ABSTRACT
Immune checkpoint inhibitors improve patient survival in multiple cancers, but immune-related adverse events, including new immunologic conditions arising during therapy, pose a significant challenge. Gastrointestinal immune-related adverse events, although common, exhibit diverse presentations. We present a case of duodenitis resembling celiac disease because of the anti–programmed cell death protein-1 antibody, pembrolizumab. Despite diagnostic uncertainty and therapeutic interventions, including gluten-free diet, symptoms stabilized even with resuming gluten. In addition, endoscopic abnormalities after pembrolizumab therapy have neither progressed nor completely resolved. This case underscores the need for investigation into the pathogenesis of immune checkpoint inhibitor–induced duodenitis, with implications for care of patients on immunotherapies.
KEYWORDS: immunotherapy, duodenitis, immune checkpoint inhibitor, autoimmune enteropathy
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, by leveraging the body's own immune system to target tumor cells.1 However, there is a risk of triggering an inappropriate immune response, referred to as immune-related adverse events (irAEs). Gastrointestinal irAEs are the most common, affecting up to 50% of all patients.2 However, gastrointestinal irAEs exhibit considerable variability in timing of onset and symptom presentation and can be challenging to identify and manage. A limited number of case reports suggest the occurrence of ICI-related enteropathies.3–5 We describe the diagnostic and therapeutic complexity of a case of ICI-induced duodenitis because of pembrolizumab, an inhibitor of anti–programmed cell death protein-1.
CASE REPORT
A 48-year-old man with clear cell renal cell carcinoma presented with 2 months of episodic nausea, vomiting, and abdominal pain. His symptoms began 6 months into adjuvant pembrolizumab monotherapy. His history included hypertension, a radical nephrectomy for his renal cell carcinoma, and stage 3a chronic kidney disease. He had no history of gastrointestinal symptoms, personal/familial autoimmune conditions, or food allergies. He followed a primarily plant-based diet, consumed 1 glass of wine weekly, and previously smoked cigarettes. His medications included losartan, vitamin D, fish oil, and turmeric supplements, with occasional acetaminophen use but no nonsteroidal anti-inflammatory drugs. Discontinuation of vitamins and supplements failed to alleviate symptoms.
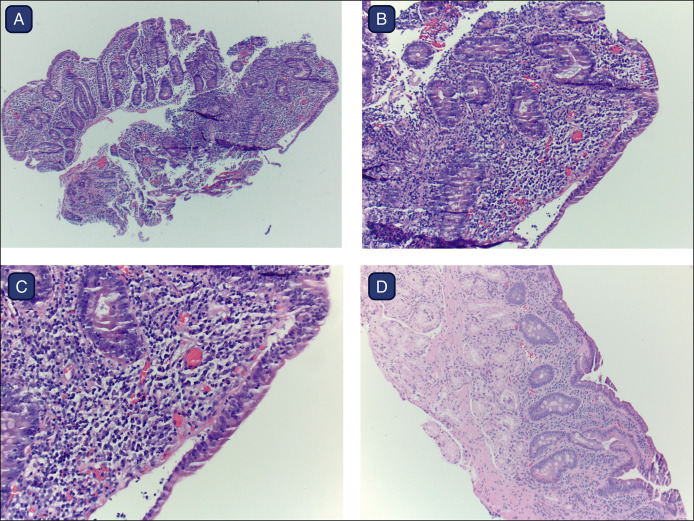
Physical examination revealed a well-nourished male with a soft, nondistended, abdomen that was nontender to palpation. Initial upper endoscopy revealed diffusely nodular duodenal mucosa. Histopathology showed focal, mild active duodenitis, villous blunting, lymphoplasmacytic infiltrate in the lamina propria, and increased intraepithelial lymphocytes (Figure 1). Colonoscopy identified a sessile serrated adenoma in the ascending colon. Laboratory studies collected 2 weeks later included negative serum tissue transglutaminase IgA antibody (<2 U/mL, ref 0–3), normal total IgA (131 mg/dL, ref 90–386), and negative endomysial IgA antibody. A preliminary diagnosis of celiac disease was made based on initial pathology, and he was advised to adhere to a gluten-free diet (GFD). However, the GFD was discontinued after negative serologic results, without significant exacerbation or improvement in symptoms.
Figure 1.
(A–D). Duodenal biopsies revealed histologic features typical of celiac disease, reflecting immune checkpoint inhibitor–induced duodenitis in this patient undergoing pembrolizumab therapy. Characteristic findings include (A) villous blunting and crypt hyperplasia, (B and C) lymphoplasmacytic infiltrate in the lamina propia and increased intraepithelial lymphocytes (hematoxylin & eosin stain, magnification at (A) 4×, (B) 10×, and (C) 20×), and (D) persistent partial villous blunting after discontinuation of pembrolizumab. Figure created using BioRender.
A secondary pathology review at our center revealed lymphocytic inflammation in the duodenum and mucosal villous blunting. Human leukocyte antigen (HLA) typing was positive for HLA-DQB1*02, an allelic variant present in more than 90% of patients with celiac disease.6 This allele has also been associated with the risk of other autoimmune conditions.7,8 Given intermittent nature of symptoms, and the anticipated benefit of continuing cancer treatment, it was decided to retrial a GFD as an initial management strategy rather than modify his ICI therapy, although further diagnostic information was gathered. The patient started on a GFD, alongside twice-daily proton pump inhibitor and daily cetirizine. Nutritional and allergy assessments revealed no clear dietary triggers. Without significant improvement, he reintroduced gluten-containing foods after 2 months, without exacerbation of symptoms, and completed his final ICI infusion by the third month. By the sixth month, he reported near resolution of his symptoms and was instructed to continue eating gluten. A follow-up endoscopy at 9 months revealed a grossly normal duodenum, partial recovery of intestinal villi architecture, with resolution of intraepithelial lymphocytosis. However, patchy villous blunting and focal gastric foveolar metaplasia persisted as nonspecific histologic abnormalities.
DISCUSSION
ICI-induced duodenitis is a rare but serious irAE. Several cases have been reported in the literature, but our patient is unique in the level of diagnostic uncertainty, with features of both ICI-induced celiac disease and ICI-induced duodenitis. ICI-induced duodenitis poses a diagnostic challenge for patients and clinicians for several reasons. First, patients may present with nonspecific gastrointestinal symptoms. Initially, our patient reported symptom improvement on a GFD, which raised suspicion of new-onset celiac disease induced by immunotherapy. However, a detailed food diary revealed no association with gluten. In addition, patients undergoing immunotherapy may receive treatment with antineoplastic agents with known gastrointestinal side effects, thereby adding further layers of complexity.9 Second, other clinical factors may obscure the interpretation of serologic markers. In this patient's case, serologies were collected after a brief period of GFD, lowering diagnostic accuracy. This may have heightened risk of a false negative result, despite a compatible haplotype. He was also taking losartan, which has been associated with enteropathies, although this was not a new medication.10 Third, histopathologic findings may not discern these disease entities.11 Distinguishing between preexisting celiac disease, ICI-induced celiac disease, ICI-induced seronegative duodenitis, and other disease mimickers requires thorough investigation.5 Previous histopathologic studies have noted differences between ICI-induced celiac disease and ICI-induced duodenitis, with the latter having more erosions, more neutrophilic infiltrates, and more CD3 and CD8 T cells in the lamina propria.12 Clinically, both entities may present with diarrhea and abdominal pain. Patients with ICI-induced celiac disease often respond to a GFD, whereas those with ICI-induced seronegative duodenitis may more often require immunosuppression.4 Further data including prospective studies are needed to better characterize both entities. Interdisciplinary collaboration between oncologists and gastroenterologists, including timely referral to endoscopy, is paramount for identifying active luminal pathology.
Our patient's diagnosis favors ICI-induced duodenitis, based on histologic improvement unrelated to gluten. However, the time to histologic remission after discontinuation of ICIs is unknown. Among patients with celiac disease, lymphoproliferative malignancy risk is believed to be more pronounced in patients with persistent villous atrophy in comparison with patients with resolution.13 Incomplete resolution of villous blunting in ICI-induced duodenitis may, therefore, be clinically significant, underscoring a role for continued endoscopic surveillance. Monitoring symptomatic and endoscopic treatment response is essential to understanding this clinical syndrome and its long-term outcomes.
ICI-induced duodenitis remains a poorly understood entity but likely stems from dysregulated immune responses triggered by immunotherapy.14 Programmed cell death protein-1 inhibitors such as pembrolizumab have been associated with increased disease activity in preexisting autoimmune conditions.15 For individuals without known autoimmune conditions, mechanisms proposed include immunotherapy inducing a transition from preautoimmunity (compatible HLA serotypes) to clinically evident autoimmunity, or the development of a de novo autoimmune disorder, without predisposing risk factors. Although the specific mechanism may remain unclear, removing a suspected inciting cause, such as adopting GFD, represents a reasonable initial approach. Although GFD may lead to symptom resolution for some patients, others may require steroid treatment or biologic anti-inflammatory agents to control symptoms.4 It is, therefore, critical to establish a comprehensive follow-up plan to monitor for treatment response and mitigate complications such as malnutrition, malabsorption, and weight loss. A stepwise treatment approach may also enable patients to avoid unnecessary immunosuppressive medication or premature discontinuation of immunotherapy.
This case describes the real-world challenge of identifying ICI-induced duodenitis and highlights the role of endoscopic surveillance even in the absence of symptoms. Further studies to elucidating pathogenesis and natural history are needed to facilitate early recognition, guide management, and prevent complications from these gastrointestinal irAEs.
DISCLOSURES
Author contributions: S. Au: data interpretation, literature review, manuscript writing, and critical revisions; W. Chen: data acquisition/interpretation; I. Vance: management of the case, data acquisition/interpretation, and critical revisions; K. Wegermann: identification and management of the case, data acquisition/interpretation, literature review, and critical revisions. All authors read and approved the final manuscript. K. Wegermann is the article guarantor.
Financial disclosure: None to report.
Previous presentation: ACG 2023 Annual Scientific Meeting; October 20–25, 2023; Vancouver, British Columbia (Abstract #1560755).
Informed consent was obtained for this case report.
Contributor Information
Wei Chen, Email: wei.chen573@duke.edu.
Iris Vance, Email: iris.vance@duke.edu.
Kara Wegermann, Email: kara.wegermann@duke.edu.
REFERENCES
- 1.Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29(5):3044–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajha E, Chaftari P, Kamal M, Maamari J, Chaftari C, Yeung SCJ. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol Rep (Oxf). 2020;8(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsaadi D, Shah NJ, Charabaty A, Atkins MB. A case of checkpoint inhibitor-induced celiac disease. J Immunother Cancer. 2019;7(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badran YR, Shih A, Leet D, et al. Immune checkpoint inhibitor-associated celiac disease. J Immunother Cancer. 2020;8(1):e000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodoraki E, Giannarakis M, Tzardi M, Koutroubakis IΕ. Pembrolizumab-induced antiTTG IgA-negative duodenitis treated with gluten withdrawal. Eur J Gastroenterol Hepatol. 2021;33(8):1130–1. [DOI] [PubMed] [Google Scholar]
- 6.Poddighe D, Rebuffi C, De Silvestri A, Capittini C. Carrier frequency of HLA-dqb1*02 allele in patients affected with celiac disease: A systematic review assessing the potential rationale of a targeted allelic genotyping as a first-line screening. World J Gastroenterol. 2020;26(12):1365–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur N, Bhadada SK, Minz RW, Dayal D, Kochhar R. Interplay between type 1 diabetes mellitus and celiac disease: Implications in treatment. Dig Dis. 2018;36(6):399–408. [DOI] [PubMed] [Google Scholar]
- 8.Noble JA. Immunogenetics of type 1 diabetes: A comprehensive review. J Autoimmun. 2015;64:101–12. [DOI] [PubMed] [Google Scholar]
- 9.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38(24):2782–97. [DOI] [PubMed] [Google Scholar]
- 10.van Gils T, Robijn RJ, Bouma G, Neefjes-Borst EA, Mulder CJJ. A pitfall in suspected (refractory) celiac disease: Losartan-induced enteropathy. Am J Gastroenterol. 2017;112(11):1754–5. [DOI] [PubMed] [Google Scholar]
- 11.Kamboj AK, Oxentenko AS. Clinical and histologic mimickers of celiac disease. Clin Transl Gastroenterol. 2017;8(8):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irshaid L, Robert ME, Zhang X. Immune checkpoint inhibitor-induced upper gastrointestinal tract inflammation shows morphologic similarities to, but is immunologically distinct from, Helicobacter pylori gastritis and celiac disease. Arch Pathol Lab Med. 2021;145(2):191–200. [DOI] [PubMed] [Google Scholar]
- 13.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: A population-based cohort study. Ann Intern Med. 2013;159(3):169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58(Suppl 7):vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizuorno Machado A, Shatila M, Liu C, et al. Immune-related adverse events after immune checkpoint inhibitor exposure in adult cancer patients with pre-existing autoimmune diseases. J Cancer Res Clin Oncol. 2023;149(9):6341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]