Abstract
Recent technological advances have improved the sensitivity and specificity of blood-based biomarkers for Alzheimer’s disease and related dementias. Accurate quantification of amyloid-ß peptide, phosphorylated tau (pTau) isoforms, as well as markers of neurodegeneration (neurofilament light chain [NfL]) and neuro-immune activation (glial fibrillary acidic protein [GFAP] and chitinase-3-like protein 1 [YKL-40]) in blood has allowed researchers to characterize neurobiological processes at scale in a cost-effective and minimally invasive manner. Although currently used primarily for research purposes, these blood-based biomarkers have the potential to be highly impactful in the clinical setting – aiding in diagnosis, predicting disease risk, and monitoring disease progression. Whereas plasma NfL has shown promise as a non-specific marker of neuronal injury, plasma pTau181, pTau217, pTau231, and GFAP have demonstrated desirable levels of sensitivity and specificity for identification of individuals with Alzheimer’s disease pathology and Alzheimer’s dementia. In this forward looking review, we (i) provide an overview of the most commonly used blood-based biomarkers for Alzheimer’s disease and related dementias, (ii) discuss how comorbid medical conditions, demographic, and genetic factors can inform the interpretation of these biomarkers, (iii) describe ongoing efforts to move blood-based biomarkers into the clinic, and (iv) highlight the central role that clinical neuropsychologists may play in contextualizing and communicating blood-based biomarker results for patients.
Keywords: Biomarkers, blood-based biomarkers, dementia, Alzheimer’s disease, prediction
INTRODUCTION
The improved accuracy, reliability, and scalability of molecular measurements brought about by recent technological advances has moved blood-based biomarkers to the forefront of Alzheimer's disease (AD) and dementia research. Core neurobiological processes that underly AD dementia, including amyloid-ß (Aβ) plaque formation, tau hyperphosphorylation and the spreading of tau neurofibrillary tangles (NFTs), are traditionally measured using positron emission tomography (PET) scans or cerebral spinal fluid (CSF) by way of lumbar puncture. Until recently, the discriminatory power of amyloid-ß and phosphorylated tau (pTau) abundance in blood was limited due to low measurement sensitivity. However, advancements in techniques, such as single molecular array (Simoa) and mass spectrometry, have allowed for ultra-sensitive quantification of multiple variations of the amyloid- ß peptide, including Aß42 and Aß40, and multiple pTau isoforms, including tau phosphorylated at threonine-181 (pTau181), threonine-217 (pTau217), and threonine-231 (pTau231). In parallel, non-specific measures of neurodegeneration, neuronal injury, and neuro-immune function (initially identified in CSF), have been developed and validated for use in blood. Blood-based biomarkers are less invasive compared to traditional CSF and PET measures of amyloid-ß and tau, as they do not require a lumbar puncture or the injection of a radiotracer, making them more feasible for certain patient populations and resource-limited clinical settings.
While there is ongoing debate about whether AD should be defined purely based on biology (amyloid-ß and phosphorylated tau) (Høilund-Carlsen et al., 2023; Jack et al., 2018), the consensus remains that the application of blood-based biomarkers for the in vivo characterization of disease-specific pathology should be used in combination with a clinical exam and cognitive (neuropsychological) and functional measurements to provide a comprehensive picture of a patient's current neurocognitive status. Doing so will allow providers to characterize and track the extent of current neurodegenerative disease. Because the performance of, and hence interest in, blood-based biomarkers for AD and related dementias (ADRD) has seemingly risen exponentially within the past several years, we anticipate that these measures will soon be as ubiquitous in clinical practice as they are currently in research. Neuropsychologists are uniquely positioned to make use of available blood-based biomarkers to enhance diagnostic and prognostic accuracy, and to help guide clinical decision making.
A biomarker can be defined as a measured characteristic that acts as an indicator of a normal biological process, a pathogenic process, or a response to an exposure or therapeutic intervention (FDA, 2016). Given the forecasted clinical utility of blood-based biomarkers for the identification, management, and treatment of age-related neurologic disease, it is essential that neuropsychologists who work with older adults understand how blood-based biomarkers can be integrated to enhance differential diagnosis and personalize treatment planning, recommendations, and patient feedback. We anticipate that blood-based biomarkers will be employed clinically for one of five uses. First, blood-based biomarkers will be used to assist with etiological diagnosis, i.e., to confirm or rule out the presence of a specific disease or disease subtype. Second, blood-based biomarkers will be applied to quantify a patient's potential for developing a specific disease or medical condition over a given time. Third, blood-based biomarkers will be used for prognostication, enabling estimation of the likelihood of disease progression, recurrence, or the emergence of a specific symptom or clinical event. Fourth, blood-based biomarkers will be employed longitudinally to continuously assess the status and evolution of a specific disease process. Lastly, blood-based biomarkers will likely be used to predict person-specific treatment responsiveness and to monitor target engagement after specific interventions have been initiated.
In the sections below, we will provide an overview of commonly used blood-based biomarkers in the setting of ADRD, review how comorbid medical conditions, demographic and genetic factors can inform the interpretation of these biomarkers, describe ongoing efforts to move blood-based biomarkers into the clinic, and highlight the central role that clinical neuropsychologists will likely play in contextualizing blood-based biomarker results for patients.
BLOOD-BASED BIOMARKERS FOR ALZHEIMER'S DISEASE AND RELATED DEMENTIAS
In recent years, blood-based biomarkers for ADRD have been extensively explored as potential alternatives to traditional, more invasive and expensive options. Biomarkers of AD pathology include two measures of brain amyloid burden: the 42 amino acid Aβ peptide (Aβ42), and a shorter 40 amino acid Aβ peptide (Aβ40). The other class of blood-based biomarkers used to quantify AD pathology include measures of pTau, including pTau181, pTau217, and pTau231. Blood-based biomarkers of biological processes not specific to any one disease include neurofilament light chain (NfL), a non-specific marker of neuronal injury that has been applied broadly in the research setting, glial fibrillary acidic protein (GFAP), a marker of astrocyte injury or reactive astrogliosis, and purported measures of neuroinflammation, such as chitinase-3-like protein 1 (YKL40) and triggering receptor expressed on myeloid cells 2 (sTREM2).
Plasma amyloid-β (Aβ)
Aβ is a product of the amyloid precursor protein (APP) that is cleaved to produce peptides of varying lengths, typically 37-43 amino acids. Aβ40 is the most abundant isoform and is expressed by healthy neurons outside the context of AD. Longer isoforms of Aβ, namely Aβ42, are expressed at higher levels within the context of AD and are more prone to self-aggregate. Aβ42 is found at high levels in brain Aβ plaques and thus it is considered a marker of brain amyloidosis (Gu & Guo, 2013). Aβ40 abundance is typically unchanged in AD, whereas Aβ42 abundance decreases in the CSF and plasma of persons with AD due to increased sequestration of brain Aβ42 by the formation of amyloid plaques, impaired CNS Aβ42 clearance, and increased Aβ42 oligomerization and aggregation into less detectable forms (Hansson, 2021). Because individual levels of Aβ production can vary considerably, Aβ40 is often used as a normalization factor for Aβ42 to account for person specific Aβ production (i.e., the Aβ42 to Aβ40 ratio), improving predictive accuracy for cortical Aβ-positivity (Hansson, Lehmann, Otto, Zetterberg, & Lewczuk, 2019; Janelidze et al., 2021).
Plasma Aβ42/40 has been shown to predict abnormal CSF Aβ42/40 with a level of accuracy that ranges from below acceptable (AUC=0.64) to excellent (AUC=0.87), depending largely on the diagnostic makeup of the sample and the assay used. Generally speaking, mass spectrometry-based approaches for measurement of plasma Aβ42/40/ have demonstrated superior estimates of CSF and PET amyloid-β compared to immunoassays (Janelidze et al., 2021; Wojdała et al., 2023). While plasma Aβ42/40 demonstrates good to excellent prediction of brain Aβ-positive status, as measured by PET imaging (e.g., AUC’s ranging from 0.72 to 0.88) (Bilgel et al., 2023; Schindler et al., 2019), other studies have found that plasma Aβ42/40 only modestly correlates with CSF Aβ42/40 (e.g., Spearman’s ρ=0.32-0.42) (Wojdała et al., 2023). It is now well established that CSF and PET measures of Aβ become abnormal approximately two decades before symptom onset in the context of AD. Two studies that compared the temporal dynamics of plasma Aβ42/40 to CSF and PET measures found that plasma Aβ42/40 begins to show significant changes at the same point in the disease course (compared to CSF) or earlier (with respect to PET), suggesting that plasma Aβ42/40 may be useful as an early AD biomarker (Bilgel et al., 2023; Palmqvist et al., 2019). Compared to CSF measures of Aβ, plasma Aβ measurements tend to have a more limited dynamic range, and show comparatively more overlap across Aβ-positive and Aβ-negative individuals (Guo et al., 2023).
Plasma phosphorylated tau (pTau)
Plasma measures of multiple pTau isoforms are now available for research use. These measures, which are believed to capture the abundance of the soluble tau protein phosphorylated at specific amino acids (e.g., threonine-181, threonine-217, and threonine-231), have proven to be strong predictors of cortical amyloid. Although plasma pTau has shown only modest correlations with PET-defined measures of tau NFTs (Coomans et al., 2023), plasma abundance of the pTau181, pTau217, and pTau231 proteins have been associated with autopsy-confirmed AD (Brickman et al., 2021; Smirnov et al., 2022), and plasma pTau181 shows comparable performance with tau PET for identification of individuals with elevated CSF Aβ (AUC=0.83 for plasma, AUC=0.87 for entorhinal tau PET) (Coomans et al., 2023). Plasma pTau’s modest correlation with tau PET, its ability to discriminate between Aβ-positive and Aβ-negative individuals with greater predictive accuracy than plasma measures of Aβ42, Aβ40, and Aβ42/40 (Smirnov et al., 2022), and the significant increase in plasma pTau181, pTau217, and pTau231 shortly after an individual becomes amyloid-PET-positive (approximately 15-20 years before symptom onset) (Barthélemy et al., 2020) suggests that at least a subset of pTau isoforms may serve as indicators of cerebral amyloidosis, even more so than as indicators of PET-defined tau NFT deposition. A direct comparison of tau isoforms found that plasma pTau217 outperforms pTau181 and pTau231, and performs similar to CSF pTau217 for prediction of amyloid-PET-positivity and tau-PET-positivity (AUCs>0.90) (Palmqvist et al., 2020; Therriault et al., 2023). Measures of pTau181, pTau217, and pTau231 do not tend to show elevations (compared to that of healthy older adults) in non-AD neurodegenerative disorders or in amyloid-negative individuals with mild cognitive impairment, indicative of the specificity of these blood-based biomarkers for AD (Ashton et al., 2021; Thijssen, La Joie, et al., 2021a).
Plasma neurofilament light chain (NfL)
In contrast to Aβ and pTau, which more specifically reflect AD neuropathologic changes, NfL is a non-specific marker of neuronal injury. NfL is a large caliber fiber axon protein that is released from neurons with the degeneration of myelinated axons (Gafson et al., 2020). Elevations in NfL can occur in a wide range of neurologic conditions in which neuronal damage occurs, including multiple sclerosis, traumatic brain injury, HIV-associated dementia, and nearly all of the most common neurodegenerative disorders (Sjögren et al., 2000). The greatest elevations in CSF NfL are observed in acute or rapidly progressing neurologic disorders, such as amyotrophic lateral sclerosis and Creutzfeldt-Jakob disease (Gaetani et al., 2019). Recently developed measures of plasma NfL show a moderate correlation with CSF NfL levels (Spearman’s ρ=0.59) (Mattsson et al., 2017). Both CSF and plasma NfL are elevated in the context of MCI and AD, compared to neurologically healthy controls, but significant elevations in NfL appear to occur in later stages of AD pathogenesis, typically within 10 years of dementia onset (de Wolf et al., 2020; Mattsson et al., 2017). As a non-specific marker of neurodegeneration, plasma NfL can be used for monitoring disease stage, disease progression, and treatment response. However, this marker is less informative with respect to differential diagnosis.
Plasma markers of neuro-immune function
Neuroinflammation, and more specifically the microglia- and astrocyte-mediated immune response to neuropathology, has been identified as a central feature of multiple neurodegenerative diseases, including AD, vascular dementia, Parkinson’s disease, and amyotrophic lateral sclerosis (Kwon & Koh, 2020). While initially only considered a response to pathology, considerable evidence places the microglia and astrocyte response to neurodegenerative pathology as a mechanistic driver of the disease process and a regulator of disease progression (Heneka et al., 2015). Hence, there is increasing interest in the identification and validation of biomarkers of neuroinflammation and other facets of neuro-immune activation. A challenge to efforts directed at neuro-immune biomarker identification in blood is that many of the candidate immune proteins are also highly expressed by peripheral immune cells, particularly in the context of inflammatory insults such as infection (Walker et al., 2023).
Plasma GFAP, an intracellular astrocytic cytoskeletal protein that reflects reactive astrogliosis or abnormal activation of astrocytes, is currently seeing widespread use in ADRD research (Chatterjee et al., 2021). Both CSF GFAP and plasma GFAP are elevated in participants on the AD continuum. However, plasma and CSF GFAP show low to moderate positive correlations (Spearman’s ρ=0.37-0.62) (Benedet et al., 2021), and plasma GFAP more accurately discriminates Aβ-positive from Aβ-negative individuals (Pereira et al., 2021). Specifically, plasma GFAP has been shown to discriminate between Aβ-positive individuals with AD dementia from Aβ-negative cognitively unimpaired individuals with excellent accuracy (AUC of 0.90) (Chatterjee, Pedrini, et al., 2023a). Additionally, plasma GFAP has been associated with postmortem brain Aβ abundance (Cousins et al., 2023) and PET-defined measures of reactive astrocytosis (Chatterjee, Doré, et al., 2023b). A second astrocytic biomarker, YKL-40, is involved in tissue remodeling and is expressed during inflammatory responses. CSF YKL-40 is considered a marker of neuroinflammation and has been found to increase with Aβ plaque accumulation (Craig-Schapiro et al., 2010) and differentiate AD from healthy controls (Wennström et al., 2015). Plasma YKL40 shows low (r=0.24) to moderate associations (ρ=0.40) with CSF YKL-40 and is not related to cortical Aβ (Craig-Schapiro et al., 2010; Giannisis et al., 2022). Plasma YKL-40 has been more strongly associated with non-AD neurodegenerative diseases than with AD dementia (Villar-Piqué et al., 2019).
Another increasingly popular marker of neuro-immune function, the soluble form of the triggering receptor expressed on myeloid cells 2 (sTREM2), is primarily expressed in microglia and regulates the microglial clearance of brain Aβ, microglial inflammatory signaling, and cell survival (Xue & Du, 2021). Although CSF abundance of sTREM2 has been associated with PET-defined microgliosis as well as clinical symptoms, the potential role of plasma sTREM2 as a biomarker for clinical use has yet to be established (Xue & Du, 2021). CSF sTREM2 has been found to have a modest positive correlation (r ~ 0.20) with plasma sTREM2, and the magnitude of this association appears to depend on the presence of CNS disease and specific sTREM2 assay used (Bekris et al., 2018; Španić Popovački et al., 2023). At least one study has found an inverse correlation between CSF and plasma sTREM2 (Park et al., 2021). While CSF sTREM2 has been found in one study to be increased in individuals with MCI and AD, compared to healthy controls, these group differences did not extend to plasma sTREM2 (Bekris et al., 2018). Despite these findings, others have found plasma sTREM2 to be positively associated with white matter hyperintensity volume, PET-defined measures of cortical tau pathology, and CSF measures of NfL (Park et al., 2021; Tsai et al., 2021). The poor and inconsistent associations between CSF sTREM2 and blood-based measures of the same protein suggest that blood sTREM2 measurements may be a proxy for peripheral, rather than central, immune activation.
BLOOD-BASED BIOMARKERS, COGNITION, AND DEMENTIA PREDICTION
Consistent with that of their CSF analogs, higher abundance of pTau181, pTau217, pTau231, NfL, and GFAP, and lower abundance of Aβ42/40 in blood have been associated with subjective and subtle cognitive decline (Baldacci et al., 2020; Bangen et al., 2021; Cullen et al., 2021; Thomas et al., 2021), poorer cognitive performance and greater rates of cognitive decline in some, but not all, studies of cognitively normal and cognitively impaired individuals (Frank et al., 2022; Hansson, 2021; Milà-Alomà et al., 2022; Rajan et al., 2020). Supporting the idea that these blood-based biomarkers may be used for disease monitoring, longitudinal increases in pTau217 and NfL have been linked to greater rates of cognitive decline independent of baseline biomarker levels (Mattsson-Carlgren et al., 2021; Mielke et al., 2019). While plasma GFAP demonstrates a consistent association with cognition (Gonzales et al., 2022), plasma YKL-40 has shown mixed results. Specifically, some studies have linked plasma YKL-40 to poorer cognitive function (Pase et al., 2020), while others have found that plasma YKL-40 does not predict cognition or cognitive decline (Brosseron et al., 2023; Craig-Schapiro et al., 2010). Unexpectedly, one study found that higher YKL-40 was associated with better memory performance (Vergallo et al., 2020). Less is known about the association of blood sTREM2 abundance with cognition, although this protein has been associated with cognitive decline in tau-positive, but not tau-negative, individuals (Tsai et al., 2021).
As expected given their associations with cognition, blood-based measures of AD pathology, neuronal injury, and neuro-immune activation are predictive of future dementia onset (Silva-Spínola et al., 2023; Simrén et al., 2021). While plasma pTau181, NfL, and GFAP can accurately predict dementia risk and tend to increase in concentration with advancing disease stage, the associations of plasma Aβ42/40 with clinically defined outcomes have been less consistent (Janelidze et al., 2016; Simrén et al., 2021; Wojdała et al., 2023). Plasma pTau181 and GFAP have been found to discriminate persons with dementia from cognitively unimpaired individuals with excellent accuracy (AUCs=0.8-0.9), whereas plasma Aβ42/40 and NfL have tended, with some exceptions, to demonstrate lower predictive accuracy in this context (AUC’s typically around 0.70) (Baiardi et al., 2022; Benussi et al., 2022; Simrén et al., 2021).
In addition to classifying dementia risk, these blood-based biomarkers have also demonstrated an ability to discriminate between dementia etiologies. pTau181, pTau217, and pTau231 can differentiate AD dementia from non-AD dementia with excellent to outstanding accuracy (AUCs=0.84-0.96) (Ashton et al., 2021; Kivisäkk et al., 2023). Though not as accurately as pTau, GFAP has been shown to differentiate AD dementia from non-AD dementia. For example, Baiardi et al., (2022) found plasma GFAP had an AUC=0.70 for discriminating AD dementia from non-AD dementia. Although Aβ42/40 is considered a marker of AD-specific disease processes, at least one study has found that Aβ42/40 poorly discriminates AD from frontotemporal dementia, or dementia with Lewy bodies (Thijssen et al., 2022). Predictive accuracy for plasma Aβ42/40 has been found to vary largely based on assay and assay characteristics (Thijssen, Verberk, et al., 2021b). Blood markers of YKL-40 and sTREM2 have shown mixed results for classification of clinical status, and YKL-40 has been found to be nonspecific with respect to dementia etiologies (Španić Popovački et al., 2023) (Ashton et al., 2019; Wilczyńska, Maciejczyk, Zalewska, & Waszkiewicz, 2021).
Several blood-based biomarkers have also been shown to predict future dementia risk and the likelihood of progression from MCI to dementia within a specified follow-up period. pTau181 and GFAP have shown excellent accuracy for discriminating persons with MCI who progress to AD dementia from persons with stable MCI (AUCs=.83) (Kivisäkk et al., 2023). Plasma NfL, by comparison, has shown comparatively weaker predictive power in this context (AUC=0.73) (Kivisäkk et al., 2023). These biomarkers can show even better predictive accuracy for MCI progression when combined, compared to their accuracy as individual predictors. For example, when GFAP and pTau181 are combined, the ability to predict progression from MCI to AD dementia improves to an AUC of 0.89 (Kivisäkk et al., 2023).
Although blood-based biomarkers have demonstrated clear utility for discrimination of dementia etiology and prediction of dementia risk, additional research is needed to establish clinically and pathologically relevant biomarker cut-points that can be used to group participants into risk bins for a particular neurocognitive outcome. The studies that have reported optimal cut-points for discriminating cortical amyloid-β positivity using plasma Aβ42/40 have yielded varied results. For example, plasma Aβ42/40 cut-points ranging from 0.076 to 0.1218 have been recommended (Feinkohl et al., 2020; Pais, Forlenza, & Diniz, 2023; Schindler et al., 2019; West et al., 2021). Given the inter-study variability in cut-points among blood-based biomarkers, use of study specific cut-points is recommended in the research context (Pais et al., 2023). Ultimately, however, the goal is to establish cut-points that are reliable enough across samples to offer accurate prediction in the clinical setting. The Alzheimer’s Association guidelines for the use of blood-based AD biomarkers recommends that cut-points be established prior to the widespread clinical use of blood-based ADRD biomarkers (Hansson et al., 2022). One of many challenges with this approach is that person-specific health, lifestyle, and environmental factors may influence the optimal cut-point (Dark et al., 2023). As discussed in the next sections, additional work is needed to begin to address this important question.
THE EFFECT OF COMORBID MEDICAL CONDITIONS
Several studies have demonstrated that comorbid medical conditions can affect levels of ADRD biomarkers in blood. For example, hyperlipidemia, hypertension, ischemic heart disease, diabetes, and chronic kidney disease have been associated with altered plasma abundance of Aβ40 and Aβ42 (Dark et al., 2023; Janelidze et al., 2016; O’Bryant, Petersen, Hall, & Johnson, 2023). Diabetes and chronic kidney disease have also been associated with higher plasma NfL, whereas a higher body mass index (BMI) has been linked to differences in pTau181, pTau217, and NfL levels (Brickman et al., 2021; Dark et al., 2023; Mielke et al., 2022; O’Bryant et al., 2023). However, some of these associations are attenuated – or even eliminated – after accounting for age and sex (Mielke et al., 2022). As a physiological regulator of protein excretion, kidney function (typically defined by estimated Glomerular Filtration Rate [eGFR] and creatine levels) is known to influence a large segment of the proteome (Tin et al., 2023). Accordingly, poorer kidney function has been associated with higher plasma levels of NfL, pTau181, and pTau217 (Janelidze, Barthélemy, He, Bateman, & Hansson, 2023; Lehmann et al., 2023; Zhang et al., 2023). Moreover, kidney function has been shown to modify pTau181’s ability to predict CSF Aβ-positivity; however, not all studies show that diagnostic accuracy is affected (Lehmann et al., 2023; Zhang et al., 2023). Further, kidney function does not show a strong association with plasma Aβ42/40, nor does it appear to affect the diagnostic accuracy or optimal cut point for this biomarker (Zhang et al., 2023). Multiple conditions, including chronic kidney disease, myocardial infarction, and stroke, have been shown to modify the optimal cut points used for pTau181 and pTau217 (Mielke et al., 2022). However, there is some evidence that the use of a pTau to total tau ratio may reduce the effect of comorbidities on pTau measurement (Janelidze et al., 2023).
THE EFFECT OF GENOTYPE
In addition to health factors and comorbid medical conditions, genotype appears to influence blood-based biomarker abundance. While not always the case (Feinkohl et al., 2020), lower Aβ42/40 (Schindler et al., 2019) and higher pTau181 and pTau231 abundance have been found in cognitively unimpaired and cognitively impaired individuals with at least one copy of the APOEε4 allele (Brickman et al., 2022; Salami et al., 2022; Snellman et al., 2023). Given that plasma Aβ42/40 and pTau are indicators of AD pathology, it follows that the major AD risk variants that influence AD pathology also influence AD biomarker level, even before individuals become symptomatic. Conversely, biomarkers that are not specific to AD pathology, including NfL and GFAP, are less sensitive to the possession of AD risk genes (Asken et al., 2020; Baldacci et al., 2020; Malek-Ahmadi et al., 2023; Snellman et al., 2023). Whether other ADRD risk variants influence abundance of these biomarkers in blood remains unknown.
THE EFFECT OF RACE/ETHNIC FACTORS AND SEX
The prevalence of AD and all-cause dementia differs by self-reported race and ethnicity, with most studies finding that Black and Hispanic individuals have elevated rates of dementia relative to non-Hispanic Whites (Mehta & Yeo, 2017; Moon, Badana, Hwang, Sears, & Haley, 2019). Despite these elevated prevalence rates, Black, Hispanic, and other non-White adults have been historically underrepresented in dementia research, including the aforementioned biomarker studies, due to reliance on clinical populations (e.g., memory center) rather than population based recruitment, and the implementation of inclusion/exclusion criteria that disproportionately selects non-White participants out of the study (Gleason et al., 2019; Raman et al., 2021). The global underrepresentation of the of non-White adults in dementia research, particularly within more invasive studies that require CSF collection and PET neuroimaging, can have multiple negative consequences on biomarker development, among which includes the limited generalizability of cut-points or biomarker-based prediction scores (Barnes & Bennett, 2014; Lim et al., 2023; Weiner et al., 2023).
Compared to White individuals with clinically defined AD dementia, autopsy and PET neuroimaging studies have demonstrated that non-White individuals with clinically defined AD dementia are more likely to have mixed pathology and are less likely to have brain amyloidosis (Barnes et al., 2015; Dark & Walker, 2023; Wilkins et al., 2022). Based on these findings, multiple studies have sought to determine whether blood-based biomarker levels differ by race or ethnicity across diagnostic strata. While several studies have found no significant differences in plasma ADRD biomarker abundance as a function of race (Brickman et al., 2021; Hall, Petersen, Johnson, & O’Bryant, 2022; Ramanan et al., 2023; Windon et al., 2022), others have found evidence for race differences (O’Bryant et al., 2022; Schindler et al., 2022). For example, White participants have been found to have lower Aβ42/40 compared to Black participants and higher NfL compared Mexican Americans (O’Bryant et al., 2023; Schindler et al., 2022). Another study found plasma abundance of Aβ40, Aβ42, Aβ42/40, and NfL to differ by race in a manner that was partially contingent upon clinically-defined disease stage, with Black participants showing lower plasma abundance of each biomarker compared to Non-Hispanic Whites and Mexican Americans (Hall et al., 2022). One study that found associations between plasma biomarkers and clinical outcomes to be stronger for White, compared to Black participants. They also found that the results did not hold after participants with chronic kidney disease were excluded, suggesting that race-based differences in ADRD biomarkers may be explained by the differential prevalence of comorbid disease across race groups (Ramanan et al., 2023). Another study that found significant differences in biomarker levels when analyses were stratified by self-reported race saw these group differences abate when analyses were stratified by genetic ancestry (Hajjar et al., 2022). These results suggest that social constructs may drive race-related differences in plasma biomarkers, rather than inherent biological differences.
It remains unclear whether blood-based biomarker concentrations differ by sex. While some studies show no difference in biomarker abundance between men and women (Baldacci et al., 2020; Mattsson et al., 2017; Triant, Lee, Hadigan, & Grinspoon, 2007), others suggest that men tend to have a more pathogenic pattern characterized by lower levels of Aβ42/40 (Schindler et al., 2019; Snellman et al., 2023) and higher levels of NfL (Lin, Lee, Wang, & Fuh, 2018). However, this pattern was reversed for blood GFAP, which tends to show higher abundance among women (Benedet et al., 2021; Saloner et al., 2023). There also exists evidence for sex differences in the association of these biomarkers with neurocognitive outcomes. For example, in women greater pTau abundance is more strongly associated with elevated cortical amyloid-β and tau, and greater medial temporal lobe atrophy and verbal memory decline compared to men (Saloner et al., 2023; Tsiknia et al., 2022).
BLOOD-BASED BIOMARKER PLATFORMS FOR CLINICAL USE
Clinical application of blood-based biomarkers remains actively debated among leading researchers, with no clear consensus among primary care providers, memory care specialists or regulatory bodies. There are estimated to be a dozen or more blood-based assays at various stages of development in the private sector, although the exact number can be difficult to assess because of intellectual property rights, delays in dissemination of internal research findings, and so forth (for a more complete list of biomarkers in development, see Hampel et al., 2023). In the United States, the FDA has not approved any blood-based assay for AD diagnosis, but approval is not necessary for such tests to be marketed to the public, clinically applied in conjunction with other diagnostic information, or utilized in research settings (for a more complete overview of biomarker types and regulatory approval steps, see Cummings & Kinney, 2022). Several blood-based laboratory developed tests (LDTs) for quantification of ADRD biomarkers have received Breakthrough Device Designation from the FDA for measurement. Breakthrough Device Designation is granted by the FDA to measures that evaluate a serious condition with the potential to offer substantial improvement over existing diagnostics. Below, we describe the blood-based biomarkers that are either currently available for clinical use or making their way to the clinic (see also Table 1).
Table 1.
Summary of blood-based biomarkers in late-stage clinical development
| Name | Company | Measures | BDD (year) | CLIA (Year) | Launch | Assay type | Context of use |
|---|---|---|---|---|---|---|---|
| PrecivityAD | C2N Diagnostics, St. Louis, MO, USA | age, Aβ42/40 and APOE proteotype | Yes (2018) | Yes (2020) | 2020 | Immunoprecipitation and liquid chromatography mass spectrometry | Adults presenting with cognitive impairment, not stand-alone, must be ordered through health care provider |
| LucentAD | Quanterix, Billerica, MA, USA | pTau181 | Yes (2021) | Yes (2018) | 2023 | Simoa® immunoassay | Adults presenting with cognitive impairment, not stand-alone, must be ordered through health care provider |
| Elecsys Amyloid Plasma Panel | Roche Diagnostics, Indianapolis, IN, USA | pTau181 and APOE proteotype | Yes (2022) | Unknown | Unknown | Cobas® immunoassay | Adults presenting with cognitive impairment, not stand-alone, ordering mechanism unknown |
| AlzoSure Predict | Diadem SpA, Milan, LOM, IT, and Palo Alto, CA, USA | p53 | Yes (2022) | Unknown | Unknown | Immunoprecipitation and liquid chromatography mass spectrometry | Inclusion criteria unknown, stand-alone status unknown, ordering mechanism unknown |
| SOBA-AD | AltPep, Seattle, WA, USA | Aβ42 (oligomers) | Yes (2022) | Yes (unknown) | Unknown | ELISA | Inclusion criteria unknown, stand-alone status unknown, ordering mechanism unknown |
| AD-Detect | Quest Diagnostics, Secaucus, NJ, USA | Aβ42/40 | unknown | Yes (unknown) | 2023 | Immunoprecipitation and liquid chromatography mass spectrometry | Adults with a family history of AD, brain trauma, other risk factors (excessive alcohol consumption) or presenting with cognitive impairment, not stand-alone, can be ordered directly by consumer |
Abbreviations: Aβ, amyloid-β; AD, Alzheimer’s disease; BDD, Breakthrough Device Designation; CLIA, Clinical Laboratory Improvement Amendments; ELISA, enzyme-linked immunoassay.
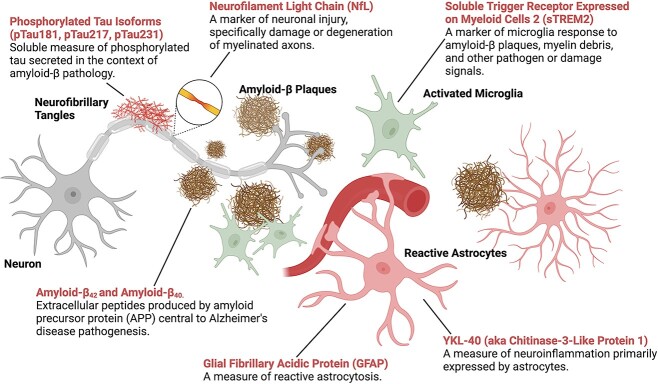
Figure 1.
Blood-based biomarkers for Alzheimer’s disease and related dementias. Biomarkers for Alzheimer’s disease pathology, neurodegeneration, and neuro-immune activation (red text) are illustrated in the context of neurons, glial cells, and Alzheimer’s disease pathology (amyloid-β plaques and tau neurofibrillary tangles). Created with BioRender.com.
PrecivityAD
Advertised as a blood-based screening measure for brain amyloid-β pathology, Precivity uses chronological age, Aβ42/40 and APOE proteotype to determine the likelihood of amyloid-β positivity in individuals being assessed for AD. Leveraging such measures to calculate an amyloid probability score (APS), the Precivity test offers AUCs of 0.88-0.90 for predicting Aβ PET status (+/-), with the Aβ42/40 measurement accounting for the majority of variance (i.e., AUCs of 0.81-0.84) (Fogelman et al., 2023; Hu et al., 2022). Results of PrecivityAD2 (which has added pTau217 to the predictive model to improve accuracy) have not been peer-reviewed, but a poster presentation from 2023 suggested it provides AUCs of 0.95-0.97 (unpublished).
LucentAD
LucentAD uses pTau181 to aid in the diagnostic evaluation of AD. Although the assay’s results have not been peer-reviewed, a poster presentation from 2022 suggested it shows an AUC of 0.90 for discriminating CSF-confirmed AD diagnoses (n=34) from age- and sex-matched cognitively normal controls (n=36) (Malyavantham et al., 2022).
Elecsys Amyloid Plasma Panel
This panel uses pTau181 and APOE proteotype to identify individuals who warrant further confirmatory AD testing with PET or CSF; results examining its discriminative performance for Aβ CSF status (+/-) showed an AUC of 0.85 (n=693) (Palmqvist et al., 2023).
AlzoSure Predict
AlzoSure Predict measures an AD-specific conformational variant of the p53 protein, resulting in a reportedly near-perfect AUC of 0.99 for discriminating neuropsychologically-defined AD from cognitively unimpaired individuals, as well as conversion to AD at 36- and 72-month follow-up time points (n=482) (Piccirella et al., 2022). AlzoSure Predict’s intended use is not yet specified.
SOBA-AD
The SOBA-AD assay, which captures a unique oligomeric form of Aβ42, has reported a near-perfect AUC of 0.99 for discriminating cognitively normal individuals from autopsy- and clinically- diagnosed MCI and AD cases (n=379) (Shea et al., 2022). The intended use for SOBA-AD is not yet reported.
AD-Detect
As the first direct to consumer product, AD-Detect uses Aβ42/40 to assess the risk of having AD pathology in adults with MCI or dementia. Notably, the marketing campaign for AD-Detect suggests it can also be applied among individuals who have a family history of AD or who have been exposed to risk factors, such as traumatic brain injury. Although the assay’s results have not been peer-reviewed, a poster presentation from 2022 reported an AUC of 0.86 for discriminating Aβ PET-positive individuals from age- and sex-matched cognitively normal controls (n=209) (Weber, Kim, Goldman, Racke, & Clarke, 2022). The direct-to-consumer approach has been met with criticism (Rogers, 2023).
CONSIDERATIONS FOR NEUROPSYCHOLOGY
Predictive and diagnostic biomarkers for ADRD will continue to make their way into the clinical setting, likely with more fervor than did CSF and PET measures. Reduced expense, desirable levels of accuracy, and minimal invasiveness are characteristics of the current wave of blood-based biomarkers that have made them practical tools for clinicians and researchers. The potential utility of plasma biomarkers in the setting of ADRD extends to multiple roles, including patient screening, informing etiological diagnoses, prediction or prognostication, and disease monitoring. Like the results of neuroradiological studies and CSF marker quantification, patient-specific blood-based biomarker information will be made available to patients by referring providers and via direct-to-consumer platforms. While we anticipate that neuropsychologists, neurologists, and other providers will be able to use the results of these blood-based biomarkers to inform their clinical assessment and treatment planning/monitoring, neuropsychologists will often be in the best position to integrate the biomarker information with a complementary characterization of cognitive and functional abilities in a manner that will determine the syndromic diagnosis and provide further support for an etiologic diagnosis and prognosis. Though the expectation is that blood-based biomarkers will be interpreted in the context of a full clinical workup, some patients will be left with questions about how to interpret the quantitative readouts and qualitative descriptions provided by blood-based biomarker platforms. These questions will inevitably make their way to neuropsychologists who work with older adults. While we anticipate that blood-based biomarkers will be valuable, particularly for screening for clinical trials or for estimating the likelihood of brain AD pathology in those with mild cognitive impairment or dementia, without appropriate guidance and context these tests have the potential to be misused and misinterpreted, particularly in the direct-to-consumer setting.
One way to limit potential harm done by blood-based biomarkers is to establish and adhere to a set of eligibility use criteria. Guidelines for the use of blood-based AD biomarkers were published in 2022 by the Alzheimer’s Association (Hansson et al., 2022). These guidelines recommend that commercially available blood-based measures of AD pathology not be used for asymptomatic or cognitively normal individuals. Using these tests as general population screening measures and as direct-to-consumer tests has also been discouraged (Hansson et al., 2022). Without the ability to take actionable steps toward treatment or risk mitigation – at the time of this writing AD disease modifying drugs are approved only for symptomatic AD patients – and the elevated likelihood of false positives in the setting of lower disease prevalence, the risk introduced by screening cognitively normal individuals may outweigh the potential benefit. Guidelines further encourage providers to consider the ramifications of disclosing biomarker results to individuals who are asymptomatic (Hansson et al., 2022). Separate studies found that participants who learned that they were positive for an AD biomarker (i.e., elevated amyloid-β or APOEε4-positive status) did not show elevated anxiety, depression, or suicidality compared to participants who learned that they were biomarker-negative (Green et al., 2009; Grill et al., 2020). However, as expected, participants told they had elevated amyloid-β levels had greater concern about their risk for AD (Grill et al., 2020).
An anticipated consequence of the rollout of diagnostic blood-based biomarkers is a sharp uptick in referrals from individuals with and without objective cognitive decrements who have been identified by blood-based biomarkers as having Alzheimer's disease pathologic changes. Accordingly, clinical neuropsychologists should be prepared to both rule out cognitive impairment when there is none, and – in the setting of meaningful cognitive decrements – make use of available blood-based biomarker measurements to inform differential diagnosis, improve prognostic accuracy, and personalize treatment recommendations.
CONCLUSION
Clinical neuropsychologists working with older adult populations should be prepared to answer fundamental questions about the potential value and utility of blood-based biomarkers. Clinical neuropsychologists will also need to be equipped to help patients and healthcare providers place biomarker findings in the appropriate context, given cognitive and functional abilities, as well as relevant psychosocial factors. As blood-based biomarkers for ADRD become more common in the clinical setting, we anticipate that neuropsychologists will be asked to play a central role in educating patients about the meaning of biomarker findings, and the limits and uncertainty surrounding these measures. Ultimately, the goal should be to provide realistic expectations and reduce unneeded patient and caregiver anxiety.
Contributor Information
Heather E Dark, Laboratory of Behavioral Neuroscience, National Institute on Aging, Intramural Research Program, Baltimore, MD, USA.
Michael R Duggan, Laboratory of Behavioral Neuroscience, National Institute on Aging, Intramural Research Program, Baltimore, MD, USA.
Keenan A Walker, Laboratory of Behavioral Neuroscience, National Institute on Aging, Intramural Research Program, Baltimore, MD, USA.
FUNDING
HED, MRD, and KAW are supported by the National Institute on Aging’s Intramural Research Program. The manuscript was supported by the National Institute on Aging’s Intramural Research Program.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Heather E. Dark (Writing – original draft, Writing – review & editing), Michael R. Duggan (Writing – original draft, Writing – review & editing), and Keenan Walker (Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing).
REFERENCES
- Ashton, N. J., Pascoal, T. A., Karikari, T. K., Benedet, A. L., Lantero-Rodriguez, J., Brinkmalm, G., et al. (2021). Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathologica, 141(5), 709–724. 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton, N. J., Suárez-Calvet, M., Heslegrave, A., Hye, A., Razquin, C., Pastor, P., et al. (2019). Plasma levels of soluble TREM2 and neurofilament light chain in TREM2 rare variant carriers. Alzheimer’s Research and Therapy, 11(1), 94. 10.1186/s13195-019-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asken, B. M., Elahi, F. M., La Joie, R., Strom, A., Staffaroni, A. M., Lindbergh, C. A., et al. (2020). Plasma Glial Fibrillary Acidic Protein Levels Differ along the Spectra of Amyloid Burden and Clinical Disease Stage. Journal of Alzheimer’s Disease, 78(1), 265–276. 10.3233/JAD-200755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiardi, S., Quadalti, C., Mammana, A., Dellavalle, S., Zenesini, C., Sambati, L., et al. (2022). Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimer’s Research and Therapy, 14(1), 153. 10.1186/s13195-022-01093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacci, F., Lista, S., Manca, M. L., Chiesa, P. A., Cavedo, E., Lemercier, P., et al. (2020). Age and sex impact plasma NFL and t-Tau trajectories in individuals with subjective memory complaints: a 3-year follow-up study. Alzheimer’s Research and Therapy, 12(1), 147. 10.1186/s13195-020-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen, K. J., Thomas, K. R., Weigand, A. J., Edmonds, E. C., Clark, A. L., Solders, S., et al. (2021). Elevated plasma neurofilament light predicts a faster rate of cognitive decline over 5 years in participants with objectively-defined subtle cognitive decline and MCI. Alzheimer’s and Dementia, 17(10), 1756–1762. 10.1002/alz.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, L. L., & Bennett, D. A. (2014). Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Affairs, 33(4), 580–586. 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, L. L., Leurgans, S., Aggarwal, N. T., Shah, R. C., Arvanitakis, Z., James, B. D., et al. (2015). Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology, 85(6), 528–534. 10.1212/WNL.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélemy, N. R., Li, Y., Joseph-Mathurin, N., Gordon, B. A., Hassenstab, J., Benzinger, T. L. S., et al. (2020). A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nature Medicine, 26(3), 398–407. 10.1038/s41591-020-0781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris, L. M., Khrestian, M., Dyne, E., Shao, Y., Pillai, J., Rao, S., et al. (2018). Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. Journal of Neuroimmunology, 319, 19–27. 10.1016/j.jneuroim.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedet, A. L., Milà-Alomà, M., Vrillon, A., Ashton, N. J., Pascoal, T. A., Lussier, F., et al. (2021). Differences between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels across the Alzheimer Disease Continuum. JAMA Neurology, 78(12), 1471–1483. 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi, A., Cantoni, V., Rivolta, J., Archetti, S., Micheli, A., Ashton, N., et al. (2022). Classification accuracy of blood-based and neurophysiological markers in the differential diagnosis of Alzheimer’s disease and frontotemporal lobar degeneration. Alzheimer’s Research and Therapy, 14(1), 155. 10.1186/s13195-022-01094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel, M., An, Y., Walker, K. A., Moghekar, A. R., Ashton, N. J., Kac, P. R., et al. (2023). Longitudinal changes in Alzheimer’s-related plasma biomarkers and brain amyloid. Alzheimer’s and Dementia., 19(10), 4335–4345. 10.1002/alz.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman, A. M., Manly, J. J., Honig, L. S., Sanchez, D., Reyes-Dumeyer, D., Lantigua, R. A., et al. (2021). Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s and Dementia, 17(8), 1353–1364. 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman, A. M., Manly, J. J., Honig, L. S., Sanchez, D., Reyes-Dumeyer, D., Lantigua, R. A., et al. (2022). Correlation of plasma and neuroimaging biomarkers in Alzheimer’s disease. Annals of Clinical and Translational Neurology, 9(5), 756–761. 10.1002/acn3.51529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseron, F., Maass, A., Kleineidam, L., Ravichandran, K. A., Kolbe, C. C., Wolfsgruber, S., et al. (2023). Serum IL-6, sAXL, and YKL-40 as systemic correlates of reduced brain structure and function in Alzheimer’s disease: results from the DELCODE study. Alzheimer’s Research and Therapy, 15(1), 13. 10.1186/s13195-022-01118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, P., Doré, V., Pedrini, S., Krishnadas, N., Thota, R., Bourgeat, P., et al. (2023b). Plasma Glial Fibrillary Acidic Protein Is Associated with 18F-SMBT-1 PET: Two Putative Astrocyte Reactivity Biomarkers for Alzheimer’s Disease. Journal of Alzheimer’s Disease, 92(2), 615–628. 10.3233/JAD-220908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, P., Pedrini, S., Doecke, J. D., Thota, R., Villemagne, V. L., Doré, V., et al. (2023a). Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer’s disease continuum: A cross-sectional and longitudinal study in the AIBL cohort. Alzheimer’s and Dementia, 19(4), 1117–1134. 10.1002/alz.12724. [DOI] [PubMed] [Google Scholar]
- Chatterjee, P., Pedrini, S., Stoops, E., Goozee, K., Villemagne, V. L., Asih, P. R., et al. (2021). Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Translational Psychiatry, 11(1). 10.1038/s41398-020-01137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans, E. M., Verberk, I. M. W., Ossenkoppele, R., Verfaillie, S. C. J., Visser, D., Gouda, M., et al. (2023). A Head-to-Head Comparison Between Plasma pTau181 and Tau PET Along the Alzheimer’s Disease Continuum. Journal of Nuclear Medicine, 64(3), 437–443. 10.2967/jnumed.122.264279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins, K. A. Q., Irwin, D. J., Chen-Plotkin, A., Shaw, L. M., Arezoumandan, S., Lee, E. B., et al. (2023). Plasma GFAP associates with secondary Alzheimer’s pathology in Lewy body disease. Annals of Clinical and Translational Neurology, 10(5), 802–813. 10.1002/acn3.51768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro, R., Perrin, R. J., Roe, C. M., Xiong, C., Carter, D., Cairns, N. J., et al. (2010). YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biological Psychiatry, 68(10), 903–912. 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, N. C., Leuzy, A., Janelidze, S., Palmqvist, S., Svenningsson, A. L., Stomrud, E., et al. (2021). Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nature Communications, 12(1), 3555. 10.1038/s41467-021-23746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J., & Kinney, J. (2022). Biomarkers for Alzheimer’s Disease: Context of Use, Qualification, and Roadmap for Clinical Implementation. Medicina (Kaunas, Lithuania), 58(7), 952. 10.3390/medicina58070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark, H. E., Paterson, C., Daya, G. N., Peng, Z., Duggan, M. R., Bilgel, M., et al. (2023). Proteomic Indicators of Health Predict Alzheimer’s Disease Biomarker Levels and Dementia Risk. Annals of Neurology, 95(2), 260–273. 10.1002/ANA.26817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark, H. E., & Walker, K. A. (2023). New IDEAS about amyloid, race and dementia disparities. Nature Reviews Neurology, 19(1), 5–6. 10.1038/s41582-022-00748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf, F., Ghanbari, M., Licher, S., McRae-McKee, K., Gras, L., Weverling, G. J., et al. (2020). Plasma tau, neurofilament light chain and amyloid-b levels and risk of dementia; a population-based cohort study. Brain, 143(4), 1220–1232. 10.1093/brain/awaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . (2016). BEST (Biomarkers, EndpointS, and other Tools) Resource. Retrieved October 2, 2023, from BEST (Biomarkers, EndpointS, and other Tools) Resource website: https://www.ncbi.nlm.nih.gov/books/NBK326791/ [PubMed]
- Feinkohl, I., Schipke, C. G., Kruppa, J., Menne, F., Winterer, G., Pischon, T., et al. (2020). Plasma Amyloid Concentration in Alzheimer’s Disease: Performance of a High-Throughput Amyloid Assay in Distinguishing Alzheimer’s Disease Cases from Controls. Journal of Alzheimer’s Disease, 74(4), 1285–1294. 10.3233/JAD-200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman, I., West, T., Braunstein, J. B., Verghese, P. B., Kirmess, K. M., Meyer, M. R., et al. (2023). Independent study demonstrates amyloid probability score accurately indicates amyloid pathology. Annals of Clinical and Translational Neurology, 10(5), 765–778. 10.1002/acn3.51763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, B., Ally, M., Brekke, B., Zetterberg, H., Blennow, K., Sugarman, M. A., et al. (2022). Plasma p-tau181 shows stronger network association to Alzheimer’s disease dementia than neurofilament light and total tau. Alzheimer’s and Dementia, 18(8), 1523–1536. 10.1002/alz.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani, L., Blennow, K., Calabresi, P., Di Filippo, M., Parnetti, L., & Zetterberg, H. (2019). Neurofilament light chain as a biomarker in neurological disorders. Journal of Neurology, Neurosurgery and Psychiatry, 90(8), 870–881. 10.1136/jnnp-2018-320106. [DOI] [PubMed] [Google Scholar]
- Gafson, A. R., Barthélemy, N. R., Bomont, P., Carare, R. O., Durham, H. D., Julien, J. P., et al. (2020). Neurofilaments: Neurobiological foundations for biomarker applications. Brain, 143(7), 1975–1998. 10.1093/brain/awaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannisis, A., Al-Grety, A., Carlsson, H., Patra, K., Twohig, D., Sando, S. B., et al. (2022). Plasma apolipoprotein E levels in longitudinally followed patients with mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Research and Therapy, 14(1), 115. 10.1186/s13195-022-01058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, C. E., Norton, D., Zuelsdorff, M., Benton, S. F., Wyman, M. F., Nystrom, N., et al. (2019). Association between enrollment factors and incident cognitive impairment in Blacks and Whites: Data from the Alzheimer’s Disease Center. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 15(12), 1533–1545. 10.1016/j.jalz.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, M. M., Wiedner, C., Wang, C. P., Liu, Q., Bis, J. C., Li, Z., et al. (2022). A population-based meta-analysis of circulating GFAP for cognition and dementia risk. Annals of Clinical and Translational Neurology, 9(10), 1574–1585. 10.1002/acn3.51652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R. C., Roberts, J. S., Cupples, L. A., Relkin, N. R., Whitehouse, P. J., Brown, T., et al. (2009). Disclosure of APOE genotype for risk of Alzheimer’s disease. The New England Journal of Medicine, 361(3), 245–254. 10.1056/NEJMOA0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, J. D., Raman, R., Ernstrom, K., Sultzer, D. L., Burns, J. M., Donohue, M. C., et al. (2020). Short-term Psychological Outcomes of Disclosing Amyloid Imaging Results to Research Participants Who Do Not Have Cognitive Impairment. JAMA Neurology, 77(12), 1504–1513. 10.1001/JAMANEUROL.2020.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, L., & Guo, Z. (2013). Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. Journal of Neurochemistry, 126(3), 305–311. 10.1111/jnc.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y., Shen, X. N., Wang, H. F., Chen, S. D., Zhang, Y. R., Chen, S. F., et al. (2023). The dynamics of plasma biomarkers across the Alzheimer’s continuum. Alzheimer’s Research and Therapy, 15(1), 31. 10.1186/s13195-023-01174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar, I., Yang, Z., Okafor, M., Liu, C., Waligorska, T., Goldstein, F. C., et al. (2022). Association of Plasma and Cerebrospinal Fluid Alzheimer Disease Biomarkers with Race and the Role of Genetic Ancestry, Vascular Comorbidities, and Neighborhood Factors. JAMA Network Open, 5(10), E2235068. 10.1001/jamanetworkopen.2022.35068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. R., Petersen, M., Johnson, L., & O’Bryant, S. E. (2022). Characterizing Plasma Biomarkers of Alzheimer’s in a Diverse Community-Based Cohort: A Cross-Sectional Study of the HAB-HD Cohort. Frontiers in Neurology, 13. 10.3389/fneur.2022.871947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel, H., Hu, Y., Cummings, J., Mattke, S., Iwatsubo, T., Nakamura, A., et al. (2023). Blood-based biomarkers for Alzheimer’s disease: Current state and future use in a transformed global healthcare landscape. Neuron, 111(18), 2781–2799. 10.1016/j.neuron.2023.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, O. (2021). Biomarkers for neurodegenerative diseases. Nature Medicine, 27(6), 954–963. 10.1038/s41591-021-01382-x. [DOI] [PubMed] [Google Scholar]
- Hansson, O., Edelmayer, R. M., Boxer, A. L., Carrillo, M. C., Mielke, M. M., Rabinovici, G. D., et al. (2022). The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s and Dementia, 18(12), 2669–2686. 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, O., Lehmann, S., Otto, M., Zetterberg, H., & Lewczuk, P. (2019). Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimer’s Research and Therapy, 11(1), 1–15. 10.1186/s13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet Neurology, 14(4), 388–405. 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høilund-Carlsen, P. F., Revheim, M. E., Costa, T., Kepp, K. P., Castellani, R. J., Perry, G., et al. (2023). FDG-PET versus Amyloid-PET Imaging for Diagnosis and Response Evaluation in Alzheimer’s Disease: Benefits and Pitfalls. Diagnostics, 13(13). 10.3390/diagnostics13132254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Kirmess, K. M., Meyer, M. R., Rabinovici, G. D., Gatsonis, C., Siegel, B. A., et al. (2022). Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings among Adults with Cognitive Impairment. JAMA Network Open, 5(4), E228392. 10.1001/jamanetworkopen.2022.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s and Dementia, 14(4), 535–562. 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze, S., Barthélemy, N. R., He, Y., Bateman, R. J., & Hansson, O. (2023). Mitigating the Associations of Kidney Dysfunction with Blood Biomarkers of Alzheimer Disease by Using Phosphorylated Tau to Total Tau Ratios. JAMA Neurology, 80(5), 516–522. 10.1001/jamaneurol.2023.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze, S., Stomrud, E., Palmqvist, S., Zetterberg, H., Van Westen, D., Jeromin, A., et al. (2016). Plasma β-amyloid in Alzheimer’s disease and vascular disease. Scientific Reports, 6(1). 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze, S., Teunissen, C. E., Zetterberg, H., Allué, J. A., Sarasa, L., Eichenlaub, U., et al. (2021). Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurology, 78(11), 1375–1382. 10.1001/jamaneurol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisäkk, P., Carlyle, B. C., Sweeney, T., Trombetta, B. A., LaCasse, K., El-Mufti, L., et al. (2023). Plasma biomarkers for diagnosis of Alzheimer’s disease and prediction of cognitive decline in individuals with mild cognitive impairment. Frontiers in Neurology, 14. 10.3389/fneur.2023.1069411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, H. S., & Koh, S. H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration, 9, 42. 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, S., Schraen-Maschke, S., Vidal, J. S., Delaby, C., Blanc, F., Paquet, C., et al. (2023). Plasma phosphorylated tau 181 predicts amyloid status and conversion to dementia stage dependent on renal function. Journal of Neurology, Neurosurgery and Psychiatry, 94(6), 411–419. 10.1136/jnnp-2022-330540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, A. C., Barnes, L. L., Weissberger, G. H., Lamar, M., Nguyen, A. L., Fenton, L., et al. (2023). Quantification of race/ethnicity representation in Alzheimer’s disease neuroimaging research in the USA: a systematic review. Communications Medicine, 3(1), 101. 10.1038/s43856-023-00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. S., Lee, W. J., Wang, S. J., & Fuh, J. L. (2018). Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Scientific Reports, 8(1), 17368. 10.1038/s41598-018-35766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi, M., Su, Y., Ghisays, V., Luo, J., Devadas, V., Chen, Y., et al. (2023). Plasma NfL is associated with the APOE ε4 allele, brain imaging measurements of neurodegeneration, and lower recall memory scores in cognitively unimpaired late-middle-aged and older adults. Alzheimer’s Research and Therapy, 15(1), 74. 10.1186/s13195-023-01221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyavantham, K., Brock, M., Brunelle, L. A., Miller, M., Marble, H., Wilson, D., et al. (2022). Validation of a fully automated lab developed test for plasma phospho-tau 181 levels for Alzheimer’s disease diagnosis. Alzheimer’s & Dementia, 18(S6), e069375. 10.1002/alz.069375. [DOI] [Google Scholar]
- Mattsson-Carlgren, N., Janelidze, S., Palmqvist, S., Cullen, N., Svenningsson, A. L., Strandberg, O., et al. (2021). Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain, 143(11), 3234–3241. 10.1093/BRAIN/AWAA286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, N., Andreasson, U., Zetterberg, H., Blennow, K., Weiner, M. W., Aisen, P., et al. (2017). Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurology, 74(5), 557–566. 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, K. M., & Yeo, G. W. (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s and Dementia, 13(1), 72–83. 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- Mielke, M. M., Dage, J. L., Frank, R. D., Algeciras-Schimnich, A., Knopman, D. S., Lowe, V. J., et al. (2022). Performance of plasma phosphorylated tau 181 and 217 in the community. Nature Medicine, 28(7), 1398–1405. 10.1038/s41591-022-01822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. M., Syrjanen, J. A., Blennow, K., Zetterberg, H., Vemuri, P., Skoog, I., et al. (2019). Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology, 93(3), E252–E260. 10.1212/WNL.0000000000007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milà-Alomà, M., Ashton, N. J., Shekari, M., Salvadó, G., Ortiz-Romero, P., Montoliu-Gaya, L., et al. (2022). Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nature Medicine, 28(9), 1797–1801. 10.1038/s41591-022-01925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, H., Badana, A. N. S., Hwang, S. Y., Sears, J. S., & Haley, W. E. (2019). Dementia Prevalence in Older Adults: Variation by Race/Ethnicity and Immigrant Status. American Journal of Geriatric Psychiatry, 27(3), 241–250. 10.1016/j.jagp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- O’Bryant, S. E., Petersen, M., Hall, J., & Johnson, L. A. (2023). Medical comorbidities and ethnicity impact plasma Alzheimer’s disease biomarkers: Important considerations for clinical trials and practice. Alzheimer’s and Dementia, 19(1), 36–43. 10.1002/alz.12647. [DOI] [PubMed] [Google Scholar]
- O’Bryant, S. E., Zhang, F., Petersen, M., Hall, J., Johnson, L. A., Yaffe, K., et al. (2022). Neurodegeneration from the AT(N) framework is different among Mexican Americans compared to non-Hispanic Whites: A Health & Aging Brain among Latino Elders (HABLE) Study. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 14(1), e12267. 10.1002/dad2.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais, M. V., Forlenza, O. V., & Diniz, B. S. (2023). Plasma Biomarkers of Alzheimer’s Disease: A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. Journal of Alzheimer’s Disease Reports, 7(1), 355–380. 10.3233/adr-230029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, S., Insel, P. S., Stomrud, E., Janelidze, S., Zetterberg, H., Brix, B., et al. (2019). Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Molecular Medicine, 11(12), e11170. 10.15252/emmm.201911170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, S., Janelidze, S., Quiroz, Y. T., Zetterberg, H., Lopera, F., Stomrud, E., et al. (2020). Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA-Journal of the American Medical Association, 324(8), 772–781. 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, S., Stomrud, E., Cullen, N., Janelidze, S., Manuilova, E., Jethwa, A., et al. (2023). An accurate fully automated panel of plasma biomarkers for Alzheimer’s disease. Alzheimer’s and Dementia, 19(4), 1204–1215. 10.1002/alz.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. H., Lee, E. H., Kim, H. J., Jo, S., Lee, S., Seo, S. W., et al. (2021). The relationship of soluble TREM2 to other biomarkers of sporadic Alzheimer’s disease. Scientific Reports, 11(1), 13050. 10.1038/s41598-021-92101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase, M. P., Himali, J. J., Bis, J. C., Beiser, A. S., Satizabal, C. L., Aparicio, H. J., et al. (2020). Plasma YKL40 as a biomarker for brain aging and injury in three community cohorts. Alzheimer’s & Dementia, 16(S5), e042094. 10.1002/alz.042094. [DOI] [Google Scholar]
- Pereira, J. B., Janelidze, S., Smith, R., Mattsson-Carlgren, N., Palmqvist, S., Teunissen, C. E., et al. (2021). Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain, 144(11), 3505–3516. 10.1093/brain/awab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirella, S., Van Neste, L., Fowler, C., Masters, C. L., Fripp, J., Doecke, J. D., et al. (2022). A Conformational Variant of p 53 (U-p53AZ) as Blood-Based Biomarker for the Prediction of the Onset of Symptomatic Alzheimer’s Disease. Journal of Prevention of Alzheimer’s Disease, 9(3), 469–479. 10.14283/jpad.2022.52. [DOI] [PubMed] [Google Scholar]
- Rajan, K. B., Aggarwal, N. T., McAninch, E. A., Weuve, J., Barnes, L. L., Wilson, R. S., et al. (2020). Remote Blood Biomarkers of Longitudinal Cognitive Outcomes in a Population Study. Annals of Neurology, 88(6), 1065–1076. 10.1002/ana.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, R., Quiroz, Y. T., Langford, O., Choi, J., Ritchie, M., Baumgartner, M., et al. (2021). Disparities by Race and Ethnicity Among Adults Recruited for a Preclinical Alzheimer Disease Trial. JAMA Network Open, 4(7), e2114364. 10.1001/jamanetworkopen.2021.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan, V. K., Graff-Radford, J., Syrjanen, J., Shir, D., Algeciras-Schimnich, A., Lucas, J., et al. (2023). Association of Plasma Biomarkers of Alzheimer Disease With Cognition and Medical Comorbidities in a Biracial Cohort. Neurology, 101(14). 10.1212/WNL.0000000000207675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, M. B. (2023). Direct-to-Consumer Alzheimer’s Blood Test Opens Pandora’s Box ALZFORUM. Retrieved October 9, 2023, from AlzForum website: https://www.alzforum.org/news/community-news/direct-consumer-alzheimers-blood-test-opens-pandoras-box
- Salami, A., Adolfsson, R., Andersson, M., Blennow, K., Lundquist, A., Adolfsson, A. N., et al. (2022). Association of APOE ϵ4 and Plasma p-tau181 with Preclinical Alzheimer’s Disease and Longitudinal Change in Hippocampus Function. Journal of Alzheimer’s Disease, 85(3), 1309–1320. 10.3233/JAD-210673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner, R., VandeVrede, L., Asken, B. M., Paolillo, E. W., Gontrum, E. Q., Wolf, A., et al. (2023). Plasma phosphorylated tau-217 exhibits sex-specific prognostication of cognitive decline and brain atrophy in cognitively unimpaired adults. Alzheimer’s and Dementia., 20(1), 376–387. 10.1002/alz.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, S. E., Bollinger, J. G., Ovod, V., Mawuenyega, K. G., Li, Y., Gordon, B. A., et al. (2019). High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology, 93(17), E1647–E1659. 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, S. E., Karikari, T. K., Ashton, N. J., Henson, R. L., Yarasheski, K. E., West, T., et al. (2022). Effect of Race on Prediction of Brain Amyloidosis by Plasma Aβ42/Aβ40, Phosphorylated Tau, and Neurofilament Light. Neurology, 99(3), E245–E257. 10.1212/WNL.0000000000200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea, D., Colasurdo, E., Smith, A., Paschall, C., Jayadev, S., Keene, C. D., et al. (2022). SOBA: Development and testing of a soluble oligomer binding assay for detection of amyloidogenic toxic oligomers. Proceedings of the National Academy of Sciences of the United States of America, 119(50), e2213157119. 10.1073/pnas.2213157119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Spínola, A., Lima, M., Leitão, M. J., Bernardes, C., Durães, J., Duro, D., et al. (2023). Blood biomarkers in mild cognitive impairment patients: Relationship between analytes and progression to Alzheimer disease dementia. European Journal of Neurology, 30(6), 1565–1573. 10.1111/ene.15762. [DOI] [PubMed] [Google Scholar]
- Simrén, J., Leuzy, A., Karikari, T. K., Hye, A., Benedet, A. L., Lantero-Rodriguez, J., et al. (2021). The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimer’s and Dementia, 17(7), 1145–1156. 10.1002/alz.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren, M., Rosengren, L., Minthon, L., Davidsson, P., Blennow, K., & Wallin, A. (2000). Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology, 54(10), 1960–1964. 10.1212/WNL.54.10.1960. [DOI] [PubMed] [Google Scholar]
- Smirnov, D. S., Ashton, N. J., Blennow, K., Zetterberg, H., Simrén, J., Lantero-Rodriguez, J., et al. (2022). Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathologica, 143(4), 487–503. 10.1007/s00401-022-02408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman, A., Ekblad, L. L., Ashton, N. J., Karikari, T. K., Lantero-Rodriguez, J., Pietilä, E., et al. (2023). Head-to-head comparison of plasma p-tau181, p-tau231 and glial fibrillary acidic protein in clinically unimpaired elderly with three levels of APOE4-related risk for Alzheimer’s disease. Neurobiology of Disease, 183, 106175. 10.1016/j.nbd.2023.106175. [DOI] [PubMed] [Google Scholar]
- Španić Popovački, E., Babić Leko, M., Langer Horvat, L., Brgić, K., Vogrinc, Ž., Boban, M., et al. (2023). Soluble TREM2 Concentrations in the Cerebrospinal Fluid Correlate with the Severity of Neurofibrillary Degeneration, Cognitive Impairment, and Inflammasome Activation in Alzheimer’s Disease. Neurology International, 15(3), 842–856. 10.3390/neurolint15030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therriault, J., Servaes, S., Tissot, C., Rahmouni, N., Ashton, N. J., Benedet, A. L., et al. (2023). Equivalence of plasma p-tau217 with cerebrospinal fluid in the diagnosis of Alzheimer’s disease. Alzheimer’s and Dementia., 19(11), 4967–4977. 10.1002/alz.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen, E. H., La Joie, R., Strom, A., Fonseca, C., Iaccarino, L., Wolf, A., et al. (2021a). Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. The Lancet Neurology, 20(9), 739–752. 10.1016/S1474-4422(21)00214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen, E. H., Verberk, I. M. W., Kindermans, J., Abramian, A., Vanbrabant, J., Ball, A. J., et al. (2022). Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 14(1), e12285. 10.1002/dad2.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen, E. H., Verberk, I. M. W., Vanbrabant, J., Koelewijn, A., Heijst, H., Scheltens, P., et al. (2021b). Highly specific and ultrasensitive plasma test detects Abeta(1–42) and Abeta(1–40) in Alzheimer’s disease. Scientific Reports, 11(1), 9736. 10.1038/s41598-021-89004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R., Bangen, K. J., Edmonds, E. C., Weigand, A. J., Walker, K. S., Bondi, M. W., et al. (2021). Objective subtle cognitive decline and plasma phosphorylated tau181: Early markers of Alzheimer’s disease-related declines. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 13(1), e12238. 10.1002/dad2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin, A., Sullivan, K. J., Walker, K. A., Bressler, J., Talluri, R., Yu, B., et al. (2023). Proteomic Analysis Identifies Circulating Proteins Associated With Plasma Amyloid-β and Incident Dementia. Biological Psychiatry Global Open Science, 3(3), 490–499. 10.1016/j.bpsgos.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant, V. A., Lee, H., Hadigan, C., & Grinspoon, S. K. (2007). Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of Clinical Endocrinology and Metabolism, 92(7), 2506–2512. 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, H. H., Chen, Y. F., Yen, R. F., Lo, Y. L., Yang, K. C., Jeng, J. S., et al. (2021). Plasma soluble TREM2 is associated with white matter lesions independent of amyloid and tau. Brain, 144(11), 3371–3380. 10.1093/brain/awab332. [DOI] [PubMed] [Google Scholar]
- Tsiknia, A. A., Edland, S. D., Sundermann, E. E., Reas, E. T., Brewer, J. B., Galasko, D., et al. (2022). Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Molecular Psychiatry, 27(10), 4314–4322. 10.1038/s41380-022-01675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergallo, A., Lista, S., Lemercier, P., Chiesa, P. A., Zetterberg, H., Blennow, K., et al. (2020). Association of plasma YKL-40 with brain amyloid-β levels, memory performance, and sex in subjective memory complainers. Neurobiology of Aging, 96, 22–32. 10.1016/j.neurobiolaging.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Villar-Piqué, A., Schmitz, M., Hermann, P., Goebel, S., Bunck, T., Varges, D., et al. (2019). Plasma YKL-40 in the spectrum of neurodegenerative dementia. Journal of Neuroinflammation, 16(1), 145. 10.1186/s12974-019-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, K. A., Le Page, L. M., Terrando, N., Duggan, M. R., Heneka, M. T., & Bettcher, B. M. (2023). The role of peripheral inflammatory insults in Alzheimer’s disease: a review and research roadmap. Molecular Neurodegeneration, 18(1), 37. 10.1186/s13024-023-00627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, D. M., Kim, J. C., Goldman, S., Racke, M. K., & Clarke, N. J. (2022). A new LC-MS/MS assay for the quantification of Aβ40 and Aβ42 in plasma: validation and clinical performance. Alzheimer’s & Dementia, 18(S6), e064182. 10.1002/alz.064182. [DOI] [Google Scholar]
- Weiner, M. W., Veitch, D. P., Miller, M. J., Aisen, P. S., Albala, B., Beckett, L. A., et al. (2023). Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimer’s and Dementia, 19(1), 307–317. 10.1002/alz.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennström, M., Surova, Y., Hall, S., Nilsson, C., Minthon, L., Hansson, O., et al. (2015). The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PLoS One, 10(8), e0135458. 10.1371/journal.pone.0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, T., Kirmess, K. M., Meyer, M. R., Holubasch, M. S., Knapik, S. S., Hu, Y., et al. (2021). A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Molecular Neurodegeneration, 16(1), 30. 10.1186/s13024-021-00451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczyńska, K., Maciejczyk, M., Zalewska, A., & Waszkiewicz, N. (2021). Serum Amyloid Biomarkers, Tau Protein and YKL-40 Utility in Detection, Differential Diagnosing, and Monitoring of Dementia. Frontiers in Psychiatry, 12. 10.3389/fpsyt.2021.725511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, C. H., Windon, C. C., Dilworth-Anderson, P., Romanoff, J., Gatsonis, C., Hanna, L., et al. (2022). Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia: A Secondary Analysis of the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) Cohort Study. JAMA Neurology., 79(11), 1139–1147. 10.1001/JAMANEUROL.2022.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windon, C., Iaccarino, L., Mundada, N., Allen, I., Boxer, A. L., Byrd, D., et al. (2022). Comparison of plasma and CSF biomarkers across ethnoracial groups in the ADNI. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 14(1), e12315. 10.1002/dad2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdała, A. L., Bellomo, G., Toja, A., Gaetani, L., Parnetti, L., & Chiasserini, D. (2023). CSF and plasma Aβ42/40 across Alzheimer’s disease continuum: Comparison of two ultrasensitive Simoa®assays targeting distinct amyloid regions. Clinical Chemistry and Laboratory Medicine., 62(2), 332–340. 10.1515/cclm-2023-0659. [DOI] [PubMed] [Google Scholar]
- Xue, F., & Du, H. (2021). TREM2 mediates microglial anti-inflammatory activations in Alzheimer’s disease: Lessons learned from transcriptomics. Cell, 10(2), 1–17. 10.3390/cells10020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., Zhang, C., Wang, Y. Y., Chen, L. A., Qiao, Y. N., Wang, Y., et al. (2023). Effect of renal function on the diagnostic performance of plasma biomarkers for Alzheimer’s disease. Frontiers in Aging Neuroscience, 15. 10.3389/fnagi.2023.1150510. [DOI] [PMC free article] [PubMed] [Google Scholar]