Abstract
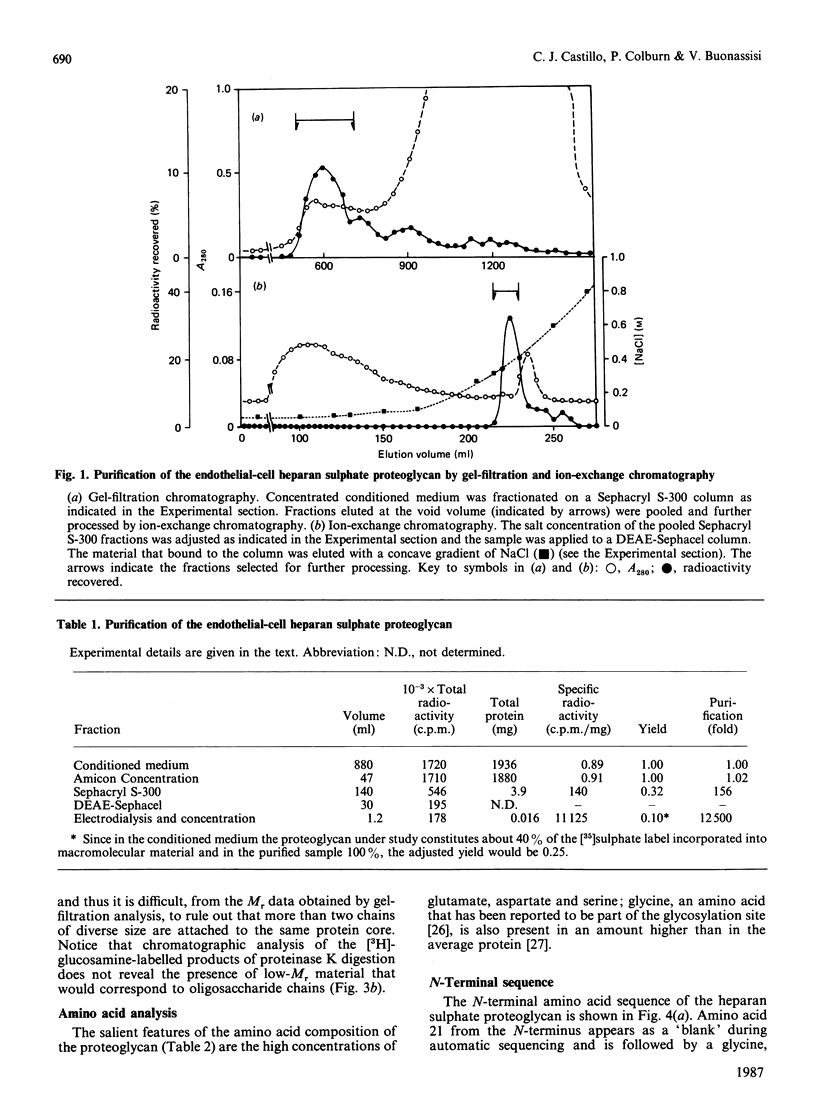
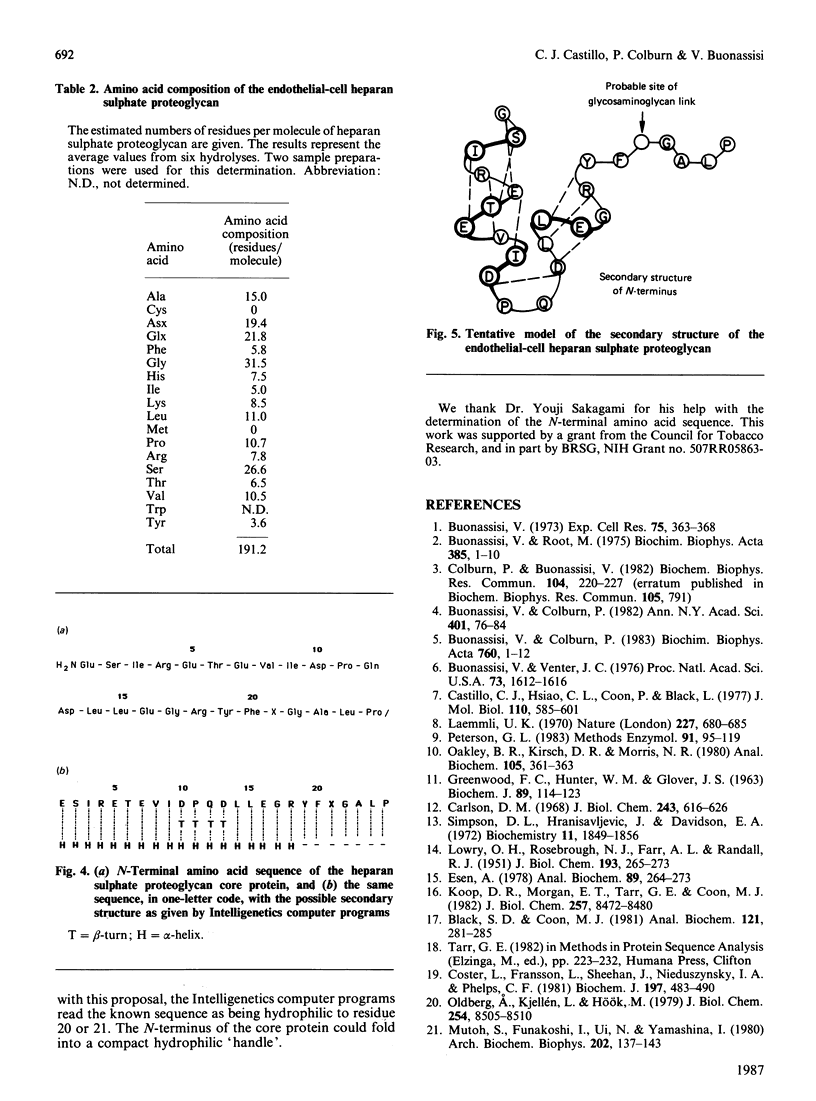
We have isolated from the conditioned medium of an established endothelial cell line a heparan sulphate proteoglycan whose involvement in the inhibition of the extrinsic coagulation pathway was reported in previous studies [Colburn & Buonassisi (1982) Biochem. Biophys. Res. Commun. 104, 220-227]. The proteoglycan was purified by gel filtration and ion-exchange chromatography, and appears to be free of contaminating proteins as determined by polyacrylamide-gel electrophoresis of the radioiodinated protein core before and after removal of the glycosaminoglycan chains by treatment with heparitinase. By this procedure the Mr of the protein core was estimated to be 22000. The N-terminal end was sequenced up to amino acid 25. The 21st residue is likely to be glycosylated. Analysis of the purified proteoglycan by gel-filtration chromatography yielded Kd values of 0.2 for the whole molecule and 0.35 for the glycosaminoglycan chains. The structure that emerges from these data is that of a heparan sulphate proteoglycan characterized by a relatively small protein core and few glycosaminoglycan chains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bella A., Jr, Danishefsky I. The dermatan sulfate-protein linkage region. J Biol Chem. 1968 May 25;243(10):2660–2664. [PubMed] [Google Scholar]
- Black S. D., Coon M. J. Simple, rapid, and highly efficient separation of amino acid phenylthiohydantoins by reversed-phase high-performance liquid chromatography. Anal Biochem. 1982 Apr;121(2):281–285. doi: 10.1016/0003-2697(82)90480-8. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Colburn P. Antibodies to the heparan sulfate proteoglycans synthesized by endothelial cell cultures. Biochim Biophys Acta. 1983 Oct 4;760(1):1–12. doi: 10.1016/0304-4165(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Colburn P. Biological significance of heparan sulfate proteoglycans. Ann N Y Acad Sci. 1982;401:76–84. doi: 10.1111/j.1749-6632.1982.tb25708.x. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Root M. Enzymatic degradation of heparin-related mucopolysaccharides from the surface of endothelial cell cultures. Biochim Biophys Acta. 1975 Mar 14;385(1):1–10. doi: 10.1016/0304-4165(75)90067-7. [DOI] [PubMed] [Google Scholar]
- Buonassisi V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp Cell Res. 1973 Feb;76(2):363–368. doi: 10.1016/0014-4827(73)90388-1. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Carlstedt I., Cöster L., Malmström A., Fransson L. A. Proteoheparan sulfate from human skin fibroblasts. Isolation and structural characterization. J Biol Chem. 1983 Oct 10;258(19):11629–11635. [PubMed] [Google Scholar]
- Castillo C. J., Hsiao C. L., Coon P., Black L. W. Identification and perperties of bacteriophage T4 capsid-formation gene products. J Mol Biol. 1977 Mar 5;110(3):585–601. doi: 10.1016/s0022-2836(77)80113-7. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Colburn P., Buonassisi V. Anti-clotting activity of endothelial cell cultures and heparan sulfate proteoglycans. Biochem Biophys Res Commun. 1982 Jan 15;104(1):220–227. doi: 10.1016/0006-291x(82)91962-3. [DOI] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A., Sheehan J., Nieduszynski I. A., Phelps C. F. Self-association of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Aug 1;197(2):483–490. doi: 10.1042/bj1970483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Ebeling W., Hennrich N., Klockow M., Metz H., Orth H. D., Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974 Aug 15;47(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03671.x. [DOI] [PubMed] [Google Scholar]
- Esen A. A simple method for quantitative, semiquantitative, and qualitative assay of protein. Anal Biochem. 1978 Aug 15;89(1):264–273. doi: 10.1016/0003-2697(78)90749-2. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mutoh S., Funakoshi I., Ui N., Yamashina I. Structural characterization of proteoheparan sulfate isolated from plasma membranes of an ascites hepatoma, AH 66. Arch Biochem Biophys. 1980 Jun;202(1):137–143. doi: 10.1016/0003-9861(80)90415-4. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Pearson C. H., Winterbottom N., Fackre D. S., Scott P. G., Carpenter M. R. The NH2-terminal amino acid sequence of bovine skin proteodermatan sulfate. J Biol Chem. 1983 Dec 25;258(24):15101–15104. [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Hranisavljevic J., Davidson E. A. Elimination and sulfite addition as a means of localization and identification of substituted seryl and threonyl residues in proteins and proteoglycans. Biochemistry. 1972 May 9;11(10):1849–1856. doi: 10.1021/bi00760a019. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Characterization of heparan sulfate proteoglycans synthesized by rat ovarian granulosa cells in culture. J Biol Chem. 1983 Nov 10;258(21):12857–12864. [PubMed] [Google Scholar]



