Abstract
Background
Epithelial ovarian cancer (EOC) is a major contributor to cancer-related illness and death among women worldwide. Obesity, a prevalent condition in many populations, has been implicated as a risk factor for various malignancies including EOC.
Objectives
This study investigated the impact of obesity on survival outcomes among women with advanced EOC in Lagos, Nigeria.
Methods
We conducted a retrospective analysis of patient medical records from a major gynaecological cancer unit of a teaching hospital in Lagos, Southwest Nigeria, to examine the relationship between body mass index (BMI) 30 kg/m2 as a measure of obesity, and progression-free (PFS) and overall survival (OS). We used Kaplan-Meier analysis stratified by patients’ BMI categories (obese versus non-obese) and compared using the Log Rank test to estimate PFS and OS. The multivariable Cox proportional hazard model was used to estimate hazard ratios (HR) of the associations between the BMI categories and survival outcomes while adjusting for all confounding clinicopathologic variables. Hypothesis tests were conducted using a two-tailed approach with a significance level of 5%.
Results
Our study showed no statistically significant association between obesity and PFS (adjusted HR = 0.62, 95% confidence interval = 0.36–1.06, p = 0.282). However, a significant association was observed between obesity with or without ascites and OS (adjusted HR = 3.58, 95% confidence interval 1.28–10.02, p = 0.015).
Conclusion
Our findings suggest that obesity negatively impacts OS in patients with EOC, thus highlighting the need to address obesity in the management of EOC by introducing comprehensive, multidisciplinary approaches incorporating weight management and personalized treatment strategies to enhance the prognosis of these patients.
Keywords: advanced EOC, body mass index, overweight, surgical debulking surgery, survival
Introduction
Epithelial ovarian cancer (EOC) accounts for the largest histological type of ovarian cancer [1] and is also the most common cause of gynaecological cancer death [1, 2]. It is often diagnosed at an advanced stage due to non-specific symptoms and a lack of effective early screening [3, 4]. Globally, EOC presents a substantial health burden [5, 6], with significant variations in incidence and outcomes based on geographical regions and population demographics [5, 6].
Despite advancements in surgical and chemotherapeutic interventions, the prognosis for women with advanced EOC remains poor, with 5-year survival rates lingering around 20%–40% worldwide [7–9]. Understanding and addressing modifiable risk factors of survival outcomes is crucial for improving prognosis and guiding treatment strategies. These risk factors include obesity, which is defined as a body mass index (BMI) of 30 kg/m² or higher [10]. It has emerged as a significant public health concern [10–12] and a potential risk factor for various cancers [13–17], including EOC [18]. The global prevalence of obesity has more than doubled since the 1980s [10, 12, 19], and it continues to rise, with significant variations across different regions [10, 12, 20]. The link between obesity and cancer outcomes is complex and multi-dimensional, encompassing hormonal imbalances, chronic inflammation, changes in adipokine levels and metabolic dysregulation [17, 21]. These factors may contribute to more aggressive tumour biology and poorer response to treatment in obese patients.
Obesity is also rising in Nigeria [22, 23], reflecting broader global trends and posing additional challenges to public health systems [11, 19, 24]. This is partly due to the high prevalence of sedentary lifestyles and the increase in processed food outlets, which is a trend observed in many African settings [23]. However, the impact of obesity on survival outcomes specifically among women with advanced EOC remains underexplored, particularly in low- and middle-income countries such as Nigeria. This study, therefore, assessed the effects of obesity on survival outcomes among women with advanced EOC managed over 10 years at a university teaching hospital in Lagos, the largest city in Nigeria, with its diverse population and healthcare infrastructure, thus providing a unique setting to study the interplay between obesity and cancer outcomes.
Patients and methods
Study design and setting
This retrospective cohort study was conducted at the gynaecological oncology unit of the Lagos University Teaching Hospital (LUTH) to extract and analyse the data of women with advanced EOC managed from January 2008 to December 2017. LUTH is the leading tertiary healthcare facility in Lagos, Southwest Nigeria. It primarily serves as a specialized referral centre for both public and private hospitals in Lagos and the neighbouring Ogun and Oyo States. The hospital's gynaecological oncology unit has four consultant gynaecological oncologists and up to 15 resident doctors. They offer a range of multidisciplinary oncology services for both in-patient and out-patient care, including the diagnosis and treatment of various premalignant and malignant diseases of the female genital tract [25, 26].
Eligibility criteria
We retrieved data from the medical records of women diagnosed and treated for EOC in the oncology unit during the review period. We additionally collected data on tumour recurrence and mortality for up to 3 years following the completion of treatment, until December 2020. Included patients were those with surgical-pathological evidence of advanced EOC (FIGO – International Federation of Gynaecology and Obstetrics stages III and IV) [27]. Also, those who had complete primary treatment comprising either preoperative neoadjuvant adjuvant chemotherapy, interval debulking surgery (IDS) and postoperative adjuvant chemotherapy (ACT) or primary debulking surgery (PDS) and postoperative (ACT); and those with complete clinical data required in the final data analyses. Women who did not have their mass index (BMI) recorded at diagnosis; those without sufficient evidence of treatment completion; and those who were lost to follow-up were excluded from the final analysis.
Study procedure and data collection
We obtained complete data for 126 women diagnosed with advanced EOC from the gynaecologic oncology ward register and their medical records. Extracted data included the patient’s age, menstrual status, parity, BMI, medical comorbidity (including hypertension, diabetes mellitus, kidney and liver disease), presence of significant ascites (up to one litre), preoperative serum CA-125 levels, type of primary surgery (PDS or IDS), surgical debulking status (optimal or suboptimal), presence of significant ascites, FIGO stage (stage III or IV), tumour histological type (Type I – endometrioid carcinoma, clear cell carcinoma, mucinous carcinoma and low-grade serous carcinomas and Type II – high-grade serous carcinomas) [28], and recurrence and timing of recurrence. Tumour recurrence was defined as either clinical or radiologic evidence of tumor regrowth, or death from any cause [4], within 3 years following the completion of primary treatment. Obesity was defined as having a BMI of 30 kg/m² or higher [29].
Study outcomes
The study endpoints were progression-free survival (PFS) defined as the period from the completion of primary treatment to the initial indication of disease progression, evidenced by either clinical or radiological detection of tumour regrowth with or without elevated serum CA125 levels; and overall survival (OS), defined as the period between the completion of primary treatment to death from any cause or the last follow-up. We censored the survival data collection after the third year of follow-up [30]. This 3-year follow-up duration falls within the scheduled period recommended for EOC patients’ surveillance in our setting comprising three monthly visits for the first 2 years, then six-monthly visits for the next 3 years, and then annually for low-risk patients. High-risk patients or those with significant comorbidities may require more frequent follow-up visits [26].
Statistical analyses
Descriptive statistics were computed for patients’ relevant socio-demographic and clinical data. For continuous variables, we used the mean and SD for normally distributed data, or the median and interquartile range for skewed data. Counts and percentages were used to report categorical variables. We used Kaplan-Meier analysis stratified by patients’ BMI categories (obese versus non-obese) and compared using the Log Rank test to estimate PFS and OS [31]. Bivariable and multivariable Cox proportional hazard models were used to estimate hazard ratios (HR) of the associations between the BMI categories and survival outcomes while adjusting for all confounding clinicopathologic variables. Using the backward stepwise conditional techniques, variables with p < 0.10 were built into the final multivariable model while a two-tailed hypothesis testing was performed at a 5% alpha level. All statistical analyses were conducted using SPSS version 29.0 for Windows (IBM Corp., Armonk, NY, USA).
Ethical considerations
Before access to the patient’s medical records and subsequent data collection, the study reported in this article was approved by the Health Research Ethics Committee of the LUTH with approval number ADM/DCST/HREC/APP/3699. The study was conducted per the Declaration of Helsinki. Strict confidentiality of patients' information was ensured during and after the completion of the study.
Results
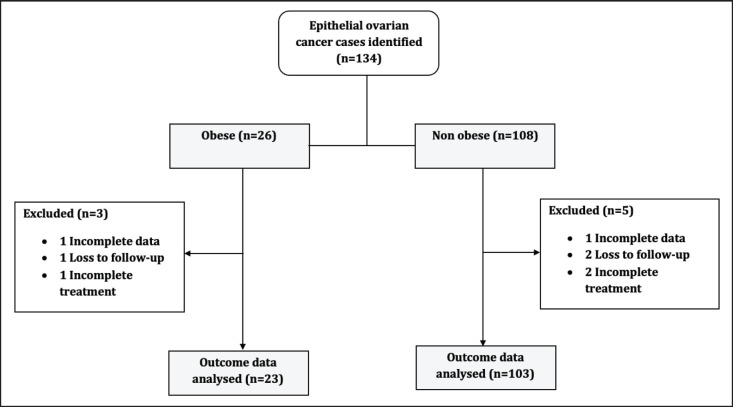
We retrieved the data of n = 134 women diagnosed with advanced EOC who had 3 years of follow-up after completing the standard first-line treatment. Of these women, n = 26 were obese while women n = 108 were non-obese (Figure 1).
Figure 1. Study patients' flow chart.
The patients’ baseline characteristics are presented in Table 1. The mean age of the women was 51.1 ± 13.3 years, and the median serum CA-125 level was 467 (246, 1,550) U/mL. Large proportions of the patients were multiparous (62.7%) and pre-menopausal (52.4%). There were statistically significant differences between obese and non-obese women with advanced EOC in terms of mean age (p = 0.013), mean serum CA 125 levels (p < 0.001), menstrual status (p = 0.005) and presence of comorbidities (p = 0.003).
Table 1. Patients baseline characteristics (n = 126).
| Characteristics | BMI category | p-value | |
|---|---|---|---|
| Obese (%) | Non-obese (%) | ||
| 23 (18.3) | 103 (81.7) | ||
| Mean age (± SD) in years | 57.3 ± 10.9 | 49.8 ± 13.4 | 0.013 |
| Median CA-125 levels (IQR) in U/mL | 1,007 (467, 1,550) | 449 (210, 1,452) | <0.001 |
| Parity | |||
| Nulliparity | 6 (26.1%) | 41 (39.8%) | 0.219 |
| Multiparity | 17 (73.9%) | 62 (60.2%) | |
| Menstrual status | |||
| Pre-menopause | 6 (26.1%) | 60 (58.3%) | 0.005 |
| Post-menopause | 17 (73.9%) | 43 (41.7%) | |
| Medical comorbidity | |||
| Yes | 11 (47.8%) | 19 (18.4%) | 0.003 |
| No | 12 (52.2%) | 84 (81.6%) | |
| Ascites | |||
| Yes | 17 (73.9%) | 54 (52.4%) | 0.060 |
| No | 6 (26.1%) | 49 (47.6%) | |
| FIGO stage | |||
| Stage 3 | 13 (56.5%) | 72 (69.9%) | 0.216 |
| Stage 4 | 10 (43.5%) | 31 (30.1%) | |
| Upfront treatment | |||
| PDS | 9 (39.1%) | 50 (48.5%) | 0.413 |
| IDS | 14 (60.9%) | 53 (51.5%) | |
| Debulking surgery status | |||
| Optimal | 4 (17.4%) | 34 (33.0%) | 0.140 |
| Suboptimal | 19 (82.6%) | 69 (67.0%) | |
| Histological subtype | |||
| Type I (LGSC and others) | 8 (34.8%) | 38 (36.9%) | 0.849 |
| Type II (HGSC) | 15 (65.2%) | 65 (63.1%) | |
BMI (body mass index), CA (cancer antigen), FIGO (International Federation of Gynaecology and Obstetrics), HGSC (high-grade serous carcinomas), IDS (interval debulking surgery), IQR (interquartile range), LGSC (low-grade serous carcinomas), PDS (primary debulking surgery) and SD (standard deviation). Type I includes endometrioid carcinoma, clear cell carcinoma, mucinous carcinoma and low-grade serous carcinomas. Type II includes high-grade serous carcinomas.
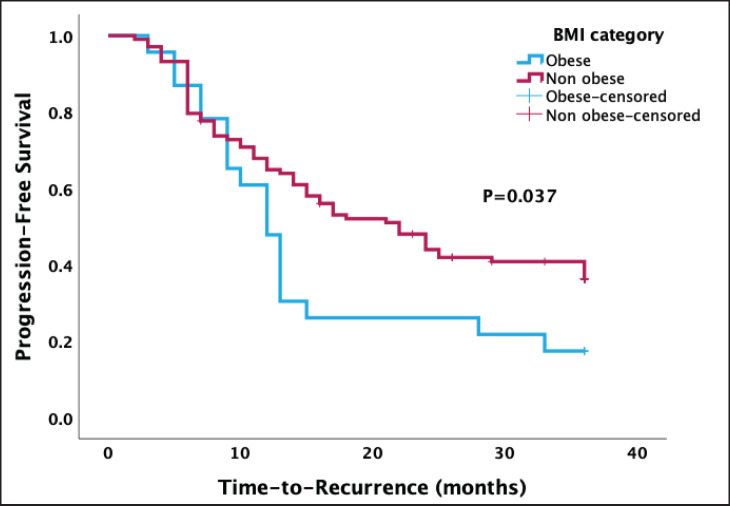
The Kaplan-Meier survival analysis recorded a median PFS of 17.0 months (95% confidence interval 10.8, 23.2). There was a statistically lower median PFS after 3 years in obese women than in non-obese women (12.0 versus 22.0 months, p = 0.037) (Figure 2).
Figure 2. Kaplan-Meier curve of PFS stratified by type of BMI categories. A significant association was found between obesity and PFS (p = 0.037).
Out of the 126 women in the study, 83 (65.9%) experienced a recurrence within 3 years after completing their primary treatment. Following adjustments for age and parity in the multivariable Cox proportional regression model, there was no statistically significant difference in PFS between obese and non-obese women (adjusted HR = 0.62, 95% confidence interval = 0.36–1.06, p = 0.282) (Table 2).
Table 2. Univariable and multivariable analyses of BMI categories and PFS.
| Factors | Number of women with recurrence within 3 years | Crude | Adjusted | |
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | ||
| BMI category | ||||
| Obese (≥30 kg/m2) | 19/23 (82.6%) | 0.044 | 0.69 (0.40–1.16) | 0.160 |
| Non-obese (<30 kg/m2) | 64/103 (62.1%) | 1.00 (reference) | ||
| Age | ||||
| <52 years | 34/62 (54.8%) | 0.005 | 1.88 (1.21–2.92) | 0.005 |
| ≥52 years | 49/64 (76.6%) | 1.00 (reference) | ||
| Parity | ||||
| Nulliparity | 25/47 (53.2%) | 0.015 | 1.50 (0.91–2.47) | 0.112 |
| Multiparity | 58/79 (73.4%) | 1.00 (reference) | ||
| Menstrual status | ||||
| Pre-menopause | 41/66 (62.1%) | 0.523 | NA | NA |
| Post-menopause | 42/60 (70.0%) | |||
| Serum CA-125 levels | ||||
| ≥470 U/mL | 39/61 (63.9%) | 0.588 | NA | NA |
| <470 U/mL | 44/65 (67.7%) | |||
| Medical comorbidity | ||||
| Yes | 23/30 (76.7%) | 0.262 | NA | NA |
| No | 60/96 (62.5%) | |||
| Presence of ascites | ||||
| Yes | 48/71 (67.6%) | 0.263 | NA | NA |
| No | 35/55 (63.6%) | |||
| FIGO stage | ||||
| Stage 3 | 54/85 (63.5%) | 0.313 | NA | NA |
| Stage 4 | 29/41 (70.7%) | |||
| Surgical debulking status | ||||
| Optimal | 28/38 (73.7%) | 0.175 | NA | NA |
| Suboptimal | 55/88 (62.5%) | |||
| Upfront surgery | ||||
| PDS | 46/67 (68.7%) | 0.396 | NA | NA |
| IDS | 37/59 (62.7%) | |||
| Histological subtype | ||||
| Type I | 27/46 (58.7%) | 0.305 | NA | NA |
| Type II | 56/80 (70.0%) | |||
BMI (body mass index), CA (cancer antigen), CI (confidence interval), FIGO (International Federation of Gynaecology and Obstetrics), HR (hazard ratio), NA (not applicable), PDS (primary debulking surgery); Type I includes low-grade serous carcinomas and other similar cancers; Type II includes high-grade serous carcinomas.
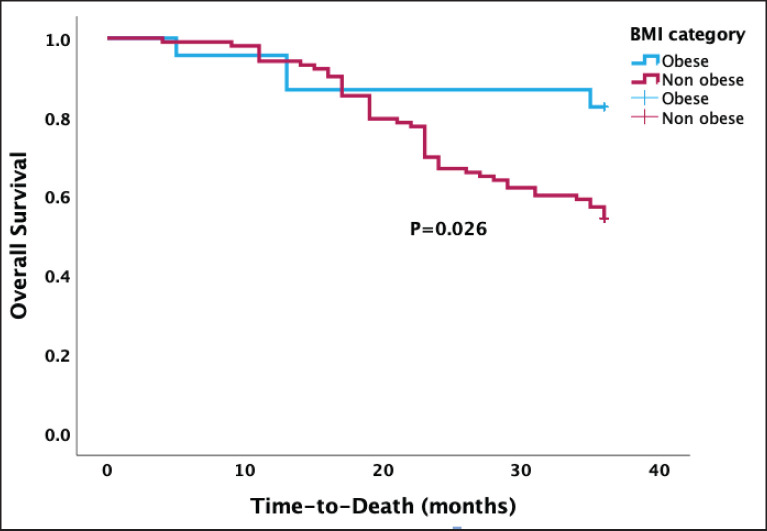
In Figure 3, the Kaplan-Meier survival analysis revealed a median OS of 30.1 months, with a 95% confidence interval ranging from 28.6 to 31.7 months. There was a statistically higher median OS after 3 years in obese than in non-obese women treatment (30.6 versus 29.6 months, p = 0.026).
Figure 3. Kaplan-Meier OS curve stratified by BMI categories showing a significant association between obesity and OS (p = 0.026).
As shown in Table 3, death was recorded in 51 (40.5%) women during the 3-year follow-up period after treatment. In the multivariable Cox proportional regression model, after adjustments for covariates including menstrual status and presence of ascites, there was a statistically significant difference in the OS between obese and non-obese women (adjusted HR = 3.58, 95% confidence interval 1.28–10.02, p = 0.015).
Table 3. Univariable and multivariable analyses of BMI categories and OS.
| Factors | Number of women that died within 3 years | Crude | Adjusted | |
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | ||
| BMI | ||||
| Obese (≥30 kg/m2) | 4/23 (17.4%) | 0.036 | 3.58 (1.28–10.02) | 0.015 |
| Non-obese (<30 kg/m2) | 47/103 (45.6%) | 1.00 (reference) | ||
| Age | ||||
| <52 years | 26/62 (41.9%) | 0.884 | NA | NA |
| ≥52 years | 25/64 (39.1%) | |||
| Parity | ||||
| Nulliparity | 17/47 (36.2%) | 0.272 | NA | NA |
| Multiparity | 34/79 (43.0%) | |||
| Menstrual status | ||||
| Pre-menopause | 33/66 (50.0%) | 0.032 | 0.65 (0.36–1.18) | 0.156 |
| Post-menopause | 18/60 (30.0%) | 1.00 (reference) | ||
| Serum CA-125 levels | ||||
| ≥470 U/mL | 21/61 (34.4%) | 0.254 | NA | NA |
| <470 U/mL | 30/65 (46.2%) | |||
| Pre-existing morbidity | ||||
| Yes | 10/30 (33.3%) | 0.672 | NA | NA |
| No | 41/96 (42.7%) | |||
| Presence of ascites | ||||
| Yes | 34/71 (47.9%) | 0.055 | 0.49 (0.27–0.87) | 0.016 |
| No | 17/55 (30.9%) | 1.00 (reference) | ||
| FIGO stage | ||||
| Stage 3 | 30/85 (35.3%) | 0.117 | NA | NA |
| Stage 4 | 21/41 (51.2%) | |||
| Upfront surgery | ||||
| PDS | 29/67 (43.3%) | 0.622 | NA | NA |
| IDS | 22/59 (37.3%) | |||
| Surgical debulking status | ||||
| Optimal | 15/38 (39.5%) | 0.943 | NA | NA |
| Sub-optimal | 36/88 (40.9%) | |||
| Histological subtype | ||||
| Type I | 20/46 (43.5%) | 0.798 | NA | NA |
| Type II | 31/80 (38.8%) | |||
BMI, body mass index; CA, cancer antigen; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NA, not applicable; PDS; primary debulking surgery; Type I includes low-grade serous carcinomas and others; Type II includes high-grade serous carcinomas
Discussion
This study investigated the impact of obesity on survival outcomes in women with advanced EOC in Lagos, Nigeria. Our results indicate that while there was no significant association between obesity and PFS, obesity was significantly associated with poorer OS among women with advanced EOC. Our study also showed that obesity with or without significant ascites at diagnosis and treatment appears to be related to poorer OS.
PFS reflects the duration during which a patient’s disease does not worsen, and it can be influenced by various factors including tumour biology, treatment efficacy and patient adherence to therapy [32]. However, our findings suggest that obesity may not directly impact the progression of EOC. This lack of association aligns with some previous studies [33–38], but contradicts the findings of others [39]. These variations in findings may be due to the time point of measurement of BMI and the cutoff points used to define obesity. Some studies used data on height and weight obtained 1 year before diagnosis or from reports of women's usual adult weight [40, 41]. In contrast, others measured BMI at diagnosis [38] and commencement of chemotherapy [34, 35]. We, therefore, propose that the weak relationship between obesity at diagnosis and progression of advanced EOC observed in our study may be due to the use of BMI which may not be an appropriate measure for evaluating the degree of obesity in ovarian cancer patients at the point of diagnosis and treatment as these patients often have ascites or cachexia [42, 43]. Second, this could be due to the lack of any difference in the extent of debulking surgery, the most important predictor of disease progression [33, 44, 45], between obese and non-obese women as observed in our study. Third, our finding may also be due to the 3-year follow-up period which may be too short to observe a significant number of cases with disease progression and death which could prevent us from also capturing the full impact of some historically strong predictors, including, residual disease status and histological subtype [32], on survival outcomes.
The significant association between obesity, in the presence or absence of significant ascites, and poorer OS in our study is consistent with a growing body of literature indicating that obesity adversely affects survival in ovarian cancer patients [39, 40, 46]. This is, however, at variance with the findings from other studies where obesity was not associated with OS [36–38, 41]. Factors that could be attributed to the association observed between obesity and OS in our study and others are – First, due to concerns about potential overdosing in obese patients with a larger body surface area, the dosing of chemotherapeutic drugs for gynecologic malignancies is often capped in some centres [47] including ours based on an empiric body surface area of either 1.8 or 2.0 m² instead of the full dosage calculation based on the body surface area as in non-obese individuals. Second, the associated medical comorbidities seen in obese individuals, such as diabetes, and cardiovascular and metabolic diseases as equally observed in our study, can complicate treatment thus leading to a reduced chemotherapy dose intensity with cumulative doses being lower in obese compared to non-obese women [39, 48]. Obese women might, therefore, be receiving less effective treatment, which could lead to a higher risk of disease progression and reduced survival [49]. Furthermore, obesity is associated with changes in tumour biology, including increased production of oestrogen, insulin resistance and chronic inflammation, all of which can promote tumour growth and metastasis leading to poorer survival outcomes in cancer patients [50].
The strength of our study is that the findings have the potential to inform clinical practice and public health strategies, emphasizing the need for comprehensive management of obese women with EOC. However, we acknowledged a few study limitations. First, the retrospective design may introduce selection and information biases. Second, historical changes in practices, exposures, or population characteristics could account for the unexpectedly high proportions of multiparous and pre-menopausal women seen in the study, and this may limit the applicability of our findings beyond the study contexts. Third, our study could be underpowered due to a lack of sample size calculation thus limiting our ability to translate the statistical to causal inference. Fourth, BMI, as a measure of obesity does not distinguish between muscle and fat mass, nor does it account for fat distribution and ascites especially when measured at diagnosis or commencement of treatment as in our study. Fifth, our study did not account for variations in chemotherapy, which could influence survival outcomes. Finally, the duration of follow-up in our study may not be long enough to capture the full impact of some strong predictors, including, residual disease status and histological subtype [46], on survival outcomes. Future prospective studies with larger sample sizes could enhance statistical power and provide more definitive insights into the relationship between obesity and other prognostic factors and survival outcomes. These studies should also extend the follow-up duration to provide a more comprehensive understanding of long-term survival outcomes related to obesity and should consider using more precise measures of body composition and obesity, and account for the types, courses and adherence to chemotherapy to provide a more comprehensive understanding of the findings of our study.
Conclusion
This study highlights that obesity is associated with significantly poorer OS in women with advanced EOC in Lagos, Nigeria, despite no observed impact on PFS. These findings underscore the importance of addressing obesity in the management of EOC by introducing comprehensive, multidisciplinary approaches incorporating weight management and personalized treatment strategies to enhance the prognosis of obese women with EOC. However, future prospective studies with larger sample sizes with extended follow-up duration could enhance statistical power and provide more definitive insights into the relationship between obesity and other prognostic factors and survival outcomes. These studies should also consider using more precise measures of body obesity and also account for the types, courses and adherence to chemotherapy to provide a more comprehensive understanding of the findings of our study.
Conflicts of interest
The authors declare no conflicts of interest.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (KSO) upon reasonable request.
Acknowledgments
The authors express their gratitude to the resident doctors of the Gynaecological Oncology Unit in the Department of Obstetrics and Gynaecology for their efforts in data retrieval and collation. The authors also thank the staff of the Medical Records Department of the hospital for their assistance in retrieving the patients' medical records.
Footnotes
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
References
- 1.Kaku T, Ogawa S, Kawano Y, et al. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36(1):9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Minig L, Zorrero C, Iserte PP, et al. Selecting the best strategy of treatment in newly diagnosed advanced-stage ovarian cancer patients. World J Methodol. 2015;5(4):196–202. doi: 10.5662/wjm.v5.i4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okunade KS, Adetuyi IE, Adenekan M, et al. Risk predictors of early recurrence in women with epithelial ovarian cancer in Lagos, Nigeria. Pan Afr Med J. 2020;36:272. doi: 10.11604/pamj.2020.36.272.17827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash Z, Menon U. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol. 2020;65:32–45. doi: 10.1016/j.bpobgyn.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 7.John-Olabode SO, Okunade KS, Olorunfemi G, et al. Pretreatment neutrophil-to-lymphocyte ratio: a prognostic biomarker of survival in patients with epithelial ovarian cancer. Cureus. 2021;13(7):e16429. doi: 10.7759/cureus.16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb PM, Jordan SJ. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. 2024;21(5):389–400. doi: 10.1038/s41571-024-00881-3. [DOI] [PubMed] [Google Scholar]
- 9.Matulonis UA, Sood AK, Fallowfield L, et al. Ovarian cancer. Nat Rev Dis Primers. 2016;2(1):16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 2021;12:706978. doi: 10.3389/fendo.2021.706978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia W, Liu F. Obesity: causes, consequences, treatments, and challenges. J Mol Cell Biol. 2021;13(7):463–465. doi: 10.1093/jmcb/mjab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellulu M, Abed Y, Rahmat A, et al. Epidemiology of obesity in developing countries: challenges and prevention. Glob Epidemic Obes. 2014;2(1):2. doi: 10.7243/2052-5966-2-2. [DOI] [Google Scholar]
- 13.Munsell MF, Sprague BL, Berry DA, et al. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe PO, O’Reilly DA, Renehan AG. Excess adiposity and gastrointestinal cancer. Br J Surg. 2014;101(12):1518–1531. doi: 10.1002/bjs.9623. [DOI] [PubMed] [Google Scholar]
- 15.Islami F, Goding Sauer A, Gapstur SM, et al. Proportion of cancer cases attributable to excess body weight by US state, 2011–2015. JAMA Oncol. 2019;5(3):384. doi: 10.1001/jamaoncol.2018.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88–112. doi: 10.3322/caac.21499. [DOI] [PubMed] [Google Scholar]
- 17.Pati S, Irfan W, Jameel A, et al. Obesity and cancer: a current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel) 2023;15(2):485. doi: 10.3390/cancers15020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9(4):e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barazzoni R, Gortan Cappellari G. Double burden of malnutrition in persons with obesity. Rev Endocr Metab Disord. 2020;21(3):307–313. doi: 10.1007/s11154-020-09578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abubakari AR, Lauder W, Agyemang C, et al. Prevalence and time trends in obesity among adult West African populations: a meta‐analysis. Obes Rev. 2008;9(4):297–311. doi: 10.1111/j.1467-789X.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu XZ, Pedersen L, Halberg N. Cellular mechanisms linking cancers to obesity. Cell Stress. 2021;5(5):55–72. doi: 10.15698/cst2021.05.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chukwuonye II, Ohagwu KA, Ogah OS, et al. Prevalence of overweight and obesity in Nigeria: systematic review and meta-analysis of population-based studies. PLoS Glob Public Health. 2022;2(6):e0000515. doi: 10.1371/journal.pgph.0000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adeloye D, Ige-Elegbede JO, Ezejimofor M, et al. Estimating the prevalence of overweight and obesity in Nigeria in 2020: a systematic review and meta-analysis. Ann Med. 2021;53(1):495–507. doi: 10.1080/07853890.2021.1897665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(3):561–568. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 25.Okunade KS, John‐Olabode S, Ohazurike EO, et al. Predictors of early mortality risk in patients with epithelial ovarian cancer. Health Sci Rep. 2022;5(4):e717. doi: 10.1002/hsr2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okunade KS, Adekanye TV, Osunwusi B, et al. Survival outcomes following interval versus primary debulking surgery in advanced epithelial ovarian cancer: a retrospective cohort study in Lagos, Southwest Nigeria. J Gynecol Surg. 2024. [DOI]
- 27.Berek JS, Renz M, Kehoe S, et al. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynecol Obstet. 2021;155(S1):61–85. doi: 10.1002/ijgo.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshiyama M, Matsumura N, Konishi I. Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics (Basel) 2017;7(1):12. doi: 10.3390/diagnostics7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weir CB, Jan A. BMI Classification Percentile and Cut Off Points. Treasure Island: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 30.Okunade KS, John-Olabode SO, Soibi-Harry AP, et al. Prognostic performance of pretreatment systemic immune-inflammation index in women with epithelial ovarian cancer. Future Sci OA. 2023;9(10):FSO897. doi: 10.2144/fsoa-2023-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 32.Andreou M, Kyprianidou M, Cortas C, et al. Prognostic factors influencing survival in ovarian cancer patients: a 10-year retrospective study. Cancers (Basel) 2023;15(24):5710. doi: 10.3390/cancers15245710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharafadeen Okunade K, Adejimi AA, Ohazurike EO, et al. Predictors of survival outcomes after primary treatment of epithelial ovarian cancer in Lagos, Nigeria. JCO Glob Oncol. 2021;7 doi: 10.1200/GO.20.00450. GO 20.00450 [ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright JD, Tian C, Mutch DG, et al. Carboplatin dosing in obese women with ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;109(3):353–358. doi: 10.1016/j.ygyno.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Bakkum-Gamez JN, Weaver AL, et al. Impact of obesity on surgical and oncologic outcomes in ovarian cancer. Gynecol Oncol. 2014;135(1):19–24. doi: 10.1016/j.ygyno.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 36.Suh DH, Kim HS, Chung HH, et al. Body mass index and survival in patients with epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(1):70–76. doi: 10.1111/j.1447-0756.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 37.Barrett SV, Paul J, Hay A, et al. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. 2008;19(5):898–902. doi: 10.1093/annonc/mdm606. [DOI] [PubMed] [Google Scholar]
- 38.Fotopoulou C, Richter R, Braicu EI, et al. Impact of obesity on operative morbidity and clinical outcome in primary epithelial ovarian cancer after optimal primary tumor debulking. Ann Surg Oncol. 2011;18(9):2629–2637. doi: 10.1245/s10434-011-1637-z. [DOI] [PubMed] [Google Scholar]
- 39.Pavelka JC, Brown RS, Karlan BY, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107(7):1520–1524. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 40.Kjærbye-Thygesen A, Frederiksen K, Høgdall EV, et al. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(4):798–803. doi: 10.1158/1055-9965.EPI-05-0897. [DOI] [PubMed] [Google Scholar]
- 41.Moysich KB, Baker JA, Menezes RJ, et al. Usual adult body mass index is not predictive of ovarian cancer survival. Cancer Epidemiol Biomarkers Prev. 2007;16(3):626–628. doi: 10.1158/1055-9965.EPI-06-1052. [DOI] [PubMed] [Google Scholar]
- 42.Delort L, Kwiatkowski F, Chalabi N, et al. Central adiposity as a major risk factor of ovarian cancer. Anticancer Res. 2009;29(12):5229–5234. [PubMed] [Google Scholar]
- 43.Brändstedt J, Nodin B, Manjer J, et al. Anthropometric factors and ovarian cancer risk in the Malmö Diet and cancer study. Cancer Epidemiol. 2011;35(5):432–437. doi: 10.1016/j.canep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Trifanescu O. Clinical prognostic factors in pre-and post-menopausal women with ovarian carcinoma. Acta Endocrinol (Bucharest) 2018;14(3):353–359. doi: 10.4183/aeb.2018.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotsopoulos J, Rosen B, Fan I, et al. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol. 2016;140(1):42–47. doi: 10.1016/j.ygyno.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Hess L, Barakat R, Tian C, et al. Weight change during chemotherapy as a potential prognostic factor for stage III epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;107(2):260–265. doi: 10.1016/j.ygyno.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modesitt SC, Nagell JR. The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60(10):683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 48.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz J, Toste B, Dizon DS. Chemotherapy toxicity in gynecologic cancer patients with a body surface area (BSA)>2 m2. Gynecol Oncol. 2009;114(1):53–56. doi: 10.1016/j.ygyno.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author (KSO) upon reasonable request.