Abstract
Results of toxicological studies indicate that phthalates and per-/polyfluoroalkyl substances (PFAS), 2 classes of endocrine-disrupting chemicals, may alter the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis. We evaluated the associations of urinary phthalate metabolites and serum PFAS during gestation and childhood with adolescent hair cortisol concentrations (pg/mg hair) at age 12 years, an integrative marker of HPA axis activity (n = 205 mother-child pairs; Cincinnati, Ohio; enrolled 2003–2006). We used quantile-based g-computation to estimate associations between mixtures of urinary phthalate metabolites or serum PFAS and hair cortisol. We also examined whether associations of individual phthalate metabolites or PFAS with cortisol varied by the timing of exposure. We found that a 1-quartile increase in all childhood phthalate metabolites was associated with 35% higher adolescent hair cortisol (phthalate mixture ψ = 0.13; 95% confidence interval: 0.03, 0.22); these associations were driven by monoethyl phthalate, monoisobutyl phthalate, and monobenzyl phthalate. We did not find evidence that phthalate metabolites during gestation or serum PFAS mixtures were related to adolescent hair cortisol concentrations. We found suggestive evidence that higher childhood concentrations of individual PFAS were related to higher and lower adolescent hair cortisol concentrations. Our results suggest that phthalate exposure during childhood may contribute to higher levels of chronic HPA axis activity.
Keywords: adolescence, cortisol, hypothalamic-pituitary-adrenocortical axis, per-/polyfluoroalkyl substances, phthalate metabolites
Abbreviations
- CI
confidence interval
- DEHP
di(2-ethylhexyl) phthalate
- HOME
Health Outcomes and Measures of the Environment
- HPA
hypothalamic-pituitary-adrenocortical
- LOD
limit of detection
- MBzP
monobenzyl phthalate
- MCNP
monocarboxynonyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MCOP
monocarboxyoctyl phthalate
- MECPP
mono-2-ethyl-5-carboxypentyl phthalate
- MEOHP
mono-2-ethyl-5-oxohexyl phthalate
- MEP
monoethyl phthalate
- MiBP
monoisobutyl phthalate
- MnBP
mono-n-butyl phthalate
- PFAS
per-/polyfluoroalkyl substances
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
Prior research suggests that exposure to phthalates and per-/polyfluoroalkyl substances (PFAS) during fetal development and early life may be associated with excess adiposity, disruption in glucose-insulin homeostasis, and metabolic syndrome during childhood (1–7). Exposure to phthalates during early brain development is also associated with behavioral, cognitive, and memory deficits (8–11). Phthalates are endocrine-disrupting chemicals added to personal care products, found in food packaging and products, and used in textiles (12–18). PFAS are a class of synthetic chemicals widely used in consumer products such as water repellant clothing, carpet, cleaning products, and food packaging (19–21). People of all ages are continuously exposed to these chemicals through everyday activities (22–28).
Early-life phthalate and PFAS exposure may contribute to adverse health by interfering with the hormone receptors that maintain hypothalamus-pituitary-adrenal (HPA) axis homeostasis and disrupting circulation of the downstream glucocorticoid cortisol (29, 30). Results of toxicological studies indicate that some individual phthalates and PFAS inhibit the action of 11β-hydroxysteroid dehydrogenase type-2 (11β-HSD2), which converts active cortisol into inactive cortisone (31–34). Further, some phthalates may interact with the glucocorticoid receptor or interfere with cortisol transport by corticosteroid-binding globulin (35, 36). However, we are not aware of any studies that have examined the associations of phthalate or PFAS mixtures with adolescent cortisol concentrations. Humans are exposed to mixtures of these chemicals, and the cumulative effect on HPA axis activity could be greater than the independent effect of any individual chemical (37).
Our objective was to examine the prospective association of gestational and childhood phthalate and PFAS mixtures with adolescent hair cortisol, an integrative measure of HPA axis activity (38). We also investigated potential periods of heightened susceptibility to phthalates and PFAS during fetal development, childhood, and adolescence. We hypothesized that phthalates and PFAS would be positively associated with hair cortisol concentrations and these associations could vary by the developmental stage of exposure (39).
METHODS
HOME Study participants
We recruited pregnant women from 9 local prenatal clinics to participate in the Health Outcomes and Measures of the Environment (HOME) Study (Cincinnati, Ohio, 2003–2006), a longitudinal pregnancy and birth cohort study. We conducted follow-up visits with their children at birth, 4 weeks, and ages 1, 2, 3, 4, 5, 8, and 12 years (40, 41). Eligibility criteria for the study included: English fluency; 18 years of age or older; 16 (standard deviation, 3) weeks’ gestation; plan to continue prenatal care and deliver at collaborating clinics and hospitals; residence in a home built in or prior to 1978 which was not a mobile or trailer home; plan to live in the greater Cincinnati metropolitan area for at least 1 year; human immunodeficiency virus–negative; not taking medications for seizures or thyroid disorders; and not diagnosed with diabetes, bipolar disorder, schizophrenia, or cancer. For this study, there were 205 eligible participants with hair cortisol concentrations, covariate information, and urinary phthalate metabolite concentrations quantified at least once in: 1) maternal biospecimens (at 16 and/or 26 weeks’ gestation) or 2) childhood biospecimens (at ages 1, 2, 3, 4, 5, and 8 years). Among 200 participants who met these eligibility criteria and reported breastfeeding duration information, 175 and 174 participants had PFAS quantified at least once in maternal or childhood serum, respectively. Therefore, the sample size varied for analyses with phthalate metabolites and serum PFAS, but participant characteristics were similar for both analyses (Web Table 1, available at https://doi.org/10.1093/aje/kwad198).
The institutional review boards (IRBs) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the participating prenatal clinics and delivery hospitals approved the HOME Study. Brown University deferred to the CCHMC IRB as the IRB of record. During face-to-face visits, women provided written informed consent for their participation and their child’s participation. Children provided written informed assent at the 12-year visit.
Urinary phthalate metabolite assessment
We previously described methods used to assess urinary phthalate metabolites during pregnancy (16 and 26 weeks’ gestation) and childhood (1, 2, 3, 4, 5, 8, and 12 years) (40–42). Briefly, we used polypropylene specimen cups to collect urine samples during pregnancy at 16- and 26 weeks’ gestation. For children who did not use the toilet, we collected urine by placing an insert in a clean diaper and later expressed the urine using a syringe. We also used training toilets lined with inserts to collect urine samples when children were learning how to use the toilet. Caregivers helped children who used the toilet to collect urine directly into the polypropylene specimen cups. All urine samples were stored at −20°C until they were shipped overnight on dry ice to the Centers for Disease Control and Prevention for the quantification of phthalate metabolites.
Using previously described methods, we quantified concentrations of mono(2-ethylhexyl) phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP), which are metabolites of di(2-ethylhexyl) phthalate, as well as monobenzyl phthalate (MBzP), monocarboxynonyl phthalate (MCNP), mono(3-carboxypropyl) phthalate (MCPP), monocarboxyoctyl phthalate (MCOP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), and monoisobutyl phthalate (MiBP) using isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry (42). We did not quantify MCNP or MCOP in urine samples collected during gestation because the methods had not yet been developed at that time. We were also not able to assess urinary concentrations of mono(2-ethylhexyl) phthalate, MnBP, or MiBP in urine samples collected at ages 1, 2, or 3 years due to phthalate contamination from the diaper inserts used in collection of the samples. Limits of detection (LOD) for phthalate metabolites were similar in prenatal and childhood analyses (Web Table 2). We created a summary di(2-ethylhexyl) phthalate (DEHP) measure by dividing mono-2-ethyl-5-hydroxyhexyl phthalate, mono-2-ethyl-5-oxohexyl phthalate, and MECPP concentrations by their respective molar mass, summing the molar metabolite concentrations, and then multiplying by the molar mass of MECPP to express the sum in units of ng/mL.
To account for individual variation in urine dilution, we quantified urinary creatinine concentrations using enzymatic methods. Next, we creatinine standardized and log10-transformed phthalate metabolite concentrations. These concentrations are prone to measurement error due to the short biological half-life of phthalate metabolites, episodic and chronic nature of phthalate exposures, and nonrandom urine sampling. Nondifferential exposure misclassification from this error could attenuate our results. Therefore, we used previously described regression-calibration approaches to correct urinary phthalate metabolite concentrations for measurement-error (10, 43). We estimated the measurement-error-corrected average of phthalate metabolite concentrations representing the gestational period with the urinary phthalate metabolites quantified at 16 and 26 weeks’ gestation. We used the area under the curve of subject-specific phthalate metabolite concentrations at ages 1, 2, 3, 4, 5, 8, and 12 years to estimate phthalate metabolite intensity during childhood up to each age.
Serum PFAS assessment
To assess serum PFAS during gestation we collected blood samples from pregnant women at approximately 16 weeks (86%). For women who did not have blood samples at 16 weeks, we used blood samples collected at 26 weeks’ gestation (9%) or within 48 hours of delivery (5%). We collected blood from children at ages 3, 8, and 12 years. We quantified 4 PFAS in serum—perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoate (PFNA), and perfluorohexane sulfonate (PFHxS)—using online solid-phase extraction coupled to high-performance liquid chromatography-isotope dilution tandem mass spectrometry at the Centers for Disease Control and Prevention. We summed the concentration of both linear and branched isomers to calculate the total concentrations of PFOS and PFOA (5, 44). Serum PFAS concentrations were log2-transformed for analyses.
Quantification of hair cortisol
We collected hair from participants during study visits at age 12 years. Staff collected a sample approximately 5 mm in diameter from the posterior vertex portion of the head and attempted to cut the hair sample as close to the scalp as possible. Samples were shipped to the University of Massachusetts for processing and cortisol analysis following the methods described in Meyer et al., with minor modifications (45). Briefly, we cut the proximal samples to a length of 3 cm to represent hair growth over roughly the preceding 3 months (based on an average hair growth rate of 1 cm/month). Each sample was weighed, washed twice with isopropanol, then ground to a fine powder using a bead mill. Cortisol was extracted into methanol, the methanol was evaporated, and the dried extract was reconstituted in assay buffer and spin-filtered to remove any residual particulate matter. Finally, each sample was analyzed in duplicate for cortisol along with standards and quality controls using the Arbor Assays DetectX enzyme-linked immunosorbent assay (ELISA), and the assay output was converted to pg cortisol per mg hair weight. The limit of detection for the cortisol assay was 45.4 pg/ml of reconstituted hair extract and all samples were above the limit of detection. The intra- and interassay coefficients of variation for this assay are <10%. Four samples were excluded from subsequent statistical analysis because their cortisol content was still above the highest standard in the ELISA after 100-fold dilution, suggesting potential contamination from the use of a corticosteroid-containing medication.
Participant characteristics and covariates
We identified participant characteristics and variables that may be related to phthalate or PFAS exposure and adolescent cortisol concentrations. During pregnancy and at study visits after birth, trained research staff collected information on maternal age, maternal education, household income, and child’s race and ethnicity using standardized interviews. We abstracted child sex from hospital medical charts.
We used these participant characteristics in directed acyclic graphs to identify confounding variables and covariates a priori based the scientific literature (Web Figures 1 and 2). We included child’s sex (categorical), child’s race/ethnicity (categorical), child’s age at hair cortisol assessment (continuous, months), maternal education (categorical), and parity (categorical) as covariates in our main adjusted models. For analyses of PFAS, we also included breastfeeding duration as a covariate. Breastfeeding could be a source of PFAS exposure that is also related to HPA axis activity.
In sensitivity analyses, we considered additional covariates that could reduce residual confounding or increase the precision of our estimates, including diet, physical activity, recent hair product use, and tobacco smoke exposure. To assess diet, we administered 24-hour food recalls on three occasions, including one weekend day, as part of the 12-year study visit. We used the Nutrition Data Systems for Research software and foods database (University of Minnesota, MN) to calculate the Healthy Eating Index (HEI) scores (46, 47). The Healthy Eating Index assesses the extent to which an individual’s diet conforms with federal dietary guidance, and higher scores indicate better diet quality. We quantified objective measures of physical activity using accelerometry. At the 12-year study visit, participants were instructed to wear an ActiGraph GT3X+ accelerometer on their wrist (24 hours/day) for 1 week and return it via postal service. We used 5-second epochs and previously published activity intensity classifications to estimate the average amount of moderate and vigorous physical activity time (minutes) per valid day (48). Because hair products could be a source of chemical exposures and breakdown hair cortisol, we asked participants about their use of hair products in the 24 hours prior to hair collection. In our analyses we considered hair products to be: shampoo, conditioner or crème rinse, hair grease, hair oil, texture balm, hair spray, hair gel, or hair treatments (e.g., bleach, perm, straightener/relaxer, or dye). To assess gestational and childhood tobacco smoke exposure, we used maternal average serum cotinine concentrations collected at 16 and 26 weeks’ gestation and children’s average serum cotinine concentration from samples collected at ages 1, 2, 3 and 4 years (49).
Statistical methods
We examined variable distributions and calculated univariate statistics for hair cortisol, urinary phthalate metabolite and serum PFAS concentrations, and covariates. We used Pearson correlation coefficients to evaluate bivariate correlations between individual phthalate metabolites and PFAS concentrations, and we evaluated differences in the geometric mean concentrations among participants with or without recent hair product use. We also compared geometric mean phthalate metabolite and PFAS concentrations between Black and White participants.
In separate models for phthalates and PFAS, we estimated unadjusted and covariate-adjusted prospective relationships between the chemical mixtures and log10-transformed hair cortisol concentrations using quantile-based g-computation (50). We used separate models representing: 1) average phthalate metabolite concentrations during gestation (measurement-error corrected, log10-transformed); 2) childhood phthalate metabolite intensity up to age 8 years (measurement-error corrected, log10-transformed); 3) average serum PFAS concentrations during gestation; and 4) average childhood serum PFAS up to age 8 years. We did not use 12-year phthalate metabolites or PFAS concentrations in the childhood mixture models because urine and serum samples were collected at the same time of hair collection. Therefore, it is possible that recent exposure would not yet impact hair cortisol concentrations. However, we included 12-year phthalate metabolite intensities and PFAS concentrations in the periods of heightened susceptibility analyses to examine both prospective and cross-sectional associations.
Using quantile-based g-computation, we estimated the overall effect on hair cortisol concentrations for a one quartile increase in all phthalate metabolite or PFAS concentrations (i.e., ψ) during either exposure period, the effects in both the positive and negative directions (i.e., directional scaled effects), and the relative contribution (i.e., weight) of each individual phthalate metabolite or PFAS to each directional scaled effects. Finally, we evaluated relationships of individual phthalate metabolites and PFAS with hair cortisol in separate multivariable linear regression models for gestation and childhood.
We also evaluated periods of heightened susceptibility to phthalates and PFAS. We used multiple informant models with generalized estimating equations (GEE) to jointly evaluate the relationships of phthalate metabolites (measurement-error corrected, log10-transformed) during gestation and at ages 1, 2, 3, 4, 5, 8, and 12 years with log10-transformed hair cortisol concentrations at age 12 years. Moreover, we used these models to examine relationships of PFAS concentrations during gestation and at ages 3, 8, and 12 years with hair cortisol. In these models, we examined whether the relationships of phthalate metabolites and PFAS with hair cortisol concentrations varied across exposure periods using the chemical-by–exposure period product terms. We considered a P for interaction of <0.05 to indicate possible differences in these relations by exposure period.
In secondary mixtures analyses, we examined whether relationships of phthalate metabolites and PFAS mixtures with hair cortisol varied by sex or race/ethnicity. We hypothesized that the relationships of these chemical mixtures with cortisol could be sex-specific due to biological differences in hormone levels and neuroendocrine development. We also hypothesized that the effect of phthalate exposure on hair cortisol could differ by race due to the intersection of two factors. Black participants may be more exposed to certain phthalates, possibly from consumer products, and have additional risk factors for higher hair cortisol concentrations, namely those related to psychosocial stressors and discrimination (51, 52). In mixtures analyses, we examine effect modification by sex or race/ethnicity in adjusted models using the R package qgcompint. We also stratified chemical mixture analyses by sex or race/ethnicity. We limited the race/ethnicity effect modification analyses to only participants who self-identified as Black or White due to the limited sample size of other racial/ethnic groups. We conducted the analyses in R, version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria), using the package qgcomp and SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Sensitivity analyses
To examine the robustness of analyses assessing childhood phthalate mixtures, we conducted sensitivity analyses further adjusting for: 1) any hair product use in the previous 24 hours; 2) average gestational and childhood cotinine concentrations; and 3) hair product use, average daily minutes of physical activity, and the Healthy Eating Index.
RESULTS
Participant characteristics
The median age of participants was 12.2 years (25th, 75th = 11.9, 12.7) and median hair cortisol concentration was 2.4 pg/mg hair (25th, 75th = 1.4, 5.5). Boys tended to have higher cortisol concentrations than girls. Participants with mothers who identified them as Black, who had a high school education or less, who had more than 1 prior pregnancy, and household income less than $45,000 per year had higher cortisol concentrations than other groups (Table 1). Participants included in our analyses had similar characteristics to those at baseline (Web Table 1). Children who recently used hair products tended to have lower hair cortisol concentrations compared with those who had not used any hair products in the prior 24 hours.
Table 1.
Participant Characteristics and Adolescent Hair Cortisol Concentrations, Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019
| Characteristic | No. | % |
Hair Cortisol (pg/mg), Median (25th, 75th) |
|---|---|---|---|
| Total | 205 | 100 | 2.4 (1.4, 5.5) |
| Child age, years | |||
| 11.0–12.0 | 65 | 31 | 2.4 (1.3, 5.2) |
| 12.1–13.0 | 108 | 53 | 2.3 (1.4, 7.0) |
| 13.1–14.0 | 32 | 16 | 2.6 (1.5, 4.6) |
| Child sex | |||
| Boys | 89 | 43 | 3.0 (1.7, 6.8) |
| Girls | 116 | 57 | 2.1 (1.3, 4.9) |
| Child race/ethnicity | |||
| Non-Hispanic White | 125 | 61 | 1.7 (1.2, 3.3) |
| Non-Hispanic Black | 68 | 33 | 7.3 (2.8, 15.8) |
| Another race | 12 | 6 | 2.0 (1.5, 2.7) |
| Maternal education | |||
| High school or less | 46 | 22 | 5.1 (1.7, 12.7) |
| Some college | 60 | 29 | 3.2 (1.4, 6.2) |
| College graduate | 99 | 48 | 1.8 (1.2, 3.3) |
| Household income, $ | |||
| <45,000 | 80 | 39 | 4.1 (1.6, 11.0) |
| 45,000–75,000 | 69 | 34 | 2.1 (1.3, 3.8) |
| >75,000 | 56 | 27 | 1.7 (1.3, 3.4) |
| Parity | |||
| 0 | 88 | 43 | 2.0 (1.3, 4.2) |
| 1 | 69 | 34 | 2.4 (1.6, 5.5) |
| >1 | 48 | 23 | 3.2 (1.5, 8.9) |
| Any hair product use in past 24 hours | |||
| No | 41 | 20 | 3.1 (1.3, 9.0) |
| Yes | 164 | 80 | 2.4 (1.4, 5.3) |
From ages 1 to 8 years, the average of the urine metabolite concentrations gradually declined for most phthalates with a more substantial decrease at age 12 years (Web Figure 3). We found weak to moderate correlation between different phthalate metabolites at the same exposure period, gestation or childhood (correlation coefficients ranging from 0.05 to 0.47; Web Tables 3–5). The same phthalate metabolites were weakly correlated across the two exposure periods (correlation coefficients ranging from −0.02 to 0.29). The highest phthalate metabolite concentration intensity at age 8 years was DEHP, followed by MEP, MCOP, MnBP, MBzP, MiBP, MCPP, and MCNP (Figure 1). Boys and girls had similar concentrations of urinary phthalate metabolites (Web Table 6). Non-Hispanic Black participants had higher concentrations of MEP compared with non-Hispanic White participants.
Figure 1.
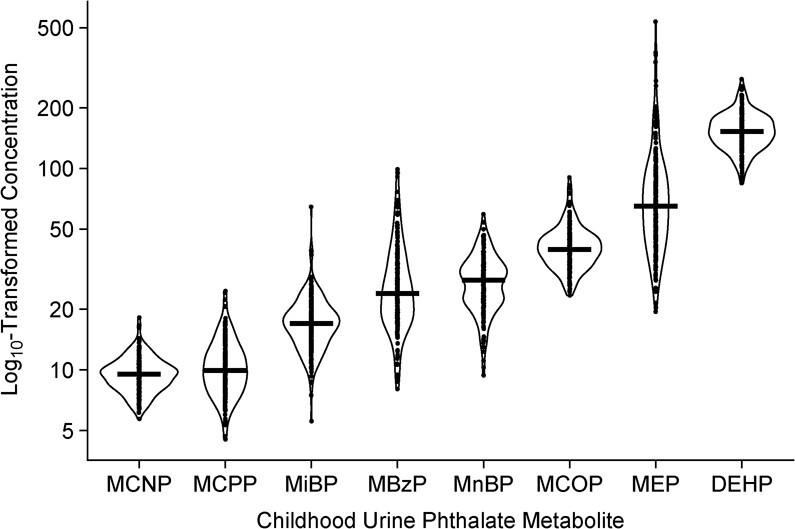
Distribution of creatinine-standardized phthalate metabolite concentration intensity (μg/g creatinine) up to age 8 years (n = 205), Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019. Solid line indicates mean of each distribution. Jittered dots are individual observations. Summary di(2-ethylhexyl) phthalate (DEHP) measure = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP) (standardized to units of ng MECPP/mL). MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, monoisobutyl phthalate; MnBP, mono-n-butyl phthalate.
Concentrations of the same PFAS were similar between gestation and childhood (Web Figure 4). We found weak to moderate correlations between different PFAS at the same exposure period (correlation coefficients ranging from 0.01 to 0.68; Web Tables 3–5). Average childhood PFAS concentrations were higher for PFOS and PFOA than PFHXS and PFNA (Figure 2). Average childhood PFAS concentrations did not meaningfully differ by sex, and White participants tended to have higher concentrations compared with Black participants (Web Table 6).
Figure 2.
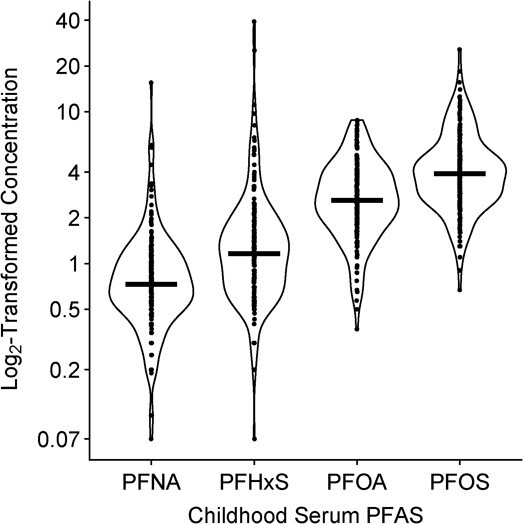
Distribution of the average childhood serum per-/polyfluoroalkyl substances (PFAS) concentrations (ng/mL) for ages 3 and 8 years (n = 174), Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019. Solid line indicates mean of each distribution. Jittered dots are individual observations. PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate.
Relationships of phthalate metabolites and PFAS with hair cortisol
During childhood, a quartile increase in all phthalate metabolite intensities was associated with 35% higher hair cortisol (adjusted ψ for log10cortisol = 0.13; 95% confidence interval (CI): 0.03, 0.22; Table 2). Based on the weights, the individual phthalate metabolites contributing most to this positive scaled effect were MEP, MiBP, and MBzP (weights = 0.34, 0.27, and 0.25, respectively; Table 3). In contrast, we did not find that higher phthalate metabolite mixtures during gestation were associated with higher hair cortisol concentrations. Moreover, higher concentrations during childhood, but not gestation, tended to be associated with higher hair cortisol in analyses of individual phthalate metabolites (Figure 3 and Web Table 7).
Table 2.
Adjusted Difference in Log10-Transformed Hair Cortisol per Quartile Increase in Gestational or Childhood Phthalate Metabolite and Per-/Polyfluoroalkyl Substances Mixture, Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019
| Gestation | Childhood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixture | All Children | All Children (n = 205) | Girls (n = 116) | Boys (n = 89) | NHB (n = 68) | NHW (n = 125) | ||||||
| Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | |
| Phthalate mixture ψa | 0.00 | −0.09, 0.10 | 0.13 | 0.03, 0.22 | 0.09 | −0.03, 0.21 | 0.16 | 0.01, 0.32 | 0.27 | 0.07, 0.47 | 0.02 | −0.08, 0.12 |
| PFAS mixture ψb | 0.01 | −0.08, 0.09 | −0.05 | −0.14, 0.05 | 0.03 | −0.09, 0.16 | −0.10 | −0.27, 0.07 | −0.05 | −0.35, 0.24 | −0.01 | −0.11, 0.09 |
Abbreviations: CI, confidence interval; NHB, non-Hispanic Black; NHW, non-Hispanic White; PFAS, per-/polyfluoroalkyl substances.
a Adjusted quantile-based g-computation model includes child sex (categorical), child race (categorical), age at hair collection (continuous), maternal education (categorical), and parity (categorical); n = 205. Sex × childhood phthalate metabolite mixture, P for interaction = 0.82. Among NHW and NHB participants (n = 179), race × childhood phthalate metabolite mixture, P for interaction = 0.026.
b Adjusted quantile-based g-computation model includes child sex (categorical), child race (categorical), age at hair collection (continuous), maternal education (categorical), parity (categorical), and breastfeeding duration (continuous); gestation n = 175, childhood n = 174. Sex × childhood PFAS mixture P for interaction = 0.81. Among NHW and NHB participants, race × childhood PFAS mixture P for interaction = 0.68.
Table 3.
Adjusted Difference in Log10-Transformed Hair Cortisol per Quartile Increase in Gestational or Childhood Phthalate Metabolite Mixture, Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019a
| Gestation | Childhood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Children | All Children (n = 205) | Girls (n = 116) | Boys (n = 89) | NHB (n = 68) | NHW (n = 125) | |||||||
| Effect Size and Weights | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive |
| Scaled effect size | −0.08 | 0.08 | −0.07 | 0.20 | −0.08 | 0.17 | −0.21 | 0.37 | −0.21 | 0.48 | −0.15 | 0.18 |
| Weights | ||||||||||||
| DEHPb | 0.28 | 0.09 | 0.46 | 0.28 | 0.06 | 0.11 | ||||||
| MBzP | 0.68 | 0.25 | 0.26 | 0.03 | 0.36 | 0.25 | ||||||
| MCNP | –c | –c | 0.04 | 0.03 | 0.29 | 0.04 | 0.41 | |||||
| MCOP | –c | –c | 0.08 | 0.11 | 0.07 | 0.23 | 0.15 | |||||
| MCPP | 0.14 | 0.01 | 0.04 | 0.18 | 0.43 | 0.34 | ||||||
| MEP | 0.25 | 0.34 | 0.45 | 0.21 | 0.09 | 0.02 | ||||||
| MiBP | 0.19 | 0.27 | 0.23 | 0.24 | 0.27 | 0.13 | ||||||
| MnBP | 0.47 | 0.92 | 0.44 | 0.71 | 0.53 | 0.59 | ||||||
Abbreviations: DEHP, di(2-ethylhexyl) phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxyoctyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MEP, monoethyl phthalate; MiBP, monoisobutyl phthalate; MnBP, mono-n-butyl phthalate; NHB, non-Hispanic Black; NHW, non-Hispanic White.
a Adjusted quantile-based g-computation model includes child sex (categorical), child race (categorical), age at hair collection (continuous), maternal education (categorical), and parity (categorical); n = 205. Weights in each direction sum to approximately 1.0 and represent the proportion that each metabolite contributes to the positive or negative scaled effect.
b Summary DEHP measure = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP).
c Phthalate metabolites not quantified in urine samples.
Figure 3.
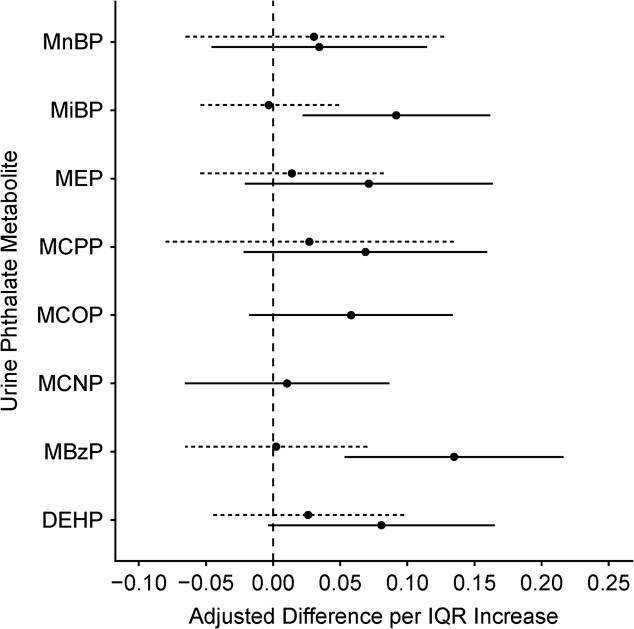
Adjusted difference in log10-transformed hair cortisol per interquartile range increase in log10-transformed phthalate metabolite concentration (n = 205), Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019. Dashed lines represent results from models with gestational phthalate metabolites and solid lines represent results with childhood phthalate metabolites. Models adjusted for child sex, child race, age at hair collection, maternal education, and parity. Phthalate metabolite interquartile ranges: di(2-ethylhexyl) phthalate (DEHP) = 0.12; monobenzyl phthalate (MBzP) = 0.27; monocarboxynonyl phthalate (MCNP) = 0.11; monocarboxyoctyl phthalate (MCOP) = 0.13; mono(3-carboxypropyl) phthalate (MCPP) = 0.18; monoethyl phthalate (MEP) = 0.31; monoisobutyl phthalate (MiBP) = 0.15; mono-n-butyl phthalate (MnBP) = 0.18. Summary DEHP measure = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP).
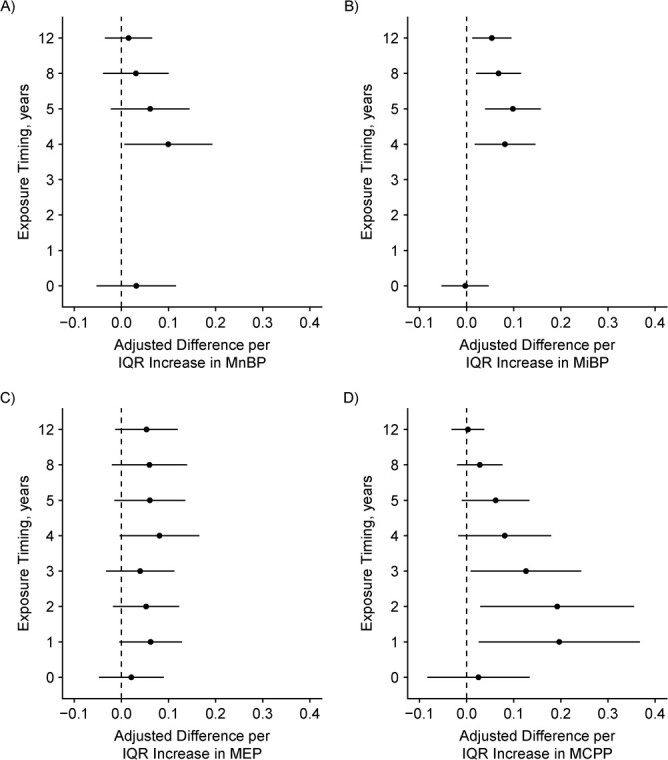
We found suggestive evidence that the associations of urinary MBzP and MCOP concentrations with adolescent hair cortisol concentrations varied by exposure period (P for heterogeneity = 0.10 and 0.06 respectively; Figure 4 and Web Table 8). For MBzP and MCOP, concentrations during infancy, compared with late childhood or adolescence, tended to be more strongly associated with higher hair cortisol concentrations.
Figure 4 . Continues.
We did not find evidence to suggest that gestational or childhood PFAS mixtures were associated with hair cortisol concentrations (Tables 2 and 4). In analyses of individual PFAS, we found slight evidence that each IQR increase of log2-transformed PFOS, PFOA, and PFHxS concentrations during childhood was associated with approximately 20% lower hair concentrations (Figure 5 and Web Table 9). We also found some evidence that an IQR increase in log2-transformed PFNA concentrations at age 8 years was associated with approximately 15% higher hair cortisol concentrations. In periods of heightened susceptibility analyses, we did not find strong evidence to suggest that the association between PFAS and hair cortisol concentrations varied by the exposure timing (P for heterogeneity = 0.15 to 0.9; Web Table 10).
Table 4.
Adjusted Difference in Log10-Transformed Hair Cortisol per Quartile Increase in Gestational or Childhood Per-/Polyfluoroalkyl Substances Mixture, Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019a
| Gestation | Childhood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Children | All Children | Girls | Boys | NHB | NHW | |||||||
| Effect Size and Weights | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive |
| Scaled effect size | −0.04 | 0.05 | −0.13 | 0.08 | −0.06 | 0.10 | −0.21 | 0.12 | −0.1 | 0.04 | −0.13 | 0.12 |
| Weights | ||||||||||||
| PFOA | 1.00 | 0.47 | 0.35 | 0.43 | 0.72 | 0.57 | ||||||
| PFOS | 0.26 | 0.43 | 0.65 | 0.57 | 0.74 | 0.16 | ||||||
| PFNA | 0.11 | 1.00 | 0.84 | 0.95 | 0.26 | 1.00 | ||||||
| PFHxS | 0.63 | 0.10 | 0.16 | 0.05 | 0.28 | 0.27 | ||||||
Abbreviations: NHB, non-Hispanic Black; NHW, non-Hispanic White; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
a Adjusted quantile-based g-computation model includes child sex (categorical), child race (categorical), age at hair collection (continuous), maternal education (categorical), parity (categorical), and breastfeeding duration (continuous); gestation n = 175, childhood n = 174. Weights in each direction sum to approximately 1.0 and represent the proportion that each metabolite contributes to the positive or negative scaled effect.
Figure 5.
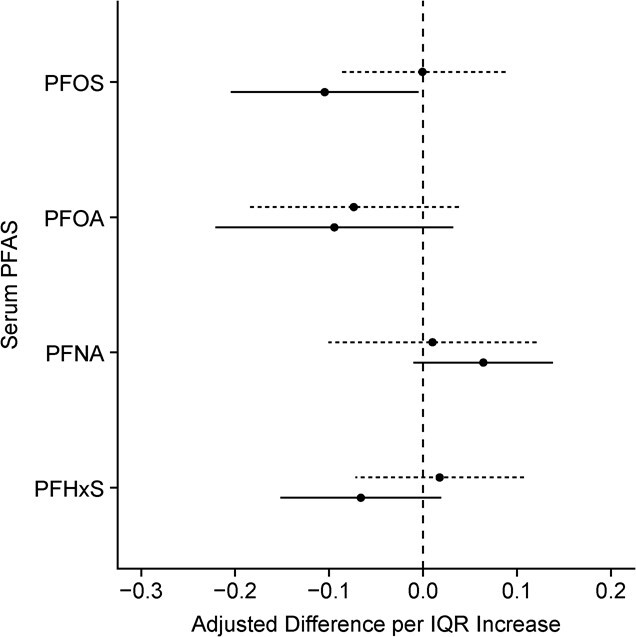
Adjusted difference in log10-transformed hair cortisol per interquartile range increase in log2-transformed serum per-/polyfluoroalkyl substances (PFAS) concentration (gestational n = 175, childhood n = 174), Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019. Dashed lines represent results from models with gestational PFAS and solid lines represent results with childhood PFAS. Models adjusted for child sex, child race, age at hair collection, maternal education, and parity. Serum PFAS interquartile ranges: perfluorooctanoate (PFOA) = 1.12; perfluorooctane sulfonate (PFOS) = 1.05; perfluorononanoate (PFNA) = 1.05; perfluorohexane sulfonate (PFHxS) = 1.42.
Figure 4.
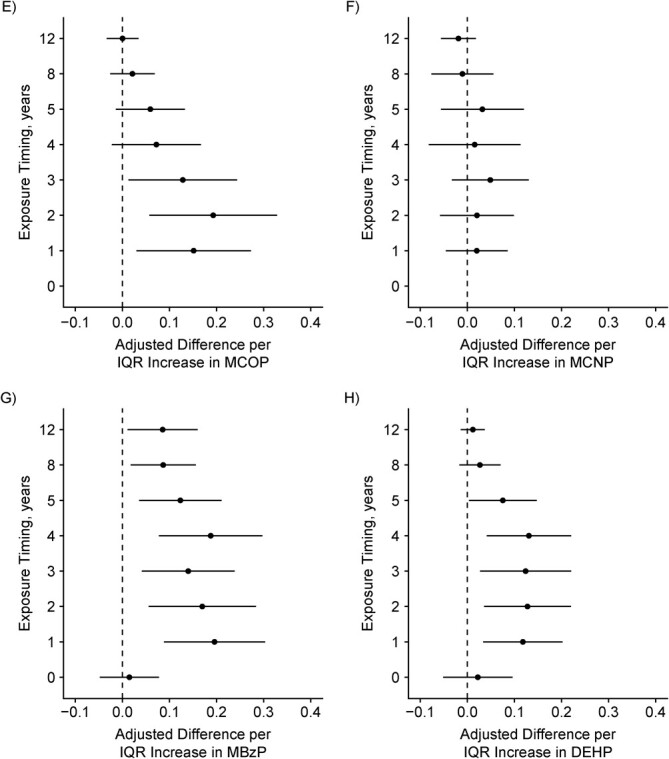
Adjusted difference in log10-transformed hair cortisol per interquartile range (IQR) increase in log10-transformed phthalate metabolite concentration during gestation and ages 1, 2, 3, 4, 5, 8, and 12 years (n = 205), Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2019. Y-axis: 0 = gestational exposure period and the rest of the points are age in years. A) Mono-n-butyl phthalate (MnBP), IQR =0.18, P for heterogeneity = 0.15; B) monoisobutyl phthalate (MiBP), IQR = 0.15, P = 0.13; C) monoethyl phthalate (MEP), IQR = 0.31, P = 0.88; D) mono(3-carboxypropyl) phthalate (MCPP), IQR = 0.18, P = 0.27; E) monocarboxyoctyl phthalate (MCOP), IQR = 0.13, P = 0.06; F) monocarboxynonyl phthalate (MCNP), IQR = 0.11, P = 0.30; G) monobenzyl phthalate (MBzP), IQR = 0.27, P = 0.10; H) summary di(2-ethylhexyl) phthalate (DEHP) = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP), IQR = 0.12, P = 0.17.
Secondary analyses of modification by sex or race/ethnicity
We did not find evidence to suggest that the association of childhood phthalate or PFAS mixtures with hair cortisol varied by sex (sex × mixture, P for interaction = 0.82 and 0.81, respectively Table 2–4). We did find that the association between childhood phthalate mixtures and hair cortisol varied by race (race × phthalate mixture, P for interaction = 0.026). In race-stratified analyses, a quartile increase in all phthalate metabolites was associated with 86% higher hair cortisol among Black participants (adjusted ψ = 0.27; 95% CI: 0.07, 0.47; Table 2); these associations were attenuated among White participants (adjusted ψ = 0.02; 95% CI: −0.08, 0.12). Among Black participants, the phthalate metabolites that contributed to the positive scaled effect based on the weights were MBzP, MiBP, and MCOP, followed by MEP and DEHP (weights = 0.36, 0.27, 0.23, 0.09, and 0.06, respectively; Table 3). The association of PFAS mixtures and hair cortisol did not vary by race (race × mixture, P for interaction = 0.68). The associations of gestational phthalate metabolite and PFAS mixtures with hair cortisol also did not vary by sex or race (Web Tables 11 and 12).
Sensitivity analyses
In sensitivity analyses, adjusting for recent hair product use, physical activity, cotinine, and Healthy Eating Index scores did not meaningfully change the relationships of phthalate metabolite mixtures with hair cortisol concentrations (Web Tables 13 and 14). Furthermore, adjusting our phthalate mixture models for PFAS concentrations did not meaningfully change our results.
DISCUSSION
We found that the mixture of urinary phthalate metabolites during childhood, but not fetal development, was associated with higher hair cortisol concentrations during adolescence. This positive association was largely driven by MEP, MiBP, and MBzP and to a lesser extent DEHP metabolites, MCNP, and MCPP. The magnitude of the association between the phthalate mixture and hair cortisol was approximately double the magnitude of the association between individual phthalate metabolites and hair cortisol. In secondary analyses, we found that these positive associations were stronger among Black participants.
We did not find evidence that higher urinary phthalate metabolites during gestation or serum PFAS mixtures during gestation or childhood were related to higher adolescent hair cortisol concentrations. We did find slight evidence that higher concentrations of individual PFAS during childhood were associated with lower (PFOS, PFOA, and PFHxS) and higher (PFNA) hair cortisol.
Our analyses are consistent with prior research and the hypothesis that phthalates affect HPA axis homeostasis. Prior studies have linked higher phthalate metabolite concentrations with higher cortisol concentrations in cord blood as well as higher urinary cortisol concentrations among infants (53, 54). Specifically, Kim et al. (53) reported that higher concentrations of several DEHP metabolites, MnBP, and MiBP during gestation and childhood were associated with higher urinary cortisol in the first 15 months of life. These associations did not differ by sex (53). Our analyses of individual phthalates also indicated a positive association between these metabolites and hair cortisol. Yet when we assessed the cumulative effect of the phthalate mixture, we did not find that MnBP contributed to the overall positive association with hair cortisol. In our analyses, the positive association between phthalate metabolite mixtures and hair cortisol was driven by several other phthalate metabolites that were moderately correlated with childhood MnBP concentrations but were not considered in the study by Kim et al. (53) Thus, it is possible that MnBP concentrations were a proxy for other phthalate metabolites.
We are not aware of any epidemiologic studies evaluating relationships of early-life PFAS exposure with adolescent cortisol. In addition to 11β-HSD2, experimental evidence suggests that PFAS may interfere with 11β-hydroxysteroid dehydrogenase type-1, which converts cortisone into cortisol (55). These isoforms are expressed in different tissues, and the degree of inhibition may differ by PFAS and exposure level (31, 55–58). Therefore, additional studies are necessary to understand the overall impacts of PFAS on HPA axis activity and the regulation of cortisol, cortisone, and cortisol metabolism during specific life stages (58–63). Epidemiological studies have examined relationships of prenatal PFAS with cortisol and cortisone during pregnancy, as well as relationships of cord blood PFAS with cortisol and cortisone concentrations (58, 62). These analyses also report some evidence of a negative association between PFOS and cortisol levels; however these results may not be generalizable to adolescence.
We hypothesized that the effect of phthalate and PFAS exposure during gestation on HPA axis activity could differ from the effects of exposure during childhood. Prior research suggests that phthalate and PFAS exposure may be capable of altering placental function and disrupting the fetal programming of the HPA axis (64–66). However, our results do not provide strong evidence that phthalate or PFAS exposure during fetal development is associated with adolescent hair cortisol concentrations, one biomarker for HPA axis activity. Instead, our results suggest that more recent phthalate and PFAS exposure may be relevant for adolescent HPA axis activity. In addition to these chemicals, evidence suggests that exposure to air pollution and green space may be related to HPA axis activity (67–69). Regulation of glucocorticoid homeostasis is essential for development, and both excessive or inadequate levels of glucocorticoids during childhood could negatively affect brain function, behavior, and health (70).
Hair cortisol is a measure of HPA axis activity that integrates systemic cortisol concentrations over a period of several months; it is more temporally stable than cortisol concentrations in saliva, blood, or urine (38, 71, 72). In adults, higher concentrations of hair cortisol have been reported among individuals with chronic pain and stress, cardiovascular disease, and some mental health conditions (73–75). Among children and adults, prior research has also reported associations of higher hair cortisol concentrations with higher body mass index, larger waist circumference, insulin resistance, and higher systolic blood pressure (71, 72, 76–79).
Consistent with our analysis, prior studies of children report higher hair cortisol concentrations among Black participants compared with participants of other races (80). Racism, causing socioeconomic disadvantage and discrimination, increases chronic and intergenerational stress. This psychosocial stress could increase HPA axis activity and cortisol. Moreover, personal care products that are frequently marketed towards Black individuals may increase phthalate exposure (16, 26, 51). Past research has reported racial disparities in phthalate exposure and their relationship with pregnancy and birth outcomes (81, 82). These associations may also be stronger among women experiencing psychosocial stress (83). Future studies evaluating multiple dimensions of racial discrimination and psychosocial stressors, phthalate exposure, and HPA axis activity among Black populations could help to improve understanding of how chemical and nonchemical stressors interact to exert an impact on health.
Our study had some limitations. First, it is possible that other metabolic factors, psychosocial stressors, or behavioral factors correlated with phthalate or PFAS exposure and hair cortisol could contribute to residual confounding. Second, phthalates are biologically nonpersistent and phthalate metabolite concentrations quantified in spot urine samples may not represent exposure across a developmental period. This potential nondifferential exposure misclassification could bias our results towards the null, especially for the periods of heightened susceptibility analyses. Third, hair cortisol concentrations could be affected by differences in individual hair growth rates, hair pigmentation, hair treatments (e.g., dyes and perms), and frequency of hair washing. Due to social and cultural norms, use of certain hair treatments may be more common among specific racial groups. Therefore, these practices may contribute to racial differences in hair cortisol concentrations. Yet a systematic review of studies evaluating determinants of hair cortisol among children found that cortisol concentrations were not related with use of hair products or frequency of hair washing when the proximal 3-cm segments of hair were used (72). Fourth, there is potential for selection bias to influence our results since some participants did not complete all follow-up visits.
Our study had some strengths. We used prospective and repeated measures of phthalate and PFAS exposure across fetal development and childhood. Moreover, we used regression-calibration approaches to reduce the impact of phthalate exposure misclassification on our main analyses. We also assessed HPA axis activity using hair cortisol, which is thought to be a measure of prolonged cortisol secretion and disruption of hormonal homeostasis. Finally, we estimated the combined effects of phthalates and PFAS using novel mixtures analyses.
In conclusion, our results—which suggest that higher urinary phthalate metabolites during childhood are associated with higher hair cortisol concentrations during adolescence—support the hypothesis that phthalate exposure may disrupt HPA axis homeostasis. This disruption could be one pathway underlying relationships of early-life phthalate exposure with cardiometabolic risk and adverse neurobehavioral outcomes (1–4, 8, 9, 11). We found some evidence that individual serum PFAS during childhood may be associated with adolescent hair cortisol, but additional studies would help to clarify our results. Future research is necessary to understand how phthalate and PFAS exposures and psychosocial stressors interact to impact HPA axis homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Christina Lee Brown Envirome Institute, Department of Medicine, Division of Environmental Medicine, University of Louisville, Kentucky, United States (Clara G. Sears); Department of Epidemiology, Brown University, Providence, Rhode Island, United States (Clara G. Sears, Yun Liu, Joseph M. Braun); Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada (Bruce P. Lanphear); Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States (Jessie P. Buckley); Department of Psychological and Brain Sciences, University of Massachusetts, Amherst, Massachusetts, United States (Jerrold Meyer); Division of General and Community Pediatrics, Cincinnati Children’s Hospital, Cincinnati, Ohio, United States (Yingying Xu); Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, United States (Aimin Chen); Department of Pediatrics, Cincinnati Children’s Hospital, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States (Kimberly Yolton); and Department of Environmental and Public Health Sciences, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States (Kimberly Yolton).
This work was supported by the National Institutes of Health (grants R01 ES032386, R01 ES035133, P01 ES011261, R01 ES014575, R01 ES020349, R01 ES027224, R01 ES025214, and 2UL1TR001425). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data for this analysis will be made available on reasonable request.
We are grateful to the participants for the time they have given to this study.
Presented at the 2021 International Society for Environmental Epidemiology Conference (online), August 23–26, 2021, New York, New York.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
J.M.B was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water. B.P.L has served as an expert witness for plaintiffs in litigation related to lead poisoning prevention cases, but he received no personal payments for his services. His institution was financially compensated for some of those cases. The other authors report no conflicts.
REFERENCES
- 1. Attina TM, Trasande L. Association of exposure to di-2-ethylhexylphthalate replacements with increased insulin resistance in adolescents from NHANES 2009–2012. J Clin Endocrinol Metab. 2015;100(7):2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoaff J, Papandonatos GD, Calafat AM, et al. Early-life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect. 2017;125(9):097008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James-Todd TM, Huang T, Seely EW, et al. The association between phthalates and metabolic syndrome: the National Health and Nutrition Examination Survey 2001–2010. Environ Health. 2016;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribeiro C, Mendes V, Peleteiro B, et al. Association between the exposure to phthalates and adiposity: a meta-analysis in children and adults. Environ Res. 2019;179(pt A):108780. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Li N, Papandonatos GD, et al. Exposure to per- and polyfluoroalkyl substances and adiposity at age 12 years: evaluating periods of susceptibility. Environ Sci Technol. 2020;54(24):16039–16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li N, Liu Y, Papandonatos GD, et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ Int. 2021;147:106344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braun JM, Eliot M, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond). 2021;45(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Q, Chen XZ, Huang X, et al. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology. 2019;73:199–212. [DOI] [PubMed] [Google Scholar]
- 9. Li N, Papandonatos GD, Calafat AM, et al. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ Res. 2019;172:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li N, Papandonatos GD, Calafat AM, et al. Gestational and childhood exposure to phthalates and child behavior. Environ Int. 2020;144:106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engel SM, Villanger GD, Nethery RC, et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort. Environ Health Perspect. 2018;126(5):057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bornehag CG, Lundgren B, Weschler CJ, et al. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect. 2005;113(10):1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlstedt F, Jönsson BAG, Bornehag CG. PVC flooring is related to human uptake of phthalates in infants. Indoor Air. 2013;23(1):32–39. [DOI] [PubMed] [Google Scholar]
- 14. Cirillo T, Fasano E, Esposito F, et al. Study on the influence of temperature, storage time and packaging type on di-n-butylphthalate and di(2-ethylhexyl)phthalate release into packed meals. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(2):403–411. [DOI] [PubMed] [Google Scholar]
- 15. Duty SM, Ackerman RM, Calafat AM, et al. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Just AC, Adibi JJ, Rundle AG, et al. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York City. J Expo Sci Environ Epidemiol. 2010;20(7):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wormuth M, Scheringer M, Vollenweider M, et al. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803–824. [DOI] [PubMed] [Google Scholar]
- 19. Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954–7961. [DOI] [PubMed] [Google Scholar]
- 20. Susmann HP, Schaider LA, Rodgers KM, et al. Dietary habits related to food packaging and population exposure to PFASs. Environ Health Perspect. 2019;127(10):107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eick SM, Goin DE, Trowbridge J, et al. Dietary predictors of prenatal per- and poly-fluoroalkyl substances exposure. J Expo Sci Environ Epidemiol. 2023;33(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun JM, Smith KW, Williams PL, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120(5):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun JM, Just AC, Williams PL, et al. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casas L, Fernández MF, Llop S, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–866. [DOI] [PubMed] [Google Scholar]
- 25. Kasper-Sonnenberg M, Koch HM, Wittsiepe J, et al. Levels of phthalate metabolites in urine among mother-child-pairs—results from the Duisburg Birth Cohort Study, Germany. Int J Hyg Environ Health. 2012;215(3):373–382. [DOI] [PubMed] [Google Scholar]
- 26. Watkins DJ, Eliot M, Sathyanarayana S, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol. 2014;48(15):8881–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whitehead HD, Venier M, Wu Y, et al. Fluorinated compounds in North American cosmetics. Environ Sci Technol Lett. 2021;8(7):538–544. [Google Scholar]
- 29. Zhang J, Yang Y, Liu W, et al. Glucocorticoid and mineralocorticoid receptors and corticosteroid homeostasis are potential targets for endocrine-disrupting chemicals. Environ Int. 2019;133(Pt A):105133. [DOI] [PubMed] [Google Scholar]
- 30. La Merrill MA, Vandenberg LN, Smith MT, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao B, Lian Q, Chu Y, et al. The inhibition of human and rat 11B-hydroxysteroid dehydrogenase 2 by perfluoroalkylated substances. J Steroid Biochem Mol Biol. 2011;125(1–2):143–147. [DOI] [PubMed] [Google Scholar]
- 32. Zhao B, Chu Y, Huang Y, et al. Structure-dependent inhibition of human and rat 11β-hydroxysteroid dehydrogenase 2 activities by phthalates. Chem Biol Interact. 2010;183(1):79–84. [DOI] [PubMed] [Google Scholar]
- 33. Ye L, Guo J, Ge RS. Environmental pollutants and hydroxysteroid dehydrogenases. Vitam Horm. 2014;94:349–390. [DOI] [PubMed] [Google Scholar]
- 34. Ma X, Lian QQ, Dong Q, et al. Environmental inhibitors of 11β-hydroxysteroid dehydrogenase type 2. Toxicology. 2011;285(3):83–89. [DOI] [PubMed] [Google Scholar]
- 35. Kolšek K, Gobec M, Mlinarič Raščan I, et al. Molecular docking revealed potential disruptors of glucocorticoid receptor-dependent reporter gene expression. Toxicol Lett. 2014;226(2):132–139. [DOI] [PubMed] [Google Scholar]
- 36. Sheikh IA, Beg MA. Endocrine disruption: in silico interactions between phthalate plasticizers and corticosteroid binding globulin. J Appl Toxicol. 2017;37(12):1471–1480. [DOI] [PubMed] [Google Scholar]
- 37. Braun JM, Gennings C, Hauser R, et al. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124(1):A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer JS, Novak MA. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13(3):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braun JM, Kalloo G, Chen A, et al. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braun JM, Buckley JP, Cecil KM, et al. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: cohort profile. BMJ Open. 2020;10(5):e034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silva M, Samandar E, Preaujr J, et al. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B. 2007;860(1):106–112. [DOI] [PubMed] [Google Scholar]
- 43. Jackson-Browne MS, Papandonatos GD, Chen A, et al. Early-life triclosan exposure and parent-reported behavior problems in 8-year-old children. Environ Int. 2019;128:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang H, Yolton K, Webster GM, et al. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8years. Environ Int. 2018;111:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyer J, Novak M, Hamel A, et al. Extraction and analysis of cortisol from human and monkey hair. J Vis Exp. 2014;83(83):e50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–1271. [PubMed] [Google Scholar]
- 48. Chandler JL, Brazendale K, Beets MW, et al. Classification of physical activity intensities using a wrist-worn accelerometer in 8-12-year-old children: wrist-worn accelerometry in children. Pediatr Obes. 2016;11(2):120–127. [DOI] [PubMed] [Google Scholar]
- 49. Bernert JT, McGuffey JE, Morrison MA, et al. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–339. [DOI] [PubMed] [Google Scholar]
- 50. Keil AP, Buckley JP, O’Brien KM, et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418.e1–418.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim JH, Lee J, Moon HB, et al. Association of phthalate exposures with urinary free cortisol and 8-hydroxy-2′-deoxyguanosine in early childhood. Sci Total Environ. 2018;627:506–513. [DOI] [PubMed] [Google Scholar]
- 54. Sun X, Li J, Jin S, et al. Associations between repeated measures of maternal urinary phthalate metabolites during pregnancy and cord blood glucocorticoids. Environ Int. 2018;121(Pt 1):471–479. [DOI] [PubMed] [Google Scholar]
- 55. Ye L, Zhao B, Cai XH, et al. The inhibitory effects of perfluoroalkyl substances on human and rat 11β-hydroxysteroid dehydrogenase 1. Chem Biol Interact. 2012;195(2):114–118. [DOI] [PubMed] [Google Scholar]
- 56. Crowley RK, Woods CP, Hughes BA, et al. Increased central adiposity and decreased subcutaneous adipose tissue 11β-hydroxysteroid dehydrogenase type 1 are associated with deterioration in glucose tolerance—a longitudinal cohort study. Clin Endocrinol (Oxf). 2019;91(1):72–81. [DOI] [PubMed] [Google Scholar]
- 57. Hassan-Smith ZK, Morgan SA, Sherlock M, et al. Gender-specific differences in skeletal muscle 11β-HSD1 expression across healthy aging. J Clin Endocrinol Metab. 2015;100(7):2673–2681. [DOI] [PubMed] [Google Scholar]
- 58. Dreyer AF, Jensen RC, Glintborg D, et al. Perfluoroalkyl substance exposure early in pregnancy was negatively associated with late pregnancy cortisone levels. J Clin Endocrinol Metab. 2020;105(8):e2834–e2844. [DOI] [PubMed] [Google Scholar]
- 59. Piekarski D, Diaz K, McNerney M. Perfluoroalkyl chemicals in neurological health and disease: human concerns and animal models. Neurotoxicology. 2020;77:155–168. [DOI] [PubMed] [Google Scholar]
- 60. Pereiro N, Moyano R, Blanco A, et al. Regulation of corticosterone secretion is modified by PFOS exposure at different levels of the hypothalamic–pituitary–adrenal axis in adult male rats. Toxicol Lett. 2014;230(2):252–262. [DOI] [PubMed] [Google Scholar]
- 61. Austin ME, Kasturi BS, Barber M, et al. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111(12):1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goudarzi H, Araki A, Itoh S, et al. The association of prenatal exposure to perfluorinated chemicals with glucocorticoid and androgenic hormones in cord blood samples: the Hokkaido Study. Environ Health Perspect. 2017;125(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu H, Pan Y, Jin S, et al. Associations of per-/polyfluoroalkyl substances with glucocorticoids and progestogens in newborns. Environ Int. 2020;140:105636. [DOI] [PubMed] [Google Scholar]
- 64. Huang Y, Li J, Garcia JM, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PloS One. 2014;9(2):e87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhu Y-D, Gao H, Huang K, et al. Prenatal phthalate exposure and placental size and shape at birth: a birth cohort study. Environ Res. 2018;160:239–246. [DOI] [PubMed] [Google Scholar]
- 66. Mose T, Mortensen H, Knudsen LE. Phthalate monoesters in perfusate from a dual placenta perfusionsystem, the placenta tissue and umbilical cord blood. Reprod Toxicol. 2007;23(1):83–91. [DOI] [PubMed] [Google Scholar]
- 67. Wing SE, Bandoli G, Telesca D, et al. Chronic exposure to inhaled, traffic-related nitrogen dioxide and a blunted cortisol response in adolescents. Environ Res. 2018;163:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verheyen VJ, Remy S, Lambrechts N, et al. Residential exposure to air pollution and access to neighborhood greenspace in relation to hair cortisol concentrations during the second and third trimester of pregnancy. Environ Health. 2021;20(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Triguero-Mas M, Gidlow CJ, Martínez D, et al. The effect of randomised exposure to different types of natural outdoor environments compared to exposure to an urban environment on people with indications of psychological distress in Catalonia. PloS One. 2017;12(3):e0172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Odermatt A, Gumy C, Atanasov AG, et al. Disruption of glucocorticoid action by environmental chemicals: potential mechanisms and relevance. J Steroid Biochem Mol Biol. 2006;102(1–5):222–231. [DOI] [PubMed] [Google Scholar]
- 71. Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. [DOI] [PubMed] [Google Scholar]
- 72. Gray NA, Dhana A, Van Der Vyver L, et al. Determinants of hair cortisol concentration in children: a systematic review. Psychoneuroendocrinology. 2018;87:204–214. [DOI] [PubMed] [Google Scholar]
- 73. Manenschijn L, Schaap L, van Schoor NM, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078–2083. [DOI] [PubMed] [Google Scholar]
- 74. Van Uum SHM, Sauvé B, Fraser LA, et al. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11(6):483–488. [DOI] [PubMed] [Google Scholar]
- 75. Staufenbiel SM, Penninx BWJH, Spijker AT, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–1235. [DOI] [PubMed] [Google Scholar]
- 76. Ling J, Kao TSA, Robbins LB. Body mass index, waist circumference and body fat are positively correlated with hair cortisol in children: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13050. [DOI] [PubMed] [Google Scholar]
- 77. Stalder T, Kirschbaum C, Alexander N, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573–2580. [DOI] [PubMed] [Google Scholar]
- 78. Petimar J, Rifas-Shiman SL, Hivert MF, et al. Childhood hair cortisol concentration and early teen cardiometabolic outcomes. Pediatr Obes. 2020;15(3):e12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vehmeijer FOL, Santos S, de Rijke YB, et al. Associations of hair cortisol concentrations with cardiometabolic risk factors in childhood. J Clin Endocrinol Metab. 2021;106(9):e3400–e3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anand KJS, Rovnaghi CR, Rigdon J, et al. Demographic and psychosocial factors associated with hair cortisol concentrations in preschool children. Pediatr Res. 2020;87(6):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. James-Todd T, Meeker J, Huang T, et al. Racial and ethnic variations in phthalate metabolite concentrations across pregnancy. J Expo Sci Environ Epidemiol. 2017;27(2):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bloom MS, Wenzel AG, Brock JW, et al. Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environ Int. 2019;127:473–486. [DOI] [PubMed] [Google Scholar]
- 83. Ferguson KK, Rosen EM, Barrett ES, et al. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ Int. 2019;133(Pt B):105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


