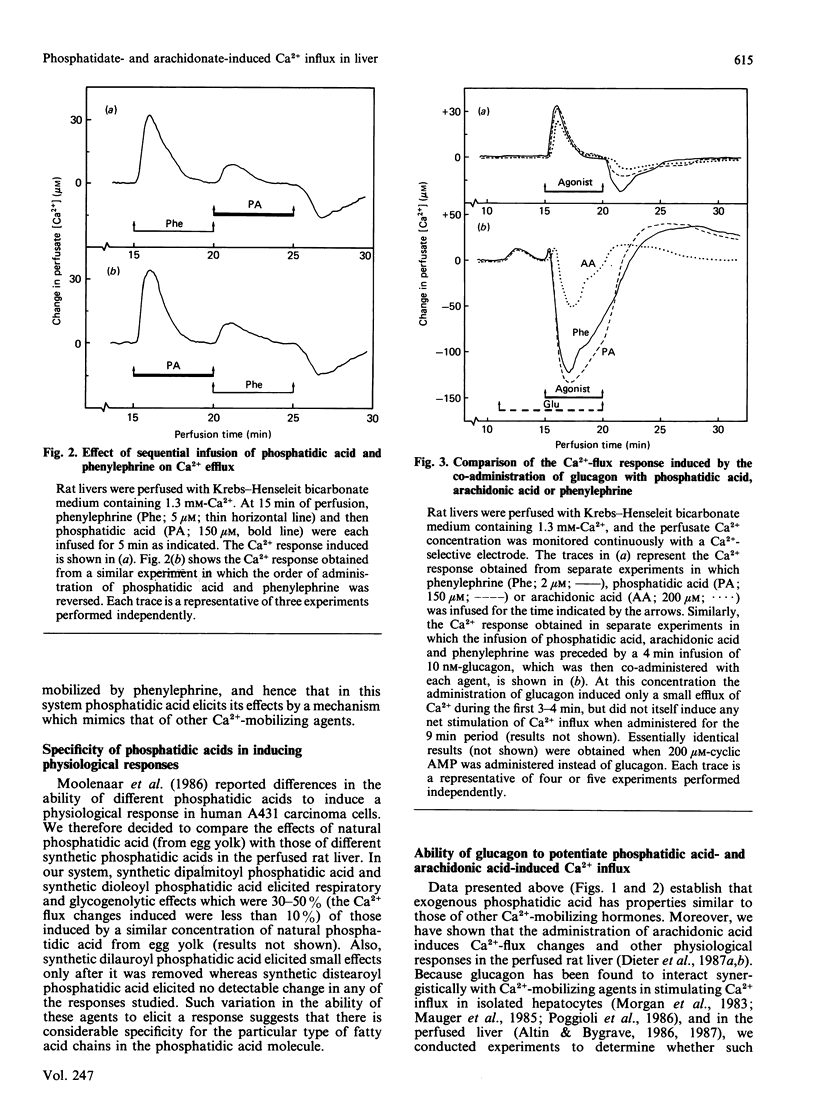
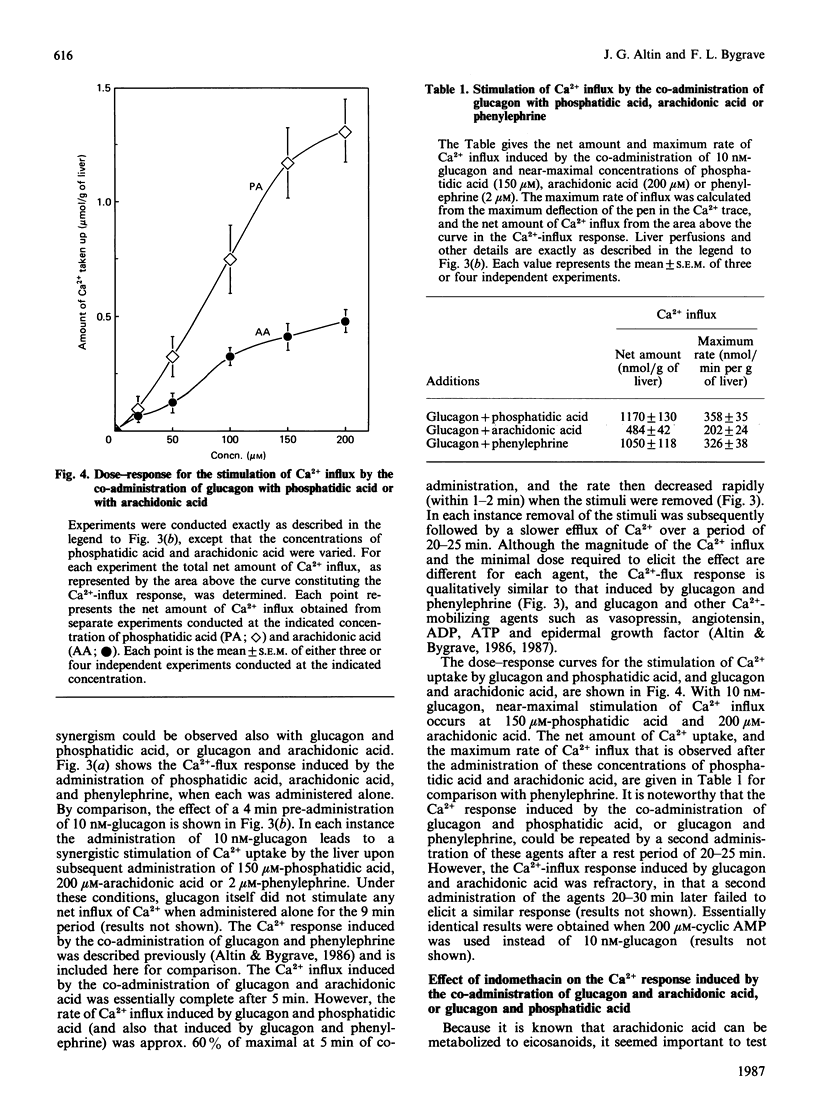
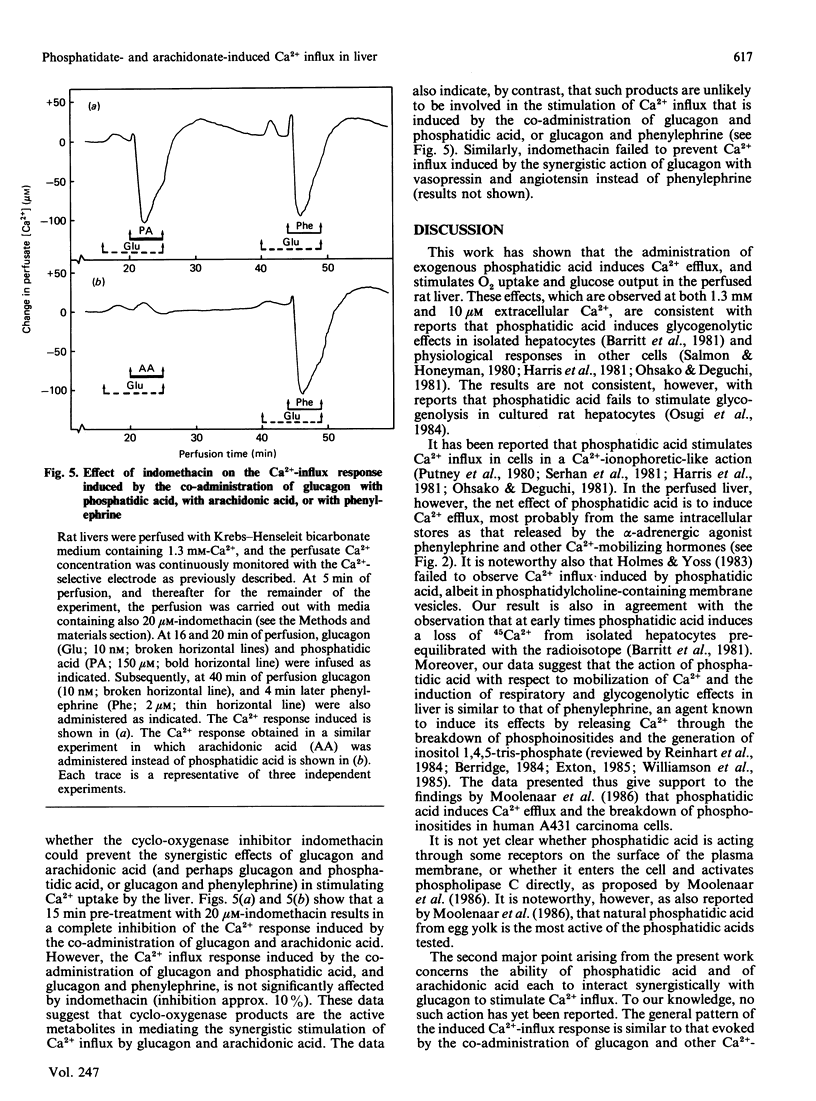
The administration of phosphatidic acid to rat livers perfused with media containing either 1.3 mM- or 10 microM-Ca2+ was followed by a stimulation of Ca2+ efflux, O2 uptake and glucose output. The responses elicited by 100 microM-phosphatidic acid were similar to those induced by the alpha-adrenergic agonist phenylephrine. Contrary to suggestions that phosphatidic acid acts like a Ca2+-ionophore, no net influx of Ca2+ was detected until the phosphatidic acid was removed. Sequential infusions of phenylephrine and phosphatidic acid indicate that the two agents release Ca2+ from the same intracellular source. The co-administration of glucagon (or cyclic AMP) and phosphatidic acid, and also of glucagon and arachidonic acid, led to a synergistic stimulation of Ca2+ uptake of the liver, a feature similar to that observed after the co-administration of glucagon and other Ca2+-mobilizing hormones [Altin & Bygrave (1986) Biochem. J. 238, 653-661]. A notable difference, however, is that the synergistic stimulation of Ca2+ uptake induced by the co-administration of glucagon and arachidonic acid was inhibited by indomethacin, whereas that induced by glucagon and phosphatidic acid, or glucagon and other Ca2+-mobilizing agents, was not. The results suggest that the synergistic action of glucagon and arachidonic acid in stimulating Ca2+ influx is mediated by prostanoids, but that of glucagon and phosphatidic acid is evoked by a mechanism similar to that of Ca2+-mobilizing agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The influx of Ca2+ induced by the administration of glucagon and Ca2+-mobilizing agents to the perfused rat liver could involve at least two separate pathways. Biochem J. 1987 Feb 15;242(1):43–50. doi: 10.1042/bj2420043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Dieter P., Bygrave F. L. Evidence that Ca2+ fluxes and respiratory, glycogenolytic and vasoconstrictive effects induced by the action of platelet-activating factor and L-alpha-lysophosphatidylcholine in the perfused rat liver are mediated by products of the cyclo-oxygenase pathway. Biochem J. 1987 Jul 1;245(1):145–150. doi: 10.1042/bj2450145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt G. J., Dalton K. A., Whiting J. A. Evidence that phosphatidic acid stimulates the uptake of calcium by liver cells but not calcium release from mitochondria. FEBS Lett. 1981 Mar 23;125(2):137–140. doi: 10.1016/0014-5793(81)80703-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K. Eicosanoids, signal molecules of liver cells. Semin Liver Dis. 1985 May;5(2):175–190. doi: 10.1055/s-2008-1063921. [DOI] [PubMed] [Google Scholar]
- Dieter P., Altin J. G., Bygrave F. L. Possible involvement of prostaglandins in vasoconstriction induced by zymosan and arachidonic acid in the perfused rat liver. FEBS Lett. 1987 Mar 9;213(1):174–178. doi: 10.1016/0014-5793(87)81486-2. [DOI] [PubMed] [Google Scholar]
- Dieter P., Altin J. G., Decker K., Bygrave F. L. Possible involvement of eicosanoids in the zymosan and arachidonic-acid-induced oxygen uptake, glycogenolysis and Ca2+ mobilization in the perfused rat liver. Eur J Biochem. 1987 Jun 1;165(2):455–460. doi: 10.1111/j.1432-1033.1987.tb11460.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P., Putney J. W., Jr Receptor-mediated metabolism of the phosphoinositides and phosphatidic acid in rat lacrimal acinar cells. Biochem J. 1984 Feb 15;218(1):187–195. doi: 10.1042/bj2180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Schmidt J., Hitzemann B. A., Hitzemann R. J. Phosphatidate as a molecular link between depolarization and neurotransmitter release in the brain. Science. 1981 Jun 12;212(4500):1290–1291. doi: 10.1126/science.7233220. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Yoss N. L. Failure of phosphatidic acid to translocate Ca2+ across phosphatidylcholine membranes. Nature. 1983 Oct 13;305(5935):637–638. doi: 10.1038/305637a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Metz S. A., Draznin B., Sussman K. E., Leitner J. W. Unmasking of arachidonate-induced insulin release by removal of extracellular calcium. Arachidonic acid mobilizes cellular calcium in rat islets of Langerhans. Biochem Biophys Res Commun. 1987 Jan 15;142(1):251–258. doi: 10.1016/0006-291x(87)90478-5. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Modulation of the alpha 1-adrenergic control of hepatocyte calcium redistribution by increases in cyclic AMP. J Biol Chem. 1983 Apr 25;258(8):5110–5116. [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Stimulation of phosphatidic acid of calcium influx and cyclic GMP synthesis in neuroblastoma cells. J Biol Chem. 1981 Nov 10;256(21):10945–10948. [PubMed] [Google Scholar]
- Osugi T., Uchida S., Watanabe Y., Yoshida H. Differences in Ca2+ mobilization induced by alpha-adrenergic agonist and phosphatidic acid in cultured hepatocytes. Life Sci. 1984 Jul 30;35(5):469–475. doi: 10.1016/0024-3205(84)90239-x. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982 Feb 25;257(4):1906–1912. [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Seyfred M. A., Wells W. W. Subcellular site and mechanism of vasopressin-stimulated hydrolysis of phosphoinositides in rat hepatocytes. J Biol Chem. 1984 Jun 25;259(12):7666–7672. [PubMed] [Google Scholar]
- Tyson C. A., Vande Zande H., Green D. E. Phospholipids as ionophores. J Biol Chem. 1976 Mar 10;251(5):1326–1332. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]


