Abstract
Postpartum readmissions (PPRs) represent a critical marker of maternal morbidity after hospital childbirth. Most severe maternal morbidity (SMM) events result in a hospital admission, but most PPRs do not have evidence of SMM. Little is known about PPR and SMM beyond the first 6 weeks postpartum. We examined the associations of maternal demographic and clinical factors with PPR within 12 months postpartum. We categorized PPR as being with or without evidence of SMM to assess whether risk factors and timing differed. Using the Oregon All Payer All Claims database, we analyzed hospital births from 2012–2017. We used log-binomial regression to estimate associations between maternal factors and PPR. Our final analytical sample included 158,653 births. Overall, 2.6% (n = 4,141) of births involved at least 1 readmission within 12 months postpartum (808 (19.5% of PPRs) with SMM). SMM at delivery was the strongest risk factor for PPR with SMM (risk ratio (RR) = 5.55, 95% confidence interval (CI): 4.14, 7.44). PPR without SMM had numerous risk factors, including any mental health diagnosis (RR = 2.10, 95% CI: 1.91, 2.30), chronic hypertension (RR = 2.17, 95% CI: 1.85, 2.55), and prepregnancy diabetes (RR = 2.85, 95% CI: 2.47, 3.30), all which were on par with SMM at delivery (RR = 1.89, 95% CI: 1.49, 2.40).
Keywords: maternal morbidity, postpartum readmissions
Abbreviations
- APAC
All Payer All Claims
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, Tenth Revision
- PPR
postpartum readmission
- RR
risk ratio
- SMM
severe maternal morbidity
Maternal mortality is a critical marker of population health (1). To address the maternal mortality crisis in the United States (2, 3), researchers and policy-makers have refocused on a critical and understudied driver of maternal mortality—severe maternal morbidity (SMM). SMM is a sentinel event defined by a composite index of acutely severe health events that indicate a serious maternal health condition, such as acute respiratory distress syndrome or acute renal failure (4). SMM can occur prenatally, but most SMM events occur during or after birth (5). Approximately 50% of maternal deaths (6) and 15% of SMM (7) happen in the postpartum period, making postpartum health and care a national priority (8, 9).
Postpartum readmission (PPR) represents an important but understudied marker of SMM and other forms of maternal morbidity. Nearly all SMM events arising postpartum result in hospital readmission (10, 11). However, PPRs without SMM, which occur more frequently than PPRs with SMM, indicate medical complexity and may represent dimensions of maternal morbidity that have not yet been characterized (12).
Numbers of PPRs occurring within the first 6 weeks postpartum have increased steadily in the United States (12) and are disproportionately concentrated within low-income populations. Medicaid recipients are up to 24% more likely to be readmitted and to experience SMM during readmission (12–14). However, we lack detailed information on PPR and its drivers. Previous studies of PPR have been limited by being conducted in single care settings (inpatient data as opposed to outpatient or emergency department data) (15) or having follow-up within a single calendar year (12, 14–19), or being carried out in a single-payer (e.g., Medicaid) (20, 21) or single-hospital (22) system. Evaluating PPR beyond the first 6 weeks postpartum is essential: 25% of maternal mortality occurs outside the traditional 6-week postpartum window (6).
While it is well established that maternal morbidity and mortality occur throughout the first year postpartum, little is known about maternal morbidity that occurs after the first 6 weeks postpartum (9, 10). However, knowledge is sorely lacking regarding PPR during the entire first year postpartum, including timing and drivers of SMM and PPR that results from other maternal factors (whether physical health conditions, mental health conditions, or social factors). It is possible that PPR without evidence of SMM has overlapping risk factors besides those for PPR with SMM, or that the drivers might differ. We do know that PPR without SMM is more prevalent than PPR with SMM (23, 24) and is associated with nonclinical risk factors, such as access to preventive care through expanded access to insurance coverage (25, 26). An improved understanding of who is most likely to experience PPR, both with and without SMM, would help in identifying interventions to improve maternal health. A deeper understanding of when PPR occurs up to 1 year postpartum can also inform recommendations for clinical care, policy (e.g., insurance coverage policy), and other social supports that people may require after experiencing pregnancy.
We assessed and compared the associations of demographic, clinical, and health-insurance–related factors with PPR, by SMM status and timing of PPR. We used Oregon’s All Payer All Claims (APAC) database, which included continuous enrollment of all individuals in the state across calendar years (including prenatal, delivery, and postpartum health care) in all care settings, regardless of type of health insurance. We hypothesized that factors associated with PPR would differ depending on the presence or absence of SMM and would differ according to the timing of PPR in the year after childbirth.
METHODS
Data source
We used Oregon APAC data from 2011–2018 (27). The APAC Reporting Program collects medical and pharmacy claims, insurance enrollment data, and demographic information from commercial health insurance plans that cover at least 5,000 people and Medicaid across the state of Oregon. Each person has a unique, deidentified person key allowing linkage of medical claims, demographic information, and enrollment data. Overall, APAC covers 3.4–3.9 million people per year, approximately 87%–98% of the Oregon population (28).
The study was approved by the institutional review board of Oregon Health & Science University. To avoid loss of privacy or confidentiality, we suppressed cell sizes of less than 11 cases.
Study population
Our analytical population included persons between 15 and 44 years of age with evidence of a hospital live birth in medical claims data. Births were identified using a previously published algorithm (29) and codes identified by the American College of Obstetricians and Gynecologists (30, 31). We included deliveries occurring from January 1, 2012, to December 31, 2017. Our inclusion criteria allowed for 12 months of data prior to delivery in order to identify comorbidity in the prenatal and preconception periods. We did not require continuous enrollment in the prenatal period.
The follow-up period for our analyses was 12 months postpartum. In order to not equate lack of health insurance enrollment (resulting in no recorded health-care claims) with the absence of PPR in the follow-up period, we further restricted our analytical population to individuals with at least 11 months of insurance enrollment postpartum. The insurance enrollment data were reported on the person-key, health insurance type (Medicaid or commercial insurance), and calendar month level. We required at least 11 of 12 calendar months of any type of insurance enrollment postpartum. Our criteria allowed people to have either commercial insurance, Medicaid coverage, or a mix of insurance plans in the postpartum period. This represents a distinct advantage of an all-payers database over single-payer databases, given that insurance discontinuities and changes are very common during the postpartum period in the United States (26, 32). We conducted a sensitivity analysis to examine how the deliveries excluded due to lack of insurance enrollment differed from the final analytical population.
Study variables: outcomes
Our primary outcome was PPR, which we identified using a quality metric method from the Oregon Health Authority. The method includes a wide range of metrics, including hospital readmissions (33). We were primarily interested in de novo readmissions separate from admissions directly related to the index childbirth. Therefore, we did not classify inpatient stays during the follow-up period for a transfer of the index birth to another hospital or a subsequent birth as a readmission. To ensure that we did not capture transfers as readmissions, we required at least 1 full day between the index birth and the subsequent readmission. Our outcome measure included inpatient readmissions occurring from 1 day to 1 year after delivery discharge and was based on the hospital admission date. While it is possible for birthing people to be readmitted multiple times to a hospital, we only considered the first readmission in our outcome measure.
We were also interested in how PPR may differ according to the presence or absence of SMM diagnosed at readmission. Therefore, we classified each readmission as with or without evidence of SMM. We identified SMM at readmission as containing at least 1 of the International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnoses or procedure codes for SMM as defined by the Centers for Disease Control and Prevention (CDC) (34). Knowledge about which SMM-defining conditions are most common at delivery hospitalization versus at readmission is currently lacking. Therefore, we assessed the frequency of all of the specific SMM indicators at childbirth hospital admissions as compared with PPRs. The 21 comorbidity indicators included in the CDC definition are not mutually exclusive, meaning that 1 hospital encounter could have more than 1 SMM indicator. In accordance with the CDC index, the measure most commonly used in SMM surveillance, we included blood transfusion in our definition of SMM. The transfusion of blood products is commonly considered a proxy measure for obstetrical hemorrhage but can be indicated for non–SMM-defining conditions.
To understand whether drivers of PPR within the traditional postpartum period (i.e., 6 weeks after childbirth) differed from drivers of PPR after the traditional postpartum period, we further classified PPR by timing. Our timing categories included 1) the traditional postpartum period (first 6 weeks after hospital delivery) and 2) the extended postpartum period (after the traditional postpartum period and up to 12 months postpartum (7–52 weeks)). For analyses, we categorized outcome measures by both the presence of SMM and the timing of PPR. For example, for PPR with evidence of SMM, the outcome measure had 3 mutually exclusive categories: no readmissions (reference), PPR with evidence of SMM in the traditional postpartum period (≤6 weeks), and PPR with evidence of SMM in the extended postpartum period (7–52 weeks). Among birthing people with multiple PPRs, we classified their PPR as occurring in the traditional postpartum period if the first PPR occurred during the first 6 weeks postpartum.
Study variables: predictors of PPR and SMM
Many factors are potentially associated with PPR, with and without SMM. To assess these associations, we included demographic factors, clinical factors, and type of health insurance as covariates based on our conceptual model and data availability. Demographic factors included maternal age (15–19, 20–24, 25–29, 30–34, or ≥40 years) and maternal residential rurality. A rurality indicator was based on Rural-Urban Commuting Area Code categories assigned to residential zip codes (35). If information on maternal residential rurality was missing, we classified the value as missing and did not drop the observation from our analysis. In other words, residence was coded as one of 3 categories: urban, rural, or missing. Zip code was missing for 3 observations in our database. In our database, information on maternal race/ethnicity was missing for nearly 50% of births. Given the high rate of missing data, we did not include maternal race/ethnicity in our analyses. In addition, we included type of health insurance (Medicaid or commercial) at birth. To obtain information on insurance type at birth, we linked the enrollment calendar month to the calendar birth month. In rare cases, people did have both Medicaid and commercial insurance during the birth month. In those instances, we prioritized Medicaid insurance.
Delivery-related covariates included mode of delivery (cesarean vs. vaginal birth), extended delivery length of stay (categorized as lengths of stay >90th percentile by mode of delivery) (36), and SMM at delivery. We included SMM at delivery as a covariate given the limited and emerging research on repeat SMM rehospitalization (23). We identified SMM at delivery using the same method as that noted above.
Finally, we examined the following morbid conditions: prepregnancy diabetes, gestational diabetes, hypertensive disorders of pregnancy (chronic hypertension, gestational hypertension, eclampsia/preeclampsia, and preeclampsia superimposed on chronic hypertension), substance use disorders, and mental health diagnoses. All comorbid conditions were identified as the presence of 1 or more ICD-9/ICD-10 diagnosis codes from 12 months prior to delivery admission through the delivery discharge date. The diagnosis codes could appear on any health-care encounter in our database, including outpatient and inpatient encounters. The relevant codes for each comorbid condition are listed in Web Table 1 (available at https://doi.org/10.1093/aje/kwac183). For mental health diagnoses, we created a binary indicator for any mental health condition. In addition, we created binary indicators for mental health diagnosis categories (depression (major depressive disorder classified under serious mental illness), anxiety, serious mental illness, or other).
Statistical analysis
We used multiple approaches to estimate associations between maternal factors and PPR, with and without SMM. First, we conducted bivariate analyses of each covariate by readmission status (any readmission within 12 months postpartum as compared with no readmission) and SMM status (readmission with SMM and readmission without SMM).
Next, we compared the frequency of SMM-defining conditions during birth hospitalizations and during readmissions. We used standardized differences to evaluate the comparability of each SMM comorbidity indicator during the birth hospitalization and during readmissions (37). Standardized differences are independent of sample size and represent a measure of the mean difference for a given covariate between 2 groups. We report the standardized differences as absolute values. To maintain subjects’ confidentiality, we do not report the count or percentage or calculate the standardized difference for any SMM comorbidity indicator with a cell size less than 11.
To estimate the unadjusted cumulative incidence ratio (risk ratio (RR)) for the association between each factor and our primary outcome (PPR with and without SMM), we utilized log-binomial regression to estimate the multivariable adjusted RR for each predictor. We identified potential confounders for each predictor of PPR and SMM through the process of creating causal diagrams (directed acyclic graphs) (38). This process of confounder identification is based on prespecified assumptions and a priori knowledge about how variables are connected and the temporal relationships between them (39). Therefore, the multivariable model for each predictor is parsimonious and only contains confounders specific to the individual predictor being studied, as identified by our directed acyclic graphs.
Many of our predictors have complex causal associations with PPR and SMM that have yet to be fully explored in the scientific literature. For instance, cesarean delivery is most commonly considered a mediator (and thus should generally not be adjusted for), and in recent work investigators have studied the mediating role of cesarean delivery as it relates to the outcome of SMM (40). This framework applies when considering a prenatal exposure (e.g., body mass index) and postpartum outcome. It underlies our decision to refrain from controlling for cesarean birth in most models, given that a majority of our predictors also precede birth (e.g., insurance type, maternal residential location, hypertensive disorders of pregnancy, diabetes, substance use disorder, and mental health conditions). The exception is extended delivery length of stay, which occurs after birth. For this variable, cesarean birth is plausibly a confounder, and thus we controlled for it.
In addition to PPR with and without SMM (dichotomous outcome measures), we assessed the timing of PPR by creating categorical outcome variables differentiating PPR within the traditional postpartum period (≤6 weeks postpartum) and PPR in the extended postpartum period (7–52 weeks postpartum). Given that our outcome variables for assessment of PPR timing were categorical rather than dichotomous, we conducted log multinomial regression (41). This method is an adaption of the log-binomial regression model for categorical outcome measures. We adjusted each factor for the same confounders as those included in the multivariate log-binomial regression models described above. We report the adjusted RRs and 95% confidence intervals (CIs) for all models.
To understand the impact of our a priori health insurance enrollment inclusion criterion (at least 11 months of postpartum insurance enrollment), we conducted a bivariate analysis comparing the excluded population with the final analytical sample. We compared demographic characteristics, clinical factors, and health insurance type across groups using Pearson’s χ2 test. Finally, we assessed the robustness of our results by conducting 2 sensitivity analyses. First, we assessed whether shortening our continuous enrollment criterion to 60 days and assessing readmissions within 60 days meaningfully changed any associations. Next, we removed transfusion of blood products from our SMM definition. Blood transfusion by itself has the lowest clinical validity as a marker for SMM (36, 41). The transfusion of blood products is often used as a proxy measure for obstetrical hemorrhage, but blood transfusion could be indicated for a variety of non–SMM-defining conditions, such as chronic anemia or hemoglobinopathy without crisis. We assessed whether the associations between covariates and PPR with and without evidence of SMM changed when we removed transfusion of blood products from the SMM definition both at birth and during PPR. All other modeling specifications remained the same as in our primary modeling approach.
For statistical comparisons, we used 2-sided tests with a 0.05 α level. All analyses were completed with R statistical software, version 4.01 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 238,329 Oregon hospital births from the period 2012–2017. We excluded 846 births (0.4%) because of implausible interdelivery intervals, 28 births (0.01%) that did not meet our maternal age criterion (15–44 years), and 43,592 births (18.3%) that did not meet our continuous postpartum insurance enrollment criterion, leaving 158,653 hospital births for analysis (Figure 1). Overall, birthing people in 2.6% (n = 4,141) of births had at least 1 PPR within 12 months (Table 1). Deliveries with any PPR were more likely to have Medicaid insurance at delivery (73.0% vs. 59.2%; P < 0.001), cesarean delivery (36.8% vs. 28.2%; P < 0.001), any hypertensive disorder of pregnancy (17.3% vs. 9.5%; P < 0.001), any SUD diagnosis (13.0% vs. 5.9%; P < 0.001), and any mental health diagnosis (14.0% vs. 7.3%; P < 0.001).
Figure 1.
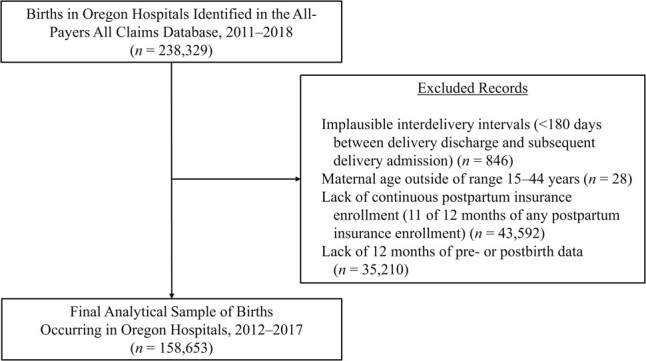
Selection of participants for a study of postpartum readmissions among Oregon hospital births, 2012–2017.
Table 1.
Maternal Characteristics for Any Postpartum Readmission and Severe Maternal Morbidity Status of Births With a Readmission Among Oregon Hospital Births (n = 158,653), 2012–2017
| PPR Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any PPR | ||||||||||
|
Total
(n = 158,653) |
No PPR
(n = 154,512) |
All PPRs
(n = 4,141) |
Evidence of SMM
(n = 808) |
No Evidence of SMM
(n = 3,333) |
||||||
| Characteristic | No. | % | No. | % | No. | % | No. | % | No. | % |
| Maternal age, years | ||||||||||
| 15–19 | 9,853 | 6.2 | 9,468 | 6.1 | 385 | 9.3 | 64 | 7.9 | 321 | 9.6 |
| 20–24 | 34,417 | 21.7 | 33,437 | 21.6 | 980 | 23.7 | 165 | 20.4 | 815 | 24.5 |
| 25–29 | 45,085 | 28.4 | 43,923 | 28.4 | 1,162 | 28.1 | 233 | 28.8 | 929 | 27.9 |
| 30–34 | 43,043 | 27.1 | 42,086 | 27.2 | 957 | 23.1 | 190 | 23.5 | 767 | 23.0 |
| 35–39 | 21,460 | 13.5 | 20,935 | 13.5 | 525 | 12.7 | 123 | 15.2 | 402 | 12.1 |
| ≥40 | 4,795 | 3.0 | 4,663 | 3.0 | 132 | 3.2 | 33 | 4.1 | 99 | 3.0 |
| Maternal residential location | ||||||||||
| Urban area | 103,353 | 65.1 | 100,742 | 65.2 | 2,611 | 63.1 | 502 | 62.1 | 2,109 | 63.3 |
| Rural area | 55,297 | 34.9 | 53,767 | 34.8 | 1,530 | 36.9 | 306 | 37.9 | 1,224 | 36.7 |
| Missing data | 3 | 0.0 | 3 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Health insurance type at delivery | ||||||||||
| Commercial | 64,165 | 40.4 | 63,048 | 40.8 | 1,117 | 27.0 | 198 | 24.5 | 919 | 27.6 |
| Medicaid | 94,488 | 59.6 | 91,464 | 59.2 | 3,024 | 73.0 | 610 | 75.5 | 2,414 | 72.4 |
| Mode of delivery | ||||||||||
| Vaginal | 113,496 | 71.5 | 110,880 | 71.8 | 2,616 | 63.2 | 490 | 60.6 | 2,126 | 63.8 |
| Cesarean | 45,157 | 28.5 | 43,632 | 28.2 | 1,525 | 36.8 | 318 | 39.4 | 1,207 | 36.2 |
| Extended delivery length of staya | 26,344 | 16.6 | 25,202 | 16.3 | 1,142 | 27.6 | 253 | 31.3 | 889 | 26.7 |
| SMM at delivery | 1,365 | 0.9 | 1,251 | 0.8 | 114 | 2.8 | 48 | 5.9 | 66 | 2.0 |
| Prepregnancy diabetes | 3,380 | 2.1 | 3,145 | 2.0 | 235 | 5.7 | 47 | 5.8 | 188 | 5.6 |
| Gestational diabetes | 16,577 | 10.4 | 16,046 | 10.4 | 531 | 12.8 | 89 | 11.0 | 442 | 13.3 |
| Any hypertensive disorder of pregnancy | 15,459 | 9.7 | 14,744 | 9.5 | 715 | 17.3 | 159 | 19.7 | 556 | 16.7 |
| Hypertensive disorders of pregnancy | ||||||||||
| Chronic hypertension | 3,676 | 2.3 | 3,478 | 2.3 | 198 | 4.8 | 46 | 5.7 | 152 | 4.6 |
| Gestational hypertension | 6,315 | 4.0 | 6,106 | 4.0 | 209 | 5.0 | 50 | 6.2 | 159 | 4.8 |
| Eclampsia/preeclampsia | 3,416 | 2.2 | 3,237 | 2.1 | 179 | 4.3 | 45 | 5.6 | 134 | 4.0 |
| Superimposedb | 2,052 | 1.3 | 1,923 | 1.2 | 129 | 3.1 | 18 | 2.2 | 111 | 3.3 |
| Substance use disorder | ||||||||||
| Any disorder | 9,686 | 6.1 | 9,146 | 5.9 | 540 | 13.0 | 122 | 15.1 | 418 | 12.5 |
| Any disorder (excluding cannabis) | 8,200 | 5.2 | 7,716 | 5.0 | 484 | 11.7 | 111 | 13.7 | 373 | 11.2 |
| Mental health diagnoses | ||||||||||
| Any diagnosis | 11,877 | 7.5 | 11,297 | 7.3 | 580 | 14.0 | 86 | 10.6 | 494 | 14.8 |
| Depression | 2,356 | 1.5 | 2,224 | 1.4 | 132 | 3.2 | 24 | 3.0 | 108 | 3.2 |
| Anxiety | 5,347 | 3.4 | 5,085 | 3.3 | 262 | 6.3 | 39 | 4.8 | 223 | 6.7 |
| Serious mental illness | 4,786 | 3.0 | 4,523 | 2.9 | 263 | 6.4 | 30 | 3.7 | 233 | 7.0 |
| Other | 2,414 | 1.5 | 2,252 | 1.5 | 162 | 3.9 | 20 | 2.5 | 142 | 4.3 |
Among readmissions, 19.5% (n = 808) had evidence of SMM. Among PPRs with evidence of SMM (Table 2), the top indicators included sepsis (41.8%), acute renal failure (11.1%), adult respiratory distress syndrome (11.3%), and transfusion of blood products (9.3%). In contrast, the top SMM indicators during delivery were transfusion of blood products (42.1%), eclampsia (15.0%), and disseminated intravascular coagulation (14.8%). Of the top indicators, the standardized difference effect sizes indicate nonnegligible differences in prevalences between SMM diagnosed at childbirth hospitalization and SMM diagnosed at PPR, with the exception of acute renal failure.
Table 2.
Indicatorsa of Severe Maternal Morbidity (CDC Definition) During Delivery Hospitalization and Postpartum Readmission for Oregon Hospital Births (n = 158,653), 2012–2017
|
Births With
Evidence of SMM (n = 1,365) |
Postpartum Readmission
With Evidence of SMM (n = 808) |
||||
|---|---|---|---|---|---|
| SMM Indicator | No. | % | No. | % |
Standardized
Difference b |
| Acute myocardial infarction | <11 | 22 | 2.7 | ||
| Aneurysm | <11 | <11 | |||
| Acute renal failure | 119 | 8.7 | 90 | 11.1 | 8.1 |
| Adult respiratory distress syndrome | 93 | 6.8 | 91 | 11.3 | 15.6 |
| Amniotic fluid embolism | <11 | <11 | |||
| Cardiac arrest/ventricular fibrillation | <11 | <11 | |||
| Conversion of cardiac rhythm | <11 | <11 | |||
| Disseminated intravascular coagulation | 202 | 14.8 | 20 | 2.5 | 45.0 |
| Eclampsia | 205 | 15.0 | 49 | 6.1 | 29.5 |
| Heart failure/arrest during surgery or procedure | 11 | 0.8 | <11 | ||
| Puerperal cerebrovascular disorders | 33 | 2.4 | 55 | 6.8 | 21.0 |
| Pulmonary edema/acute heart failure | 35 | 2.6 | 70 | 8.7 | 26.7 |
| Severe anesthesia complications | 14 | 1.0 | <11 | ||
| Sepsis | 74 | 5.4 | 338 | 41.8 | 94.9 |
| Shock | 100 | 7.3 | 63 | 7.8 | 1.8 |
| Sickle cell disease with crisis | <11 | <11 | |||
| Air and thrombotic embolism | 31 | 2.3 | 71 | 8.8 | 28.8 |
| Blood products transfusion | 575 | 42.1 | 75 | 9.3 | 81.1 |
| Hysterectomy | 80 | 5.9 | 75 | 9.3 | 13.0 |
| Temporary tracheostomy | <11 | <11 | |||
| Ventilation | 28 | 2.1 | 33 | 4.1 | 11.8 |
Abbreviations: CDC, Centers for Disease Control and Prevention; SMM, severe maternal morbidity.
a SMM indicators are not mutually exclusive, meaning that 1 person could have evidence of multiple comorbidity indicators; therefore, the percentages will not add to 100%. For any cell counts less than 11, we suppressed percentages and did not calculate standardized differences.
b An absolute standardized difference less than 10 may be indicative of a negligible difference in the prevalence of the SMM indicator between births with evidence of SMM and readmissions with evidence of SMM.
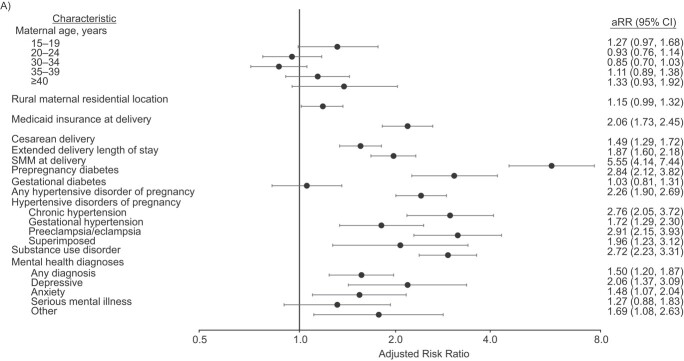
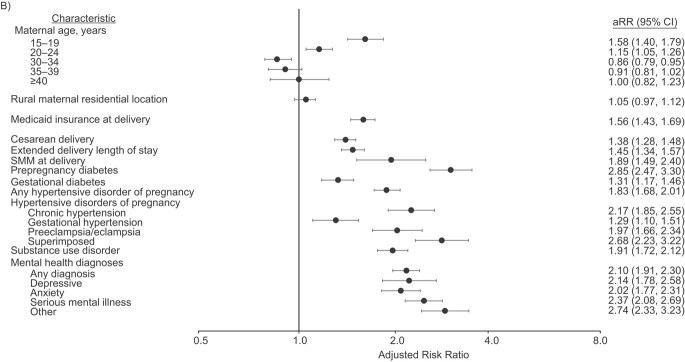
The strongest risk factor for PPR with SMM was SMM at delivery (RR = 5.55, 95% CI: 4.14, 7.44; Figure 2, Web Table 2). SMM at delivery was also associated with an increased risk of PPR without SMM (RR = 1.89, 95% CI: 1.49, 2.40) but was not the strongest risk factor. We found that PPR without SMM had numerous factors with measures of association on par with SMM at delivery, including any mental health diagnosis (RR = 2.10, 95% CI: 1.91, 2.30), substance use disorder (excluding cannabis) (RR = 2.01, 95% CI:1.80, 2.24), chronic hypertension (RR = 2.17, 95% CI: 1.85, 2.55), and prepregnancy diabetes (RR = 2.85, 95% CI: 2.47, 3.30).
Figure 2 .
Continues
Figure 2.
Adjusted risk ratios (aRRs) for postpartum readmissions with evidence of severe maternal morbidity (SMM) (A) and postpartum readmissions without evidence of SMM (B) among Oregon hospital births (n = 158,653), 2012–2017. The reference category for maternal age was 25–29 years. The model for maternal residential location (MRL) adjusted for maternal age; the reference location was urban. The model for type of health insurance at delivery adjusted for maternal age, MRL, any substance use disorder (SUD), and any mental health (MH) diagnosis; the reference category was commercial insurance. The model for mode of delivery adjusted for maternal age, MRL, insurance type at delivery, any SUD, any MH diagnosis, any diabetes, and any hypertensive disorders of pregnancy (HDP); the reference category was vaginal delivery. The model for extended length of stay adjusted for maternal age, MRL, insurance type at delivery, any SUD, any MH diagnosis, any diabetes, HDP, and mode of delivery; the reference category was no. The model for SMM at delivery adjusted for maternal age, MRL, insurance type at delivery, any diabetes, and HDP; the reference category for this and all following categories was no evidence. The model for prepregnancy diabetes/gestational diabetes adjusted for maternal age, MRL, any SUD, and any MH diagnosis; the model for HDP adjusted for maternal age, MRL, any SUD, and any MH diagnosis; the model for SUD adjusted for maternal age, MRL, and any MH diagnosis; and the model for MH diagnoses adjusted for maternal age and MRL. Bars, 95% confidence intervals (CIs).
When considering the timing of PPR, a modestly higher proportion of PPR with SMM occurred during the traditional postpartum period (n = 352; 43.6%) as compared with PPR without SMM (n = 1,259; 37.8%). SMM at delivery was the strongest risk factor for PPR with SMM in the traditional postpartum period (RR = 8.03, 95% CI: 5.49, 11.75; Table 3), followed by preclampsia/eclampsia (RR = 4.49, 95% CI: 3.07, 6.58). For PPR without SMM, chronic hypertension was the strongest risk factor in the traditional postpartum period (RR = 2.70, 95% CI: 2.12, 3.44) but not in the extended postpartum period (RR = 1.88, 95% CI: 1.51, 2.33). In addition, any mental health diagnosis (RR = 2.46, 95% CI: 2.19, 2.75) and prepregnancy diabetes (RR = 3.14, 95% CI: 2.63, 3.76) were among the strongest risk factors for PPR without SMM in the extended postpartum period.
Table 3.
Adjusted Risk Ratios for Postpartum Readmission With or Without Evidence of Severe Maternal Morbidity, by Readmission Timing, Among Oregon Hospital Births (n = 158,653), 2012–2017
| PPR With Evidence of SMM | PPR Without Evidence of SMM | |||||||
|---|---|---|---|---|---|---|---|---|
|
≤6 Weeks PP
(n = 352) |
7–52 Weeks PP
(n = 456) |
≤6 Weeks PP
(n = 1,259) |
7–52 Weeks PP
(n = 2,074) |
|||||
| Characteristic | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI |
| Maternal age, years | ||||||||
| 15–19 | 1.53 | 1.01, 2.31 | 1.11 | 0.76, 1.61 | 1.50 | 1.21, 1.85 | 1.64 | 1.40, 1.91 |
| 20–24 | 0.92 | 0.67, 1.27 | 0.93 | 0.72, 1.21 | 0.98 | 0.84, 1.15 | 1.25 | 1.11, 1.40 |
| 25–29 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 30–34 | 0.96 | 0.72, 1.30 | 0.78 | 0.61, 1.00 | 0.85 | 0.73, 0.99 | 0.87 | 0.78, 0.99 |
| 35–39 | 1.66 | 1.22, 2.26 | 0.75 | 0.55, 1.04 | 1.15 | 0.96, 1.37 | 0.77 | 0.65, 0.90 |
| ≥40 | 1.14 | 0.61, 2.12 | 1.46 | 0.93, 2.28 | 1.36 | 1.02, 1.83 | 0.78 | 0.58, 1.05 |
| Maternal residential locationa | ||||||||
| Urban | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Rural | 1.24 | 1.00, 1.54 | 1.08 | 0.89, 1.31 | 1.00 | 0.89, 1.13 | 1.07 | 0.98, 1.17 |
| Health insurance type at deliveryb | ||||||||
| Commercial | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Medicaid | 1.81 | 1.41, 2.34 | 2.29 | 1.80, 2.90 | 1.10 | 0.97, 1.25 | 2.00 | 1.79, 2.24 |
| Model of deliveryc | ||||||||
| Vaginal | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Cesarean | 1.66 | 1.33, 2.06 | 1.37 | 1.13, 1.67 | 1.69 | 1.50, 1.89 | 1.21 | 1.10, 1.33 |
| Extended delivery length of stayd | 1.99 | 1.57, 2.51 | 1.78 | 1.44, 2.19 | 1.53 | 1.34, 1.74 | 1.41 | 1.27, 1.56 |
| SMM at deliverye | 8.03 | 5.49, 11.75 | 3.72 | 2.31, 5.97 | 2.40 | 1.70, 3.38 | 1.57 | 1.12, 2.21 |
| Prepregnancy diabetesf | 1.60 | 0.90, 2.86 | 3.88 | 2.74, 5.49 | 2.42 | 1.88, 3.12 | 3.14 | 2.63, 3.76 |
| Gestational diabetesf | 1.15 | 0.81, 1.62 | 0.93 | 0.66, 1.31 | 1.14 | 0.95, 1.37 | 1.41 | 1.23, 1.62 |
| Any hypertensive disorder of pregnancyg | ||||||||
| Any disorder | 2.67 | 2.08, 3.43 | 1.96 | 1.54, 2.5 | 2.42 | 2.12, 2.77 | 1.51 | 1.34, 1.71 |
| No evidence | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Hypertensive disorders of pregnancyg | ||||||||
| Chronic hypertension | 2.95 | 1.89, 4.59 | 2.62 | 1.75, 3.93 | 2.70 | 2.12, 3.44 | 1.88 | 1.51, 2.33 |
| Gestational hypertension | 1.81 | 1.17, 2.79 | 1.66 | 1.13, 2.44 | 1.70 | 1.35, 2.15 | 1.06 | 0.85, 1.32 |
| Preeclampsia/eclampsia | 4.49 | 3.07, 6.58 | 1.78 | 1.08, 2.93 | 2.64 | 2.05, 3.39 | 1.60 | 1.26, 2.03 |
| Superimposed | 1.82 | 0.86, 3.86 | 2.06 | 1.13, 3.74 | 3.76 | 2.88, 4.91 | 2.09 | 1.61, 2.72 |
| No evidence | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Substance use disorderh | ||||||||
| Any disorder | 2.68 | 1.97, 3.65 | 2.75 | 2.12, 3.56 | 1.57 | 1.30, 1.90 | 2.10 | 1.86, 2.38 |
| Any disorder (excluding cannabis) | 2.90 | 2.11, 3.99 | 2.9 | 2.21, 3.79 | 1.68 | 1.37, 2.04 | 2.19 | 1.92, 2.50 |
| Mental health diagnosesi | ||||||||
| Any diagnosis | 1.22 | 0.85, 1.77 | 1.71 | 1.29, 2.27 | 1.53 | 1.29, 1.83 | 2.46 | 2.19, 2.75 |
| Depression | 1.38 | 0.66, 2.92 | 2.58 | 1.59, 4.18 | 1.33 | 0.90, 1.98 | 2.63 | 2.12, 3.26 |
| Anxiety | 1.40 | 0.85, 2.31 | 1.54 | 1.01, 2.34 | 1.58 | 1.24, 2.02 | 2.29 | 1.95, 2.68 |
| Serious mental illness | 0.87 | 0.45, 1.69 | 1.58 | 1.02, 2.45 | 1.85 | 1.45, 2.35 | 2.68 | 2.29, 3.14 |
| Other | 1.37 | 0.65, 2.89 | 1.93 | 1.11, 3.36 | 1.96 | 1.42, 2.70 | 3.22 | 2.65, 3.90 |
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; HDP, hypertensive disorders of pregnancy; MH, mental health; MRL, maternal residential location; PP, postpartum; PPR, postpartum readmission; SMM, severe maternal morbidity; SUD, substance use disorder.
a The model adjusted for maternal age.
b The model adjusted for maternal age, MRL, any SUD, and any MH diagnosis.
c The model adjusted for maternal age, MRL, insurance type at delivery, any SUD, any MH diagnosis, any diabetes, and any HDP.
d The model adjusted for maternal age, MRL, insurance type at delivery, any SUD, any MH diagnosis, any diabetes, HDP, and mode of delivery.
e The model adjusted for maternal age, MRL, insurance type at delivery, any diabetes, and HDP.
f The model adjusted for maternal age, MRL, any SUD, and any MH diagnosis.
g The models adjusted for maternal age, MRL, any SUD, and any MH diagnosis.
h The model adjusted for maternal age, MRL, and any MH diagnosis.
i The model adjusted for maternal age and MRL.
Finally, our comparison of demographic and clinical characteristics of the excluded population (people with less than 11 months of postpartum insurance coverage) showed some key differences (Web Table 3). We found that the excluded population was younger (32.3% were aged 15–24 years as compared with 27.9% in the analytical sample; P < 0.001) and more likely to have Medicaid insurance at the time of the birth (67.9% vs. 59.6%; P < 0.001). When shortening our continuous enrollment criteria to 60 days and assessing readmissions within 60 days, we found that Medicaid insurance at delivery (RR = 1.11, 95% CI: 0.99, 1.24) and gestational diabetes (RR = 1.14, 95% CI: 0.97, 1.34) were no longer statistically significantly associated with PPR without evidence of SMM (Web Table 4). Further, we observed that the association between any mental health diagnosis and PPR with evidence of SMM (RR = 1.36, 95% CI: 1.00, 1.86) was no longer statistically significant. When excluding transfusion of blood products from our SMM definition, we did not observe meaningful differences between covariates and PPR with and without SMM (Web Table 5).
DISCUSSION
Working with a database that covered all hospital births in a state spanning an 8-year period and across health insurance types, we examined the frequency, timing, and drivers of PPR with and without SMM. Our findings add nuance to what is known about both PPR and SMM. We found that birthing people with evidence of any SMM indicator at delivery were 5 times more likely to be rehospitalized for any SMM within 12 months postpartum compared with birthing people without evidence of SMM at delivery. We also found that SMM at delivery was driven by transfusion of blood products, whereas SMM during readmission was driven by sepsis.
The associations between maternal factors and postpartum health outcomes are complex, and our study sheds light on associations between PPR, SMM, and their overlap. We found further evidence of a strong association between SMM at childbirth and rehospitalization for SMM. Preventing birthing people from experiencing this life-threatening outcome more than once in the perinatal period should be a high priority. Our findings show that SMM rehospitalization is more likely to occur in the traditional postpartum period, with an 8-fold higher risk for those with SMM at delivery. Our association, while indicating a very high risk for SMM rehospitalization, is less pronounced than recent findings from a commercially insured population that found SMM at delivery led to a 12-fold higher risk of rehospitalization (42). Yet SMM at delivery still poses a risk for PPR with SMM up to 12 months postpartum. The risk of rehospitalization past the first 6 weeks is more than 3 times higher for birthing people with SMM at delivery than without. Our sensitivity analysis that removed transfusions of blood products from the SMM definition only modestly attenuated the risk for SMM rehospitalization and did not change our conclusions. In future research, investigators should examine the role of postpartum preventive care in reducing SMM rehospitalization, particularly the timing and frequency of outpatient care.
We also found that the prevalence of SMM-defining conditions differed if SMM was diagnosed at childbirth versus at readmission. The most common SMM indicator diagnosed at childbirth was transfusion of blood products (42.1%), followed by disseminated intravascular coagulation and eclampsia. None of these were among the most common SMM-defining conditions diagnosed at readmission; sepsis was the most common (41.8%). This demonstrates that the profile of maternal morbidity occurring at PPR cannot be assumed to reflect morbidity occurring during childbirth. SMM is a composite measure. Defining specific strategies to prevent the progression of maternal morbidity will require targeted interventions depending on when SMM is diagnosed.
Two important and understudied postpartum risk factors are substance use disorders and mental health diagnoses. We found that both factors were associated with PPR with and without SMM. However, the strength of the associations between substance use disorder and any mental health diagnoses and PPR without SMM varied depending on the timing of the PPR. Strikingly, the magnitudes of association for substance use and mental health diagnoses and PPR without SMM were on par with those of traditional clinical comorbid conditions such as chronic hypertension and prepregnancy diabetes. The complexity and heterogeneity of PPR require further study, as reflected by recent critiques of the face validity of PPR as a quality marker (43). In addition to the critical importance of SMM, factors that require ongoing management are important to consider when devising strategies to prevent deterioration of maternal well-being to the point of requiring hospitalization.
Strengths of our study include the use of a multiyear, multipayer, multicare setting database and examining PPR in the extended postpartum period. Our use of the APAC database helped us overcome the limitations of prior studies that were limited to 1 care setting (inpatient data as opposed to outpatient or emergency department data) (12, 15, 16) or 1 payer (20, 21). Our study also had limitations. First, claims data are limited to people who have health insurance at the time of any health-care encounter. Approximately 50% of pregnant people gain Medicaid insurance because of their pregnancy and lose coverage 60 days after delivery (26, 44). Our study excluded people who had insurance at delivery but did not have at least 11 months of continuous insurance coverage postpartum (22% of total births in our sample)—an important and vulnerable population that warrants further study. By excluding persons with intermittent insurance coverage postpartum, we may have been excluding those at higher risk for PPR, thus underestimating the total PPR prevalence. Notably, our sensitivity analysis that shortened the continuous enrollment criterion to 60 days postpartum did not change our overall conclusions. In addition, we were unable to reliably capture out-of-hospital births (e.g., births taking place at a birth center or at home) because they are often paid for outside of insurance plans. Out-of-hospital births account for approximately 4% of births in Oregon (45). The association between birth setting and PPR and SMM warrants further study.
Next, the current scientific literature demonstrates that interpersonal and structural racism are drivers of maternal health inequities (46, 47). However, the Oregon APAC database was missing information on race and ethnicity for 50% of our population and thus could not be reliably used to address this factor in our analyses. In addition, our analyses relied on documentation of comorbidity in health-care encounter records using ICD-9/ICD-10 codes, which is often underestimated in administrative claims data. If comorbidity was underestimated, our results may have been biased towards the null. Even with population-based data, rare events impose limitations; for example, we were unable to compare SMM indicators, except for the 12 most common. Finally, our analysis was limited to Oregon, a state with less racial and ethnic diversity than other regions in the United States (48), which reduces the generalizability of our findings.
Our study shows that the associations between maternal factors and postpartum morbidity are complex. As maternal health research progresses, more work will be needed to understand the spectrum of maternal morbidity and ultimately prevent it. In particular, more research is needed to understand the proximal health factors that drive PPR, such as continuous insurance coverage and access to preventive and mental health care. This work will enable clinicians and policy-makers to reduce maternal morbidity and mortality, helping to promote healthy pregnancy, delivery, and well-being for all pregnant people.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, Oregon Health & Science University and Portland State University, Portland, Oregon, United States (Menolly Kaufman, Jonathan M. Snowden); Department of Health Promotion and Community Health, School of Public Health, Oregon Health & Science University and Portland State University, Portland, Oregon, United States (Dawn Richardson); Center for Health Systems Effectiveness, Oregon Health & Science University, Portland, Oregon, United States (Menolly Kaufman, K. John McConnell, Maria I. Rodriguez); Departments of Pediatrics and Obstetrics and Gynecology, School of Medicine, Stanford University, Stanford, California, United States (Suzan L. Carmichael); and Department of Obstetrics and Gynecology, Oregon Health & Science University, Portland, Oregon, United States (Maria I. Rodriguez, Jonathan M. Snowden).
The data in this study contain personal health information and are not available for public use.
This work was presented at the 34th annual meeting of the Society for Pediatric and Perinatal Epidemiologic Research (virtual), June 21 and 22, 2021.
M.I.R. reports receiving personal fees from the American College of Obstetricians and Gynecologists, Bayer Corporation (Whippany, New Jersey), and Merck & Co., Inc. (Kenilworth, New Jersey) outside the scope of this work. The Oregon Health & Science University institutional review board manages these potential conflicts of interest.
REFERENCES
- 1. Rossen LM, Womack LS, Hoyert DL, et al. The impact of the pregnancy checkbox and misclassification on maternal mortality trends in the United States, 1999–2017. Vital Health Stat 3. 2020;(44):1–61. [PubMed] [Google Scholar]
- 2. Shaw D, Guise JM, Shah N, et al. Drivers of maternity care in high-income countries: can health systems support woman-centred care? Lancet. 2016;388(10057):2282–2295. [DOI] [PubMed] [Google Scholar]
- 3. Hoyert DL. Maternal mortality rates in the United States, 2019. (NCHS Health E-Stats). Hyattsville, MD: National Center for Health Statistics; 2021. 10.15620/cdc:103855. Accessed April 9, 2022. [DOI] [Google Scholar]
- 4. Creanga AA, Berg CJ, Ko JY, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health Larchmt. 2014;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray KE, Wallace ER, Nelson KR, et al. Population-based study of risk factors for severe maternal morbidity. Paediatr Perinat Epidemiol. 2012;26(6):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stuebe A, Moore JE, Mittal P, et al. Extending Medicaid coverage for postpartum moms [Web log post]. 10.1377/hblog20190501.254675/full. Published May 6, 2019. Accessed April 9, 2022. [DOI]
- 7. Centers for Disease Control and Prevention . Severe Maternal Morbidity After Delivery Discharge Among U.S. Women, 2010–2014. Atlanta, GA: Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/smm-after-delivery-discharge-among-us-women/index.htm. Accessed April 9, 2022. [Google Scholar]
- 8. American College of Obstetricians and Gynecologists . ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131(5):e140–e150. [DOI] [PubMed] [Google Scholar]
- 9. National Academies of Sciences, Engineering, Medicine . Birth Settings in America: Outcomes, Quality, Access, and Choice. Washington, DC: National Academies Press; 2020. [PubMed] [Google Scholar]
- 10. Harvey EM, Ahmed S, Manning SE, et al. Severe maternal morbidity at delivery and risk of hospital encounters within 6 weeks and 1 year postpartum. J Womens Health Larchmt. 2018;27(2):140–147. [DOI] [PubMed] [Google Scholar]
- 11. Matas JL, Mitchell LE, Sharma SV, et al. Severe maternal morbidity at delivery and postpartum readmission in the United States. Paediatr Perinat Epidemiol. 2021;35(6):627–634. [DOI] [PubMed] [Google Scholar]
- 12. Clapp MA, Little SE, Zheng J, et al. A multi-state analysis of postpartum readmissions in the United States. Am J Obstet Gynecol. 2016;215(1):113.e1–113.e10. [DOI] [PubMed] [Google Scholar]
- 13. Leonard SA, Main EK, Scott KA, et al. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aziz A, Gyamfi-Bannerman C, Siddiq Z, et al. Maternal outcomes by race during postpartum readmissions. Am J Obstet Gynecol. 2019;220(5):484.e1–484.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clapp MA, Little SE, Zheng J, et al. The relative effects of patient and hospital factors on postpartum readmissions. J Perinatol. 2018;38(7):804–812. [DOI] [PubMed] [Google Scholar]
- 16. Clapp MA, Little SE, Zheng J, et al. Hospital-level variation in postpartum readmissions. JAMA. 2017;317(20):2128–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mourad M, Wen T, Friedman AM, et al. Postpartum readmissions among women with diabetes. Obstet Gynecol. 2020;135(1):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wagner JL, White RS, Tangel V, et al. Socioeconomic, racial, and ethnic disparities in postpartum readmissions in patients with preeclampsia: a multi-state analysis, 2007–2014. J Racial Ethn Health Disparities. 2019;6(4):806–820. [DOI] [PubMed] [Google Scholar]
- 19. Wen T, Overton EE, Sheen JJ, et al. Risk for postpartum readmissions and associated complications based on maternal age. J Matern Fetal Neonatal Med. 2021;34(9):1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ehrenthal DB, Gelinas K, Paul DA, et al. Postpartum emergency department visits and inpatient readmissions in a Medicaid population of mothers. J Womens Health Larchmt. 2017;26(9):984–991. [DOI] [PubMed] [Google Scholar]
- 21. Cartus AR, Jarlenski MP, Himes KP, et al. Adverse cardiovascular events following severe maternal morbidity. Am J Epidemiol. 2021;191(1):126–136. [DOI] [PubMed] [Google Scholar]
- 22. Brousseau EC, Danilack V, Cai F, et al. Emergency department visits for postpartum complications. J Womens Health Larchmt. 2018;27(3):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Cox S, Kuklina EV, et al. Assessment of incidence and factors associated with severe maternal morbidity after delivery discharge among women in the US. JAMA Netw Open. 2021;4(2):e2036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Girsen AI, Sie L, Carmichael SL, et al. Rate and causes of severe maternal morbidity at readmission: California births in 2008–2012. J Perinatol. 2020;40(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gordon SH, Sommers BD, Wilson IB, et al. Effects of Medicaid expansion on postpartum coverage and outpatient utilization. Health Aff (Millwood). 2020;39(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daw JR, Hatfield LA, Swartz K, et al. Women in the United States experience high rates of coverage ‘churn’ in months before and after childbirth. Health Aff (Millwood). 2017;36(4):598–606. [DOI] [PubMed] [Google Scholar]
- 27. Oregon Health Authority . Oregon All Payer All Claims Database (APAC): An Overview. Salem, OR: Oregon Health Authority; 2018. https://www.oregon.gov/oha/HPA/ANALYTICS/APAC%20Page%20Docs/APAC-Overview.pdf. Accessed April 9, 2022. [Google Scholar]
- 28. Oregon Health Authority . Oregon All Payer All Claims Database (APAC): Frequently Asked Questions. Salem, OR: Oregon Health Authority; 2018. https://www.oregon.gov/oha/HPA/ANALYTICS/APAC%20Page%20Docs/APAC-FAQ.pdf. Accessed April 9, 2022. [Google Scholar]
- 29. Ailes EC, Simeone RM, Dawson AL, et al. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res A Clin Mol Teratol. 2016;106(11):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American College of Obstetricians and Gynecologists . What are the documentation requirements for vaginal deliveries? https://www.acog.org/practice-management/coding/coding-library/documentation-requirements-for-vaginal-deliveries. Accessed April 9, 2022.
- 31. Ballard D Jr. Simplify coding by knowing what is packaged into obstetrics care. https://www.aapc.com/blog/25857-from-antepartum-to-postpartum-get-the-cpt-ob-basics/. Published 2013. Accessed April 9, 2022.
- 32. D’Angelo DV, Le B, O’Neil ME, et al. Patterns of health insurance coverage around the time of pregnancy among women with live-born infants—Pregnancy Risk Assessment Monitoring System, 29 states, 2009. MMWR Surveill Summ. 2015;64(4):1–19. [PMC free article] [PubMed] [Google Scholar]
- 33. Oregon Health Authority . Ambulatory Care: Emergency Department and Outpatient Utilization. Salem, OR: Oregon Health Authority; 2018. https://www.oregon.gov/oha/HPA/ANALYTICS/CCOMetrics/2019-Ambulatory-Care-Outpatient-ED-Utilization.pdf. Accessed April 9, 2022. [Google Scholar]
- 34. Centers for Disease Control and Prevention . How does CDC identify severe maternal morbidity? https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Published 2019. Accessed April 9, 2022.
- 35. Economic Research Service, US Department of Agriculture . 2010 Rural-Urban Commuting Area (RUCA) codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Updated August 17, 2020. Accessed April 9, 2022.
- 36. Snowden JM, Lyndon A, Kan P, et al. Severe maternal morbidity: a comparison of definitions and data sources. Am J Epidemiol. 2021;190(9):1890–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 39. Hernán MA, Hernández-Díaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. [DOI] [PubMed] [Google Scholar]
- 40. Leonard SA, Carmichael SL, Main EK, et al. Risk of severe maternal morbidity in relation to prepregnancy body mass index: roles of maternal co-morbidities and caesarean birth. Paediatr Perinat Epidemiol. 2020;34(4):460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214(5):643.e1–643.e10. [DOI] [PubMed] [Google Scholar]
- 42. Black CM, Vesco KK, Mehta V, et al. Hospital readmission following delivery with and without severe maternal morbidity. J Womens Health Larchmt. 2021;30(12):1736–1743. [DOI] [PubMed] [Google Scholar]
- 43. Combs CA, Goffman D, Pettker CM. Society for Maternal-Fetal Medicine special statement: a critique of postpartum readmission rate as a quality metric. Am J Obstet Gynecol. 2022;226(4):B2–B9. [DOI] [PubMed] [Google Scholar]
- 44. Daw JR, Winkelman TNA, Dalton VK, et al. Medicaid expansion improved perinatal insurance continuity for low-income women. Health Aff (Millwood). 2020;39(9):1531–1539. [DOI] [PubMed] [Google Scholar]
- 45. Snowden JM, Tilden EL, Snyder J, et al. Planned out-of-hospital birth and birth outcomes. N Engl J Med. 2015;373(27):2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor JK. Structural racism and maternal health among Black women. J Law Med Ethics. 2020;48(3):506–517. [DOI] [PubMed] [Google Scholar]
- 48. Lee B, Martin M, Matthews S, et al. State-level changes in US racial and ethnic diversity, 1980 to 2015: a universal trend? Demogr Res. 2017;37(33):1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kametas NA, Nzelu D, Nicolaides KH. Chronic hypertension and superimposed preeclampsia: screening and diagnosis. Am J Obstet Gynecol. 2021;226(2 suppl):S1182–S1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.