Abstract
Objectives
Novel histopathologic prognostic factors are needed to identify patients with follicular lymphoma (FL) at risk of inferior outcomes. Our primary objective was to evaluate the Ki-67 proliferative index in follicular and interfollicular areas in tissue biopsy specimens from patients with newly diagnosed FL and correlate with clinical outcomes. Our secondary objective was to correlate PD-L1 and LAG-3 with clinical outcomes.
Methods
Seventy cases of low-grade FL from the University of Minnesota were evaluated with Ki-67 immunohistochemical stain. Ki-67 expression as a continuous variable was interpreted digitally and manually in follicular and interfollicular areas. Progression-free survival (PFS) and overall survival (OS) were analyzed by Cox regression, and hazard ratios (HRs) per 10-point increase in Ki-67 were calculated.
Results
Progression-free survival at 4 years was 28% (95% CI, 19%-41%). Interfollicular, but not follicular, Ki-67 was associated with PFS by manual (HR, 1.33; P = .01) and digital (HR, 1.38; P = .02) analysis. Digital and manual Ki-67 were only moderately correlated but demonstrated similar effects on PFS. At 4 years, OS was 90% with no association with follicular or interfollicular Ki-67 proliferation.
Conclusions
Higher interfollicular Ki-67 by either digital or manual analysis is associated with a poorer PFS in patients with low-grade FL. These results suggest further validation of this marker is warranted to improve pathologic risk stratification at FL diagnosis. PD-L1 and LAG-3 were not associated with PFS or OS.
Keywords: follicular lymphoma, Ki-67, proliferative index, immunohistochemistry, LAG-3, PD-L1, prognosis
Key points.
Ki-67 proliferative index may be similar or markedly different in the follicular and interfollicular areas in follicular lymphoma (FL) biopsy specimens.
Higher interfollicular Ki-67 is associated with a poorer progression-free survival in patients with low-grade FL.
Both manual and digital methods of Ki-67 proliferative index assessment are predictive, so pathologists can choose the method that works best with their workflow.
INTRODUCTION
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma in the Western hemisphere.1 Clinical, laboratory, and pathologic features guide the decision of whether and when to start treatment, and they guide the choice of therapy. Typical FL is composed of neoplastic follicles with a mixture of centrocytes and centroblasts. The number of centroblasts per high-power field (HPF) is used for grading FL, with grades 1, 2, and 3A being treated similarly and grade 3B being treated more like diffuse large B-cell lymphoma. In addition to pathologic grade, the Follicular Lymphoma International Prognostic Index (FLIPI) is calculated for patients—it uses age, laboratory testing, and extent of involvement to risk stratify patients into low (score 0-1), intermediate (score 2), and high (score 3-5) risk.
Despite the current tools available to guide therapy, some low-risk patients have a more aggressive course; therefore, additional prognostic factors are needed to identify patients who might benefit from upfront therapy. The proliferation rate by a Ki-67 immunohistochemical stain has been suggested as one such factor; however, the association of a Ki-67 proliferative index (PI) with recurrence, progression, and/or survival is conflicting in the published literature.2-6 Most studies have assessed the PI only within follicles; however, in our observation, significant differences in the PI in follicular areas and interfollicular areas are common. Only a single study has assessed the follicular and interfollicular areas; however, this study was based on manual assessment and only looked at patients with FL who received first-line treatment.6 Approximately half of the prior studies used digital imaging analysis, while the other half used manual counting; none of the studies compared the two. Manual counting or estimates can be subjective, time-consuming, and/or dependent on the observer’s level of expertise but are commonly used since digital analysis is not available at all pathology practices.
While Ki-67 is a marker of cell proliferation, LAG-3 and PD-L1 are checkpoint inhibitors that are a surrogate marker of the tumor environment, T-cell exhaustion, and immunotherapy response.7-9 The significance of PD-1 and its ligands PD-L1 and PD-L2 in FL is controversial, and response to checkpoint inhibitor therapy is still under investigation, with early studies suggesting some response.7-12 LAG-3 (CD223) is also described as a marker of T-cell exhaustion in lymphoma, and rare studies suggest that PD-1 and LAG-3 blockade reverse this state.7,13 Yang et al7 suggest that LAG-3 is a better predictor of reduced T-cell function and the exhausted state than PD-1 and is a predictor of poor outcome, although it is also associated with grade. LAG-3 has been mostly assessed in the setting of diffuse large B-cell lymphoma, but a study with 27 FL cases showed negativity for LAG-3 on the lymphoma cells but some staining on background immune cells.13
In this study, our primary objective was to assess whether the Ki-67 PI of FL samples, as measured in both inter- and intrafollicular areas, is associated with clinical outcome. Our secondary objective was to assess whether Ki-67 PI scoring could be improved with digital image analysis. Finally, we also examined markers of T-cell exhaustion (PD-L1 and LAG-3) by immunohistochemistry (IHC) to see if they could provide additional prognostic information.
METHODS
After institutional review board approval from the University of Minnesota, we performed a retrospective analysis of all patients with newly diagnosed FL evaluated at the University of Minnesota Medical Center, Fairview from January 2003 to April 2016. Cases were excluded if they lacked follow-up, lacked material for performing immunohistochemical stains, or were grade 3B at diagnosis. All remaining cases underwent morphologic examination, medical record review, and Ki-67, PD-L1, and LAG-3 immunohistochemical staining as tissue allowed.
Immunohistochemical Stains
For antigen retrieval, slides were incubated in 6.0 pH buffer at 95°C to 98°C for 39 minutes. Subsequent steps were automated on the Nemesis platform (Biocare Medical). Endogenous peroxidase activity was quenched by slide immersion in 3% hydrogen peroxide solution for 10 minutes. A serum-free blocking solution (Background Sniper; Biocare Medical) was placed on sections for 10 minutes, and then slides were incubated for 60 minutes at room temperature in primary antibody: PD-L1 (CAL10, rabbit monoclonal, 1:100; Biocare Medical), Ki-67 (SP6, rabbit monoclonal, 1:400; ThermoScientific), or LAG-3 (D2G40, rabbit monoclonal, 1:100; Cell Signaling). Detection was performed with the Novocastra Novolink Polymer Kit (Leica Microsystems). Slides then were incubated for 5 minutes with diaminobenzidine (Biolegend) and counterstained for 5 minutes with hematoxylin (Biocare Medical).
Manual Assessment of Immunohistochemistry
All cases were reviewed by two pathologists: Ki-67 (S.Y. and A.N.), and PD-L1 and LAG-3 (M.A.L. and A.N.). For Ki-67 scoring, 10 consecutive HPFs (40) in follicular and interfollicular areas with the highest staining were separately counted and averaged; if there was no appreciable difference, the same percentage was used for both. For PD-L1 and LAG-3 scoring, 10 random follicular area HPFs (50, oil immersion lens) with maximum staining were counted and averaged. As less than 1% of lymphocytes stained with PD-L1 in all cases but 1, PD-L1 staining in tissue histiocytes was graded as negative, weak only, or positive.
Digital Analysis of Ki-67
Ki-67–stained slides were scanned in the Aperio E slide manager and analyzed using Aperio image analysis Nuclear Version 9 basic algorithm (Leica Biosystems Imaging). Each case was annotated, and analysis was performed in best representative areas. In cases with sufficient tissue, an area of analysis of 1.50 +/– 0.3 mm2 was set, while for cases with small tissue size, such as biopsy specimens, the maximum available area was analyzed. For cases with well-defined follicular and interfollicular areas and sufficient tissue, an area of analysis of 1.50 +/– 0.3 mm2 was performed for both. A total of 61 cases were available for digital analysis, with only 59 having both interfollicular and follicular data; 35 of the cases had a different Ki-67 percentage between follicular and interfollicular areas. Intensity of positive nuclear staining was quantified as 0, 1+, 2+, and 3+, where 0 signifies no staining and 1+, 2+, and 3+ signify weak, moderate, and strong positive staining, respectively. The analysis generated the following data for each annotated area: total number and percentage of 3+, 2+, 1+, and 0+ nuclei; average percentage and number of positive nuclei; and area of analysis.
Statistical Analysis
Three time-to-event outcomes were analyzed using Cox regression: (1) overall survival (OS) from date of diagnosis; (2) progression-free survival (PFS), defined as time from diagnosis to death or initiation of first treatment; and (3) time from first to second treatment (excluding patients who never received a first treatment). Patients who did not have the event of interest were censored at the time of last known contact. Four independent variables were studied individually with each outcome: PD-L1 and LAG-3 were modeled as categorical predictors while follicular and interfollicular Ki-67 PI were modeled as continuous predictors. Restricted cubic spline functions were used to assess nonlinear effects; however, nonlinear effects were negligible and are not reported to improve interpretability of effect sizes. A sensitivity analysis for the association of Ki-67 with PFS was performed, which was adjusted for FLIPI. The number of events for each outcome precluded additional multivariable analyses. Statistical hypothesis tests were not adjusted for multiple testing, but all tests were prespecified and all are reported. Time-to-event estimates were calculated using the Kaplan-Meier method with log-transformed confidence intervals. Statistical analysis was performed using R version 3.4 (R Foundation for Statistical Computing).
RESULTS
We identified 137 cases of FL; however, after applying exclusion criteria, 70 cases were available for Ki-67 staining and interpretation, and 65 cases were available for PD-L1 and LAG-3 staining and interpretation Figure 1. Biopsy types included 52 incisional/excisional biopsy specimens, 6 small gastrointestinal (GI) or conjunctival biopsy specimens, and 12 core needle biopsy specimens; however, small biopsy types were more likely to be excluded due to insufficient tissue. Additionally, needle core biopsy specimens were more likely to be abdominal/retroperitoneal/pelvic (42%, n = 5 of 9) compared with incisional/excisional biopsy specimens (23%, n = 12 of 52). The study cohort had a median age of 59 years (interquartile range [IQR], 51-66 years; range, 29-89 years), 91% had low-grade lymphoma, and 45% were stage 4. B symptoms and elevated lactate dehydrogenase levels were noted in only 6 patients, all of whom were grades 1 to 2. Additional patient characteristics are described in Table 1.
FIGURE 1.
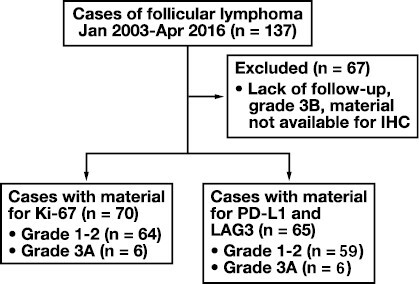
Study design. Out of 137 cases of follicular lymphoma, 67 met the exclusion criteria, leaving 70 available for immunohistochemistry. All 70 were stained for Ki-67; however, only 65 had sufficient material for PD-L1 and LAG-3 staining.
TABLE 1.
Patient Characteristics
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Female | 35 (50.0) |
| Male | 35 (50.0) |
| Grade | |
| 1 and 2 | 64 (91.4) |
| 3A | 6 (8.6) |
| Diagnosis | |
| 2003-2007 (pre-R era) | 11 (16.0) |
| 2008-2013 (post-R era) | 59 (84.0) |
| Stage | |
| I | 13 (18.6) |
| II | 13 (18.6) |
| III | 9 (12.9) |
| IV | 31 (44.3) |
| Unknown | 4 (5.7) |
| FLIPI risk | |
| Low (0-1) | 25 (35.7) |
| Intermediate (2) | 21 (30.0) |
| High (3-5) | 18 (25.7) |
| Unknown | 6 (8.6) |
| Symptoms | |
| B symptoms | 6 (8.6) |
FLIPI, Follicular Lymphoma International Prognostic Index; R, rituximab.
Nine patients underwent transformation (6 diffuse large B-cell lymphoma, 1 blastoid B-cell neoplasm, and 2 unknown), and 14 died during follow-up, 7 due to complications of FL Table 2. Four-year OS for all patients was 90% (95% CI, 82%-98%). There was no significant association of interfollicular or follicular Ki-67, LAG-3, or PD-L1 with OS (P = .56, .61, .38, and .38 respectively); however, because of the low number of deaths, the power to detect differences was limited.
TABLE 2.
Patient Treatment and Outcomes
| Characteristic | No. (%) |
|---|---|
| Treatment | |
| Observation onlya | 22 (32.9) |
| Observation followed by treatment | 7 (10.0) |
| Surgery only | 1 (1.4) |
| Radiation | 5 (7.1) |
| Low-intensity chemotherapy | 18 (25.7) |
| Aggressive chemotherapy | 16 (23.0) |
| Unknown | 1 (1.4) |
| Outcome | |
| Transformation | 9 (12.9) |
| DLBCL | 6 (8.6) |
| Blastoid B-cell neoplasm | 1 (1.4) |
| Unknown | 2 (2.8) |
| Death | 14 (20.0) |
| 4-year OS | 63 (90.0) |
DLBCL, diffuse large B-cell lymphoma; OS, overall survival.
aIncludes 1 patient treated for hepatitis C.
There was at least an absolute 10% difference in Ki-67 staining between follicular and interfollicular areas in 43 (61%) samples based on manual assessment Figure 2. The median follicular Ki-67 PI was 25 (IQR, 15-40; range, 1-80), and the median interfollicular area (IFA) Ki-67 PI was 10 (IQR, 5-15; range, 0-80). There was little to no association between Ki-67 PI and FLIPI score, presence of B symptoms, LAG-3, or PD-L1. Both Ki-67 measures were higher among stage III patients (n = 9) but similar among stages I, II, and IV; however, the number of patients in these subsets is small. Among surviving patients (n = 56), the median follow-up time was 48 months (IQR, 24-61; range, 1-140).
FIGURE 2.
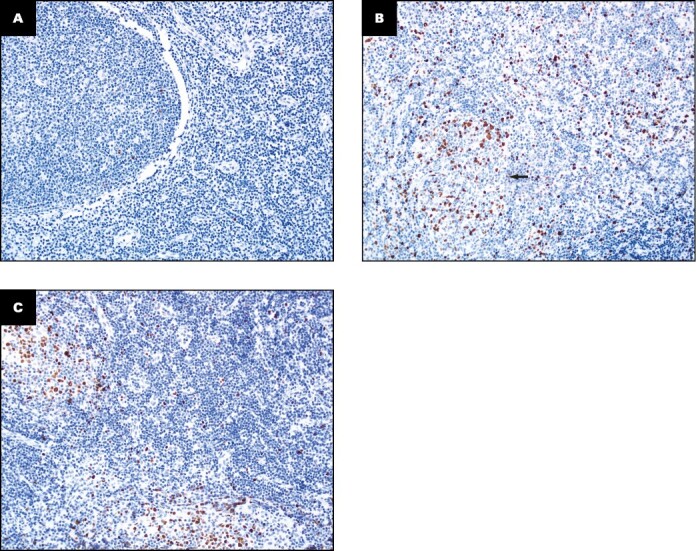
Examples of Ki-67 staining in follicular lymphoma cases (x20). A, Homogeneously low Ki-67 in follicular (3%) and interfollicular areas (1%). B, Homogeneously high Ki-67 in follicular and interfollicular areas (30%); arrow denotes follicle. C, Significantly higher Ki-67 in the follicular areas (32%) compared to the interfollicular areas (7%). A and B have the same Ki-67 score for both areas, whereas C does not.
Thirty (43%) patients were initially observed or only treated for concurrent hepatitis (n = 2) or by surgery (n = 1), and 7 went on to require therapy, with 3 requiring additional treatment. Five (7%) patients were initially treated with radiation therapy and another patient was referred for this therapy but declined treatment (included in the observation group). Treatment data were missing about the initial approach for 1 patient. Aggressive chemotherapy (R-CVP [rituximab, cyclophosphamide, vincristine, prednisolone], R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone], R-HyperCVAD [rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone]) was used upfront for 16 (23%) patients, of whom 10 were stage IV. The remaining 18 (26%) patients had less intensive therapy at diagnosis. There were 46 patients who were given a first treatment at some point, and 57% (95% CI, 43%-75%) were alive without having a second treatment within 4 years. This percentage was not associated with follicular Ki-67 (P = .60), IFA Ki-67 (P = .82), LAG-3 (P = .29), or PD-LI (P = .66).
Four-year PFS for all patients was 28% (95% CI, 19%-41%). Most events occurred within the first year, and median PFS was 2 months. The manual interfollicular Ki-67 PI was associated with PFS, and the hazard ratio (HR) associated with a 10-point increase in interfollicular area Ki-67 PI was 1.33 (95% CI, 1.08-1.64; P = .01) and 1.32 adjusted for FLIPI Table 3, Figure 3A-3B. This translates to a predicted 4-year PFS of 34% for an IFA Ki-67 of 5 (the 25th percentile) decreasing to 24% predicted 4-year PFS for an interfollicular Ki-67 of 15 (the 75th percentile). However, a similar effect was not seen with the follicular Ki-67 PI and PFS (P = .35).
TABLE 3.
Hazard Ratios for Overall Survival and Progression-Free Survival
| Hazard ratio (95% CI; P value) for 10-point increase in Ki-67 PI | ||
|---|---|---|
| Characteristic | Manual review (n = 70) | Digital analysis (n = 62) |
| Overall survival | ||
| Follicular area Ki-67 | 0.93 (.71-1.21; .58) | 0.79 (.47-1.34; .39) |
| Interfollicular area Ki-67 | 0.85 (.46-1.59; .62) | 1.08 (.59-1.99; .80) |
| Progression-free survival | ||
| Follicular area Ki-67 | 0.94 (.82-1.07; .35) | 1.10 (.87-1.40; .42) |
| Interfollicular area Ki-67 | 1.33 (1.08-1.64; .01) | 1.38 (1.06-1.80; .02) |
PI, proliferative index.
FIGURE 3.
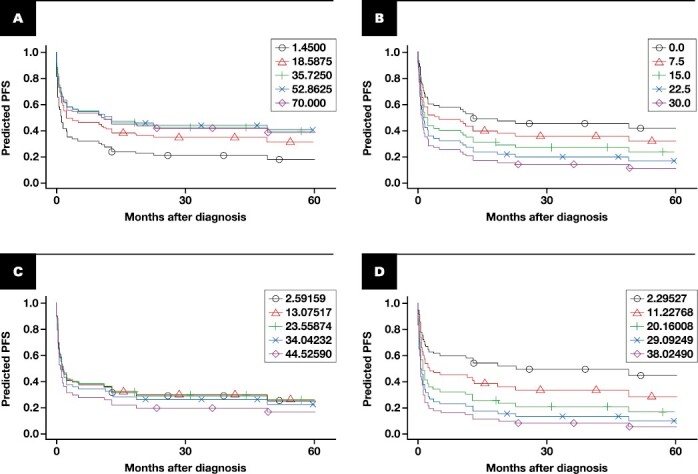
Progression-free survival (PFS) by manual follicular (A) and interfollicular (B) Ki-67 percentage and by digital follicular (C) and interfollicular (D) Ki-67 percentage. These are predicted survival curves from univariable Cox models, one for each of the 4 variables modeled as continuous, linear effects. The values within each plot correspond to the 5th, 25th, 50th, 75th, and 95th percentiles of each variable’s distribution within our cohort.
Digital Ki-67 showed similar results and was measured for 61 cases, 59 of which had separate follicular and interfollicular PI values and 35 of which had different follicular and interfollicular results. Due to limited tissue in some cases, the overall average area of analysis was 1.38 for follicular and 1.45 for interfollicular areas. The digital PI values were only moderately correlated with the respective manual PI (Spearman correlation of 0.55 for follicular and 0.44 for interfollicular); however, despite this moderate correlation, associations with OS and PFS were similar Table 3, Figure 3C-3D. For OS, the HR associated with a 10-unit increase of digital follicular Ki-67 was 0.79 (95% CI, .47-1.34; P = .39) and 1.08 for digital interfollicular Ki-67 (95% CI, .59-1.99; P = .80). For PFS, the HR associated with a 10-unit increase of digital follicular Ki-67 was 1.10 (95% CI, .87-1.40; P = .42) and 1.38 for digital interfollicular Ki-67 (95% CI, 1.06-1.80; P = .02).
All cases showed fewer PD-L1–positive cells than the control tonsil germinal centers. Control tonsil showed PD-L1–positive histiocytes and lymphocytes within germinal centers (based on morphology) and appeared polarized, with higher positivity in the light zones. In contrast, FL cases had minimal positivity in tumor cells (<1% in all cases); however, histiocytes were usually positive (59 of 65 cases) with variable staining intensity. There was no significant association between PFS or OS and PD-L1.
LAG-3 staining varied from less than 1% to 15%; however, most cases (93%) were 5% or less, and only 8 (7%) cases were more than 5%. Control tonsil showed rare scattered cells positive for LAG-3 with focal collections in paracortical areas. There was no significant association between PFS or OS and LAG-3.
DISCUSSION
In our study, there was a statistically significant association of interfollicular Ki-67 PI and PFS by both digital and manual despite only moderate correlation between digital analysis and manual counting. There was not a significant association with interfollicular Ki-67 PI and OS or follicular Ki-67 PI with either PFS or OS.
The interest in using Ki-67 PI as a prognostic marker in FL goes back at least 2 decades when Martin et al14 evaluated 106 aggressively treated FL cases and found no effect of Ki-67 PI on multivariate analysis. Studies since have tried to refine this biomarker to improve prognostication of low-grade FL with mixed results. Table 4 summarizes the literature and the current study. In 2005, Wang et al2 detected longer disease-specific survival with low PI (<30%, P = .01) with borderline significantly longer OS (P = .05). In 2007, Koster and colleagues3 showed that the PI predicted longer PFS and OS as a continuous variable. The median PI in grade 1 and 2 FL (n = 45) was 16.1, compared with 24.2 in grade 3 (n = 6; P = .02). Kedmi et al4 in 2014 found longer time to first treatment with low PI (<30%, P = .004, 19 vs 62 months) but no difference in OS. In 2020, Sohani et al5 demonstrated inferior PFS with high PI (≥30%; HR, 2.47; 95% CI, 1.33-4.61) but no change in OS. However, a study by Klapper et al6 in 2007 showed no association with time to treatment failure or OS. Our results support a correlation with Ki-67 PI and PFS; however, instead of using a strict cutoff, our study demonstrates a linear effect with worse PFS the higher the PI, similar to the study by Koster et al.3
TABLE 4.
Literature Summary of Ki-67 in Follicular Lymphoma
| Study | Year | Image analysis | Ki-67 clone, manufacturer | Rituximab era | Location | No. | Cutoff, % | Results |
|---|---|---|---|---|---|---|---|---|
| Martin et al14 | 1995 | Cell Analysis Systems 200 | A047, Dako | Pre | Follicular | 106 | <40 | No effect on PFS or OS in multivariate analysis |
| Wang et al2 | 2005 | None | Unk, Zymed | Pre and post | Unka | 142 | <30 | Longer disease-specific survival (P = .01) Borderline longer OS (P = .05) |
| Koster et al3 | 2007 | None | MIB1, Dako | Pre | Follicular | 51 | Continuous above/below median | Longer PFS (P = .016) and borderline OS (P = .053) Longer PFS (P = .006) and OS (P = .002) |
| Klapper et al6 | 2007 | None | Unk, Zymed | Pre | Follicular, interfollicular | 158 | Continuous | No effect on time to treatment failuresb No effect on OSb |
| Kedmi et al4 | 2014 | ScanScope Xt, Aperio | MIB1, Immunotech | Pre and post | Follicular | 129 | <30 | Longer time to first treatment (19 vs 62 mo, P = .004) No effect on OS |
| Sohani et al5 | 2021 | Definiens software | MIB1, Unk | Post | Follicular | 154 | >30 | Inferior PFS at 24 mo (HR, 2.47; 95% CI, 1.33-4.61) |
| Current study | 2023 | Aperio | SP6, Thermo Scientific | Post | Follicular, interfollicular | 70 | Continuous | Inferior PFS with higher interfollicular PI (P = .02) No effect OS (P = .42) or follicular PI OS (P = .39) or PFS (0.80) |
HR, hazard ratio; OS, overall survival; PI, proliferative index; PFS, progression-free survival; unk, unknown.
aFollicular may be inferred from figures.
bNodal advanced stage treated first line within a randomized trial.
Our study found significance only for interfollicular Ki-67 PI. Koster et al3 restricted the evaluation of Ki-67 PI to the follicular areas because of a concern for interference from T cells in the interfollicular areas, and most studies since have followed their lead. However, T cells are not restricted to interfollicular areas, and some cases of FL have a high proportion of T cells in follicles. Furthermore, neoplastic B cells can spread to the interfollicular areas, and 2 studies have shown inferior PFS or time to treatment failure with the amount of neoplastic B cells (as demonstrated by CD10 and/or BCL6) in the interfollicular compartment, suggesting interfollicular PI might be important despite the potential effect of admixed T cells.5,6 It may be of interest in future studies to do dual staining with Ki-67 and B-cell and T-cell markers to determine to what extent T-cell staining contributes to the Ki-67 PI within and outside of follicles. Only the Klapper et al6 study looked at interfollicular PI, and they found no association with time to treatment failure or OS for either inter- or intrafollicular PI. The Klapper et al6 study included a cohort who universally received treatment (except for a single patient who refused), which may explain why the results of this study were negative compared to other studies. The Kedmi et al4 study only showed a prognostic significance for patients who were not treated at diagnosis, and our study as well as others have shown effects on PFS, which includes time to first treatment.3-5 Therefore, it is possible that PI may be most predictive in patients who do not require or receive upfront therapy, explaining the negative results in the Klapper et al6 study. Preanalytic and analytic factors could also play a role in the differing results by study.15 Immunohistochemical clones often have different performance and IHC is affected by preanalytic factors such as cold ischemia time, fixative, time to fixation, and both tissue and IHC processing; 4 different IHC clones were used among the 6 published (and the current) studies Table 4. The literature is inconsistent in reporting the Ki-67 clone much less other analytic and preanalytic factors, so it is possible that our unique process accounts for differences from other studies. Future studies should document and report analytic and preanalytic factors as able. In addition, subset analysis of patients with FL who do not need upfront therapy may be useful in future studies.
Studies assessing Ki-67 PI in FL have been a mix of manual assessment and digital imaging. Our study showed that although manual assessment of Ki-67 was not well correlated with digital assessment, either method of Ki-67 assessment showed a correlation with PFS. Therefore, our results suggest that either method may be used for prognostic purposes depending on availability and workflow considerations.
Possible reasons for discrepancies between methods include different areas used for analysis, different interpretation of faint staining, and observer variability. Kedmi et al4 noted the high variability of PI within a single case between follicles, with some follicles well above and some follicles well below the study cutoff of 30%, demonstrating that sampling and selection of blocks and areas to determine the PI may affect results.4 It is possible this biologic variability, sampling, and intraobserver variability would have more effect when using a strict cutoff rather than a continuous variable as we did in this study.
PD-L1 and LAG-3 do not show association with OS or PFS in our cohort of patients. In addition to the above findings, there was no significant correlation of Ki-67, PD-L1, and LAG-3 with each other. The expression of PD-1 or PD-L1 is complex and not consistent in the literature.16 A study published in 2017 looked at PD-1 and LAG-3 expression through flow cytometry, mass spectrometry, and cytotoxic assay.10 They found that intratumoral PD-1+LAG-3+ T cells exhibited reduced function in vitro but PD-1+LAG-3– T cells were immunologically functional.10 Blockade of both PD-1 and LAG-3 enhanced the function of intratumoral CD8+ T cells. In their study, LA-3 expression of intratumoral T cells correlated with a poor outcome in patients with FL.10 Similar to other studies, we found minimal to absent PD-L1 staining of tumor cells or T cells in FL; however, we did note staining in macrophages.13,17 This staining was variable and not associated with outcomes. We did not find any association of LAG-3 expression with outcomes, although the number of cases with more than 5% LAG-3 staining was small.
Our data suggest that interfollicular area Ki-67 score, by either manual estimation or digital counting, at the time of initial diagnosis is prognostic to predict PFS in patients with FL, and the relationship appears to be linear and does not change when adjusting for FLIPI score. Ki-67 score in the follicular area was not significantly associated with outcomes in our study. We propose that the interfollicular Ki-67 PI be taken into account when assessing FL by Ki-67 and that future studies should assess both inter- and intrafollicular Ki-67 PI. Additionally, it may be worth a subset analysis of patients not receiving first-line therapy. Both manual and digital methods of Ki-67 PI assessment are predictive, so pathologists can choose the method that works best with their workflow.
Acknowledgments
We thank Dina El-Rayes, MD (study coordinator), Dave Ankarlo, MS (study coordinator), and Brian Dunnette (Digital Imaging & IT support) for their help and time with this project. This research received immunohistochemical staining assistance from the University of Minnesota’s Biorepository and Laboratory Services program and was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Contributor Information
Aqsa Nasir, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, US.
Livia Hegerova, Fred Hutchinson Cancer Center, University of Washington Department of Medicine, Division of Hematology, Seattle, WA, US.
Hira Yousaf, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, US.
Colleen L Forster, BioNet, Biorepository and Laboratory Services, University of Minnesota, Minneapolis, MN, US.
Ryan Shanley, Masonic Cancer Center Biostatistic Core, Department of Biostatistics, University of Minnesota, Minneapolis, MN, US.
Michael A Linden, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, US.
Veronika Bachanova, Department of Medicine, University of Minnesota, Minneapolis, MN, US.
Sophia Yohe, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, US.
Conflict of interest disclosure
The authors have nothing to disclose.
REFERENCES
- 1. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063. 10.1182/blood-2015-11-624288 [DOI] [PubMed] [Google Scholar]
- 2. Wang SA, Wang L, Hochberg EP, Muzikansky A, Harris NL, Hasserjian RP. Low histologic grade follicular lymphoma with high proliferation index: morphologic and clinical features. Am J Surg Pathol. 2005;29(11):1490-1496. 10.1097/01.pas.0000172191.87176.3b [DOI] [PubMed] [Google Scholar]
- 3. Koster A, Tromp HA, Raemaekers JM, et al. The prognostic significance of the intra-follicular tumor cell proliferative rate in follicular lymphoma. Haematologica. 2007;92(2):184-190. 10.3324/haematol.10384 [DOI] [PubMed] [Google Scholar]
- 4. Kedmi M, Hedvat CV, Maragulia J, Zhang Z, Zelenetz AD. Association of quantitative assessment of the intrafollicular proliferation index with outcome in follicular lymphoma. Br J Haematol. 2014;164(5):646-652. 10.1111/bjh.12667 [DOI] [PubMed] [Google Scholar]
- 5. Sohani AR, Maurer MJ, Giri S, et al. Biomarkers for risk stratification in patients with previously untreated follicular lymphoma receiving anti-CD20-based biological therapy. Am J Surg Pathol. 2021;45(3):384-393. 10.1097/PAS.0000000000001609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klapper W, Hoster E, Rölver L, et al. ; German Low Grade Lymphoma Study Group. Tumor sclerosis but not cell proliferation or malignancy grade is a prognostic marker in advanced-stage follicular lymphoma: the German Low Grade Lymphoma Study Group. J Clin Oncol. 2007;25(22):3330-3336. 10.1200/JCO.2006.10.5833 [DOI] [PubMed] [Google Scholar]
- 7. Yang ZZ, Kim HJ, Villasboas JC, et al. Expression of LAG-3 defines exhaustion of intratumoral PD-1. Oncotarget. 2017;8(37):61425-61439. 10.18632/oncotarget.18251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sutamtewagul G, Link BK. Novel treatment approaches and future perspectives in follicular lymphoma. Ther Adv Hematol. 2019;10:2040620718820510. 10.1177/2040620718820510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704. 10.1200/JCO.2015.65.9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang ZZ, Grote DM, Ziesmer SC, Xiu B, Novak AJ, Ansell SM. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5(2):e281. 10.1038/bcj.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470-1476. 10.1200/JCO.2008.18.0513 [DOI] [PubMed] [Google Scholar]
- 12. Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol. 2011;42(4):552-557. 10.1016/j.humpath.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 13. Laurent C, Charmpi K, Gravelle P, et al. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4(8):e1026530. 10.1080/2162402X.2015.1026530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin AR, Weisenburger DD, Chan WC, et al. Prognostic value of cellular proliferation and histologic grade in follicular lymphoma. Blood. 1995;85(12):3671-3678. [PubMed] [Google Scholar]
- 15. Compton CC, Robb JA, Anderson MW, et al. Preanalytics and precision pathology: pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch Pathol Lab Med. 2019;143(11):1346-1363. 10.5858/arpa.2019-0009-SA [DOI] [PubMed] [Google Scholar]
- 16. Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68-83. 10.1182/blood-2017-07-740993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horlad H, Ma C, Yano H, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016;107(11):1696-1704. 10.1111/cas.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]


