ABSTRACT
Background
Unbalanced levels of serum total cholesterol (TC) and its subgroups are called dyslipidemia. Several anthropometric indices have been developed to provide a more accurate assessment of body shape and the health risks associated with obesity. In this study, we used the random forest model (RF), decision tree (DT), and logistic regression (LR) to predict total cholesterol based on new anthropometric indices in a sex‐stratified analysis.
Method
Our sample size was 9639 people in which anthropometric parameters were measured for the participants and data regarding the demographic and laboratory data were obtained. Aiding the machine learning, DT, LR, and RF were drawn to build a measurement prediction model.
Results
Anthropometric and other related variables were compared between both TC <200 and TC ≥200 groups. In both males and females, Lipid Accumulation Product (LAP) had the greatest effect on the risk of TC increase. According to results of the RF model, LAP and Visceral Adiposity Index (VAI) were significant variables for men. VAI also had a stronger correlation with HDL‐C and triglyceride. We identified specific anthropometric thresholds based on DT analysis that could be used to classify individuals at high or low risk of elevated TC levels. The RF model determined that the most important variables for both genders were VAI and LAP.
Conclusion
We tend to present a picture of the Persian population's anthropometric factors and their association with TC level and possible risk factors. Various anthropometric indices indicated different predictive power for TC levels in the Persian population.
Keywords: anthropometric indices, machine learning methods, predictor, total cholesterol
Logistic Regression (LR), Decision Tree (DT), and Random Forest (RF) were utilized to examine the data collected from these individuals who had high or normal TG levels. LAP and VAI are key novel factors related to the concentration of TG. Factors such as age are associated with the level of TC. RF model was shown to have a higher accuracy in predicting the TG level in both males and females as the sensitivity was higher in training of the RF model and higher in testing in the DT model.
1. Introduction
Impaired levels of total cholesterol (TC), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C), either separately or together, are referred to as dyslipidemia [1]. High blood cholesterol is a significant risk factor for coronary heart disease (CHD) [2, 3, 4]. Furthermore, serum total cholesterol concentration is included in all recent risk calculations and is thought to be a crucial component for the prediction of atherosclerotic cardiovascular disease (ASCVD) [5, 6].
It might not always be possible to obtain laboratory testing, particularly in developing nations [7], or for health checks conducted outside established healthcare institutions [8]. However, as low‐income nations account for the bulk of cardiovascular disease cases worldwide, these nations must employ preventive measures [9, 10]. Finding relationships between anthropometric measures (AMs) and laboratory results is of tremendous interest to the medical community [11] since it would result in patient examination methods that are less intrusive [12]. The literature provides examples of this, including the relationship between AMs and atherogenic indicators [13], cardiovascular risk [14, 15], and assessment of diabetes [16, 17]. AMs can also correlate with other AMs in the body of the patient, which helps assess dosing for ICU patients as well as predict height and weight from other parameters [18].
Most people agree that an increase in Body Mass Index (BMI) corresponds with an increase in total cholesterol [19, 20, 21]. However, several researches have not found a connection between cholesterol and BMI [22, 23, 24, 25]. In recent years, several anthropometric indices have been developed to provide a more accurate assessment of body shape and obesity‐related health risks. The waist‐to‐hip ratio (WHR) and waist‐to‐height ratio (WHtR) are commonly used markers of abdominal obesity, strongly associated with metabolic disorders [26]. In the last few decades, new anthropometric indices were developed to provide a more accurate depiction of body shape [27, 28, 29]. The Body Roundness Index (BRI) can be related to visceral obesity tissue and is valuable in assessing well‐being conditions [29]. The Visceral Obesity Index (VAI) is another useful marker that takes into account BMI, waist circumference (WC), and measurements of TG and HDL‐C. It provides a more accurate estimation of the cardio‐metabolic risk associated with visceral obesity [28, 30]. The Lipid Accumulation Product (LAP) [31] is derived from WC and TG levels and is more effective than BMI in identifying individuals at risk for cardiovascular disease [32]. The Abdominal Volume Index (AVI) is a practical method for assessing overall abdominal volume [33] and is a reliable diagnostic tool for metabolic syndrome [34]. It is also associated with atherogenic dyslipidemia [35]. In addition, a formula called the Body Adiposity Index (BAI) calculates a person's adiposity without requiring them to know their weight [36]. Numbers related to Body Surface Area (BSA) are commonly utilized in the medical field, mostly to calculate chemotherapy dosages and calculate the cardiac output index [37].
In this study, we used the Random Forest (RF), Decision Tree (DT), and Logistic Regression (LR) Model to build a prediction model for TC based on these new anthropometric indices in a gender‐stratified analysis.
2. Methods
2.1. Study Population
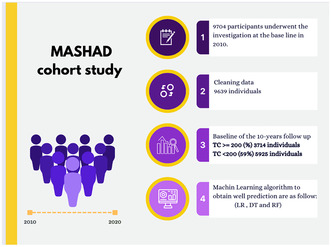
In this cross‐sectional study, participants were selected from the Mashhad Stroke and Heart Atherosclerotic Disorder (MASHAD) study (N = 9704), a cross‐sectional from north‐eastern Iran [38]. A total of 3714 participants with total cholesterol ≥200 (TC ≥200) as well as those who were healthy in the first phase of our study (N = 5925). Approximately 38.2% of individuals in Mashhad with TC ≥200 was estimated according to Ministry of Health data. The study involved participants from three districts of Mashhad, grouped into nine areas around Mashhad Healthcare Center sections. In 2010, baseline screening began with a 79% response rate through stratified cluster random sampling. Eligible candidates were then invited for a physical examination meeting, with inclusion criteria consisting of males and females aged 35–65. A total of 9704 individuals within this age range were initially included but following the exclusion of ineligible candidates, 9639 participants were included in the main analysis (Figure 1). Participants were informed of the data usage and provided written consent. The research protocol was approved by the Ethical Committee of Mashhad University of Medical Sciences.
FIGURE 1.
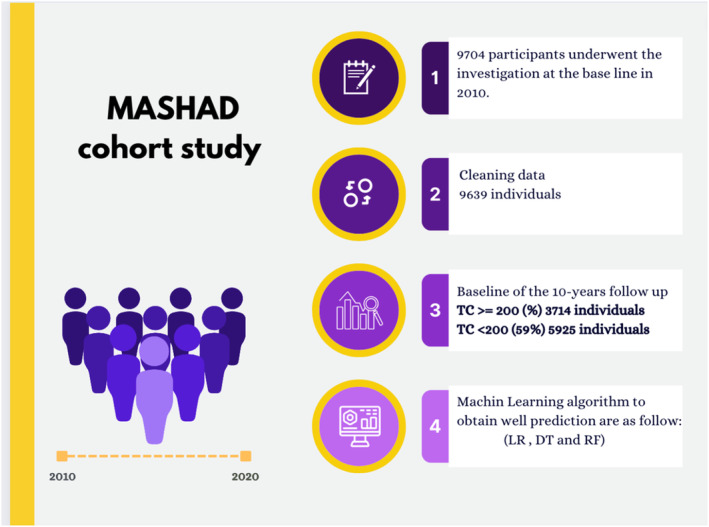
Flowchart of this study.
2.2. Baseline Examination
Blood samples were obtained from participants between 8 and 10 a.m. following a 14‐h overnight fast, through venepuncture of an antecubital vein. The collection of blood samples was done in vacuum tubes (20 mL) from contributors in an upright position, following a standardized protocol. Subsequently, all blood samples underwent centrifugation at temperatures ranging from 20°C to 25°C within 30 to 45 min to separate the serum from whole blood into six 0.5‐mL aliquots, which were then sent to the Bu Ali Research Institute in Mashhad. In cases where serum TG concentrations were below 400 mg/dL, the low‐LDL‐C was calculated based on the serum TC, and HDL‐C concentrations measured in mg/dL using the Fried Ewald formula [39].
Anthropometric measurements were conducted by a registered nurse, encompassing factors such as weight, height, waist height, WHR, BRI, BAI, AVI, VAI, LAP, BMI, and BSA. Participants were required to wear light clothing and no shoes during these measurements. Following the guidelines from the World Health Organization, BMI was determined using Quetelet's index: BMI (kg/m2) = weight/height2 [3] and classified based on WHO criteria into underweight (<18), normal (18–25), overweight (25–30), and obese (≥30) categories. The calculations for BMI, BAI, BRI, BSA, AVI, LAP, and VAI were performed using the provided formulas in Table 1.
TABLE 1.
Measurement of anthropometric indices.
Abbreviations: AVI, abdominal volume index; BAI, Body Adiposity Index; BMI, Body Mass Index; BRI, body roundness index; BSA, body surface area; HDL, high‐density lipoproteins; LAP, lipid accumulation product; TG, triglyceride; VAI, Visceral Adiposity Index.
2.3. Statistical Analysis
The primary software utilized for the analysis was the R Statistical Software (v4.1.2) [40], with IBM SPSS Statistics (Version 27) also playing a key role in the analysis. Descriptive statistics were used to present continuous and normally distributed variables as mean ± SD, median (Q1, Q3) for continuous and non‐normally distributed variables, and percentages for categorical variables. A significance level of <0.05 was considered statistically significant.
For comparisons between groups, t‐tests were employed for continuous and normally distributed variables, while the Mann–Whitney U test was used for non‐normally distributed variables, specifically to compare the mean and median levels between TC <200 and TC ≥200 in male and female subgroups. The association between categorical variables and binary outcomes was assessed using the chi‐square test.
The dataset was split into training and testing subsets (75% and 25%, respectively) using the holdout method. To evaluate multicollinearity among independent variables, correlation coefficients, and variance inflation factor (VIF) computations were conducted.
The ability of anthropometric indices including BMI, LAP, BAI, AVI, Waist high, Waist hip, BSA, BRI, and VAI (were calculated using the formulas in Table 2) to indicate the presence of TC <200 and TC ≥200 was measured by the receiver‐operating characteristic (ROC) analysis.
TABLE 2.
Baseline characteristics of males and females.
| Variables | All, N = 9704 | Males, N = 3847 | p | Females, N = 5792 | p | ||
|---|---|---|---|---|---|---|---|
| TC <200, N = 2534 | TC ≥200, N = 1313 | TC <200, N = 3391 | TC ≥200, N = 2401 | ||||
| BRI | 5.45 ± 1.91 a | 4.45 ± 1.39 | 4.79 ± 1.42 | ≤0.001 | 5.84 ± 1.91 | 6.32 ± 2.01 | ≤0.001 |
| AVI | 18.52 ± 4.60 | 17.56 ± 4.07 | 18.48 ± 4.11 | ≤0.001 | 18.54 ± 4.74 | 19.52 ± 4.95 | ≤0.001 |
| BAI | 33.31 ± 6.47 | 28.04 ± 4.09 | 28.75 ± 4.14 | ≤0.001 | 36.30 ± 5.43 | 37.17 ± 5.56 | ≤0.001 |
| Age | 48.08 ± 8.26 | 48.57 ± 8.48 | 49.51 ± 8.34 | 0.001 | 45.94 ± 7.78 | 49.79 ± 7.99 | ≤0.001 |
| Waist high | 59.50 ± 8.26 | 55.10 ± 6.66 | 56.73 ± 6.45 | ≤0.001 | 61.22 ± 8.15 | 63.26 ± 8.21 | ≤0.001 |
| BSA | 1.78 ± 0.19 | 1.86 ± 0.18 | 1.88 ± 0.17 | ≤0.001 | 1.72 ± 0.17 | 1.73 ± 0.16 | 0.045 |
| Waist hip | 0.92 (0.87, 0.97) b | 0.92 (0.88, 0.96) | 0.94 (0.90, 0.97) | ≤0.001 | 0.91 (0.85, 0.96) | 0.93 (0.87, 0.98) | ≤0.001 |
| VAI | 2.14 (1.37, 3.30) | 1.74 (1.10, 2.75) | 2.16 (1.41, 3.37) | ≤0.001 | 2.07 (1.37, 3.09) | 2.69 (1.83, 4.10) | ≤0.001 |
| LAP | 46.16 (27.64, 73.52) | 34.76 (19.75, 58.23) | 52.66 (32.83, 83.24) | ≤0.001 | 41.77 (25.92, 64.49) | 62.84 (40.85, 96.93) | ≤0.001 |
| BMI | |||||||
| Underweight (<18) | 93 (0.96) c | 55 (2.17) | 7 (0.53) | ≤0.001 | 20 (0.59) | 11 (0.46) | ≤0.001 |
| Normal (18–25) | 2582 (26.61) | 971 (38.32) | 403 (30.69) | 784 (23.12) | 401 (16.70) | ||
| Overweight (25–30) | 4091 (42.16) | 1098 (43.33) | 645 (49.12) | 1329 (39.19) | 999 (41.61) | ||
| Obese (>30) | 2912 (30.01) | 404 (15.94) | 254 (19.35) | 1246 (36.74) | 989 (41.19) | ||
Abbreviations: AVI, abdominal volume index; BAI, Body Adiposity Index; BMI, Body Mass Index; BRI, body roundness index; BSA, body surface area; LAP, lipid accumulation product; VAI, Visceral Adiposity Index.
Mean ± SD for continuous and normal variables and p value of two sample t test.
Median (Q1, Q3) for continuous and abnormal variables and p value of Mann Whitney U test.
Count (percentage) for categorical variables and p value of chi square test.
As we used LR to compute the odds ratios (OR) with their 95% confidence interval, for comparing the model's superiority in the fit test, the deviance as a likelihood ratio statistic was applied.
2.4. Decision Tree
A machine learning technique known as DT was employed in this study to develop a predictive model for anthropometric measurements using the available data. Decision trees, as a type of nonparametric method, are named after the target variable they aim to predict. The main objective when creating a decision tree model is to predict outcomes based on input predictor variables. These tree‐building algorithms establish splitting criteria at internal nodes to construct the tree structure [41, 42, 43]. Each node's split is designed to minimize impurity within that node. In cases where a split does not improve impurity reduction, the node remains unchanged and becomes a leaf node. Successful splits that effectively reduce impurity are chosen based on the greatest impurity reduction, leading to the creation of two branches and new nodes. The Gini index is a common criterion used for splitting decisions.
Within the context of the Classification and Regression Trees (CART) method, the selection of splitting variables at internal nodes is guided by maximizing the Gini index to build a binary tree. The importance of predictor variables is indicated by subsequent splits, starting with all observations at the root node. The specific performance metrics, such as specificity, accuracy, and precision, of the decision tree algorithm were evaluated using ROC curves in the R software version 4.0.5. Additionally, the confusion matrix of the decision trees was examined to further assess their performance.
2.5. Random Forest
The dataset underwent a data mining process, and a neural network was utilized to construct a predictive model for TC ≥200 measurements. Within this context, an RF algorithm, which employs an ensemble of decision trees for predictive analysis, was employed. The goal of an RF is to reduce overfitting and improve generalization performance by combining the predictions of multiple DTs.
To create a random forest, a set of DTs are trained on randomly sampled subsets of the data. During training, each tree is trained independently of the others and is allowed to make a prediction based on only a subset of the available features. This process is known as feature bagging, and it helps to reduce the correlation between the trees and improve the overall performance of the forest. When making a prediction, each decision tree in the forest independently produces a prediction, and the final prediction is made by taking the majority vote of all the trees. This method of combining predictions is known as bagging, and it helps to reduce the variance of the predictions and improve the accuracy of the model.
Random Forests have several advantages over other machine learning algorithms. They are highly accurate, even when dealing with noisy or missing data, and they can handle large datasets with many features. Additionally, they are resistant to overfitting, which makes them a popular choice for many applications. In summary, an RF is a powerful machine learning algorithm that combines the predictions of multiple DTs to make accurate predictions. By using feature bagging and bagging to reduce the variance of the predictions and improve generalization performance, RFs can handle large datasets and are resistant to overfitting.
In this paper, we have divided the train and test data into a ratio of 75% and 25%, respectively. To implement the random forest, we used the R software, and the confusion matrix of the random forest was used to evaluate the accuracy, precision, and sensitivity other result the obtained results are reported below.
3. Result
3.1. Characteristics of Population
Table 2 provides a summary of the participants' baseline clinical characteristics. It shows men and women whose clinical characteristics them are divided into those with TC <200 and those with TC ≥200. It shows that among 9639 eligible participants, 5925 with TC <200 and 3714 with TC ≥200 was observed. The distribution of sexual groups in the TC <200 subsample consists of 42.7% male and 57.2% female. Overall age was 48.08 ± 8.26, but the average age of subjects in subsamples with TC <200 for males was higher than for females (48.57 ± 8.48 vs. 45.94 ± 7.78). The table gives evidence for significant differences between men and women for most variables. The anthropometric and other variables were compared between both with TC <200 and TC ≥200 groups using the t‐test, for continuous and normal variables, Mann–Whitney U test for continuous and abnormal variables, and a chi‐square test for categorical variables. All variables had significant differences between the two groups (p value < 0.001), except the BSA of females in the subgroup of TC <200 versus TC ≥200 (p value = 0.04). Also, in participants with TC ≥200, 49.12% of males and 41.61% of females were from overweight subjects. Note that the difference in the total number of subgroups and the frequencies of BMI in total is due to missing data.
Binary response variables (TC <200 and TC ≥200) association with anthropometric predictors has been done by three data mining techniques. Hence, our primary aim focused on predicting TC <200 and TC ≥200 by employing LR, DT, and RF models, with a specific emphasis on identifying the relevant anthropometric indicators associated with these outcomes. To achieve this goal, the dataset was partitioned into two distinct subsets for the DT and RF models: a training dataset and a test dataset in a 25%–75% ratio. The training dataset was employed for model development in both DT and RF approaches, while the test dataset (25%) was used for validation purposes.
3.2. Anthropometric Measurements Relation With TC (TC <200 and TC ≥200) Using LR Model
The results of logistic regression in Model A and Model B are reported in Table 3. The models included the effect of having VAI, BAI, age, and BMI. Models show the odd ratio of parameters and their 95% CIs with TC ≥200 response variable among men and women. Other anthropometric indices were excluded because of multicollinearity. Also, logistic models were fitted for each subgroup with an accuracy of 63% and 66% for females and males, respectively. The results in Model A showed that all variables were significant (p < 0.05) except BMI Overweight (25–30) and BMI Obese (>30). The results of regression in Model A showed that the Waist hip (OR = 4.17, 95% CI = [1.43, 12.16]) and for every unit increase in VAI and BAI were related to an increased odd of TC ≥200 by 13% and 2%, respectively (OR = 1.13 [1.09, 1.17] and OR = 1.02 [1.007, 1.05]). The odd ratio for BMI Underweight (<18) is <1 (OR = 0.41 [0.18, 0.92]), which means the lower value of BMI reduces the chance of TC ≥200. Other anthropometric indices were excluded because of multicollinearity. The obtained results in model B shows that by increasing one unit in VAI and Age the odd of TC ≥200 increased 12% and 5%, respectively (VAI OR = 1.12 CI 95% [1.09, 1.15] and Age OR = 1.05 CI 95% [1.04, 1.06]). The OR of BMI in the case of Overweight (25–30) is 1.238. It reveals that increasing a unit of BMI in the range of (25–30) increased the odds of TC ≥200. The other cases of BMI were not significant.
TABLE 3.
Regression models with binary cholesterol as response variable.
| Variables | Model A, N = 9639 | Model B (Males), N = 3847 | Model C (Females), N = 5792 | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | |
| Sex | 1.14 (1.01, 1.29) | 0.029 | — | — | ||
| VAI | 1.13 (1.11, 1.16) | 0.000 | 1.13 (1.09, 1.17) | ≤0.001 | 1.13 (1.09, 1.15) | ≤0.001 |
| BAI | 1.02 (1.01, 1.04) | 0.000 | 1.02 (1.01, 1.05) | 0.010 | 1.02 (1.01, 1.03) | 0.001 |
| Age | 1.04 (1.03, 1.04) | 0.000 | 1.01 (1.01, 1.02) | 0.050 | 1.05 (1.04, 1.06) | ≤0.001 |
| Waist hip | 2.37 (1.32, 4.25) | 0.004 | 4.17 (1.43, 12.16) | 0.009 | 1.60 (0.78, 3.26) | 0.193 |
| BMI | ||||||
| Underweight (<18) | 0.69 (0.41, 1.18) | 0.173 | 0.41 (0.18, 0.92) | 0.031 | 1.18 (0.54, 2.56) | 0.677 |
| Normal (18–25) | Ref | — | Ref | — | Ref | — |
| Overweight (25–30) | 1.17 (1.04, 1.32) | 0.009 | 1.08 (0.91, 1.30) | 0.374 | 1.23 (1.05, 1.45) | 0.010 |
| Obese (>30) | 1.01 (0.86, 1.19) | 0.870 | 0.97 (0.73, 1.28) | 0.848 | 1.03 (0.83, 1.27) | 0.784 |
| Accuracy | 63% | 66% | 63% | |||
3.3. Anthropometric Measurements Relation to TC Using DT Model
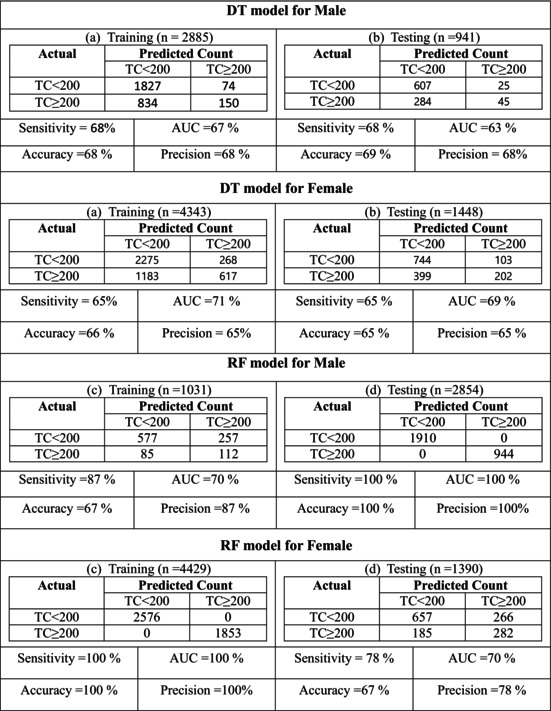
Table 4 presents the results of the DT training concerning biochemical factors and clinical features categorized by gender. Through the DT algorithm, binary risk factors (TC <200 vs. TC ≥200) were identified and categorized accordingly. In the DT model, the initial variable (root) is deemed to hold the highest significance in classifying the data, with subsequent factors showing progressively diminishing importance.
TABLE 4.
DT Rules for TC <200 and TC ≥200.
| Number | Rules | TC <200 | TC ≥200 |
|---|---|---|---|
| Males | |||
| 1 | LAP ≥36.02 & Age ≥47 & LAP ≥81.59 & VAI <3.46 & BMI <31.56 | 0.08 | 0.92 |
| 2 | LAP ≥36.02 & Age ≥47 & LAP ≥81.59 & VAI <3.46 & BMI ≥31.56 | 0.31 | 0.69 |
| 3 | LAP ≥36.02 & Age ≥47 & LAP ≥81.59 & VAI ≥3.46 & LAP ≥228.51 | 0.16 | 0.84 |
| 4 | LAP ≥36.02 & Age ≥47 & LAP ≥81.59 & VAI ≥3.46& LAP<228.51 | 0.52 | 0.48 |
| 5 | LAP ≥36.02 & Age ≥47 & LAP <81.59 & BAI <43.44 & BMI <21.12 | 0.30 | 0.70 |
| 6 | LAP ≥36.02 & Age ≥47 & LAP <81.59 & BAI <43.438 & BMI_ ≥21.12 | 0.60 | 0.40 |
| 7 | LAP ≥36.02 & Age ≥47 & LAP <81.59 & BAI ≥43.44 | 0.27 | 0.73 |
| 8 | LAP ≥36.02 & Age <47 & LAP ≥96.26 & VAI ≥9.64 | 0.20 | 0.80 |
| 9 | LAP ≥36.02 & Age <47 & LAP ≥96.26 & VAI <9.64 | 0.47 | 0.53 |
| 10 | LAP ≥36.02 & Age <47 & LAP <96.26 & Age <37 | 0.52 | 0.47 |
| 11 | LAP ≥36.02 & Age <47 & LAP <96.26 & Age ≥37 | 0.64 | 0.36 |
| 12 | LAP <36.02 & BMI ≥18.62 & VAI ≥1.19 | 0.74 | 0.26 |
| 13 | LAP <36.02 & BMI ≥18.62 & VAI <1.19 & Waist hip <0.85 | 0.73 | 0.27 |
| 14 | LAP <36.02 & BMI ≥18.62 & VAI <1.19 & Waist hip ≥0.85 | 0.83 | 0.17 |
| 15 | LAP <36.02 & BMI <18.62 | 0.91 | 0.09 |
| Females | |||
| 1 | LAP ≥44.17 &Age ≥49 & LAP ≥86.88 & VAI <3.35 & BMI <32.97 | 0.02 | 0.98 |
| 2 | LAP ≥44.17 & Age ≥49 & LAP ≥86.88 & VAI <3.345 & BMI ≥32.97 | 0.16 | 0.84 |
| 3 | LAP ≥44.17 & Age ≥49 & LAP ≥86.88 & VAI ≥3.35 & LAP ≥118.02 | 0.20 | 0.80 |
| 4 | LAP ≥44.17 & Age ≥49 & LAP ≥86.88 & VAI ≥3.35 & LAP <118.02 | 0.40 | 0.60 |
| 5 | LAP ≥44.17 & Age ≥49 & LAP <86.88 & BAI <30.38 & BMI ≥23.96 | 0.20 | 0.80 |
| 6 | LAP ≥44.17 & Age ≥49 & LAP <86.88 & BAI <30.38 & BMI <23.96 | 0.41 | 0.59 |
| 7 | LAP ≥44.17 & Age ≥49 & LAP <86.88 & BAI ≥30.38 & AVI <17.54 | 0.34 | 0.66 |
| 8 | LAP ≥44.17 & Age ≥49 & LAP <86.88 & BAI ≥30.38 & AVI ≥17.54 | 0.52 | 0.48 |
| 9 | LAP ≥44.17 & Age <49 & LAP ≥124.46 & VAI <4.68 | 0.19 | 0.81 |
| 10 | LAP ≥44.17 & Age <49 & LAP ≥124.46 & VAI ≥4.68 | 0.46 | 0.54 |
| 11 | LAP ≥44.17& Age <49 & LAP <124.46 &Age ≥44 | 0.56 | 0.44 |
| 12 | LAP ≥44.167 & Age <49 & LAP <124.46 & Age <44 | 0.60 | 0.40 |
| 13 | LAP <44.17 & Age ≥48 &AVI <13.50 & LAP ≥34.28 | 0.10 | 0.90 |
| 14 | LAP <44.17 & Age ≥48 & AVI <13.50& LAP <34.28 | 0.50 | 0.50 |
| 15 | LAP <44.17 & Age ≥48 & AVI ≥13.50 & Waist hip <1.01 | 0.63 | 0.37 |
| 16 | LAP <44.17 & Age ≥48 & AVI ≥13.50 & Waist hip ≥1.1 | 0.77 | 0.23 |
| 17 | LAP <44.17 or Missing & Age <48 or Missing & Waist hip <0.70 or Missing | 0.51 | 0.49 |
| 18 | LAP <44.17 & Age <48 & Waist hip ≥0.70 & AVI <14.19 | 0.78 | 0.22 |
| 19 | LAP <44.17 & Age <48 & Waist hip ≥0.70 & AVI ≥14.19 | 0.84 | 0.16 |
In males, LAP exhibited the most significant impact on the risk of developing elevated TC levels. Within the subgroup characterized by LAP ≥36.02, Age ≥47, LAP ≥81.59, VAI <3.46, and BMI <31, 92% of the population displayed higher TC levels (indicating the highest risk of Cholesterol ≥200). Conversely, among individuals with LAP <36.02 and BMI <18.62, 91% were identified as having lower TC levels (suggesting the lowest risk of Cholesterol <200).
Similarly, for females, LAP emerged as the key factor influencing the risk of TC development. Among those in the subgroup with LAP ≥44, Age ≥49, LAP ≥86, VAI <3.35, and BMI <32.97, 98% were at the highest risk of elevated TC levels. In contrast, individuals with LAP <44.17, Age <48, Waist hip ≥0.70, and AVI ≥14 showed that 84% had lower TC levels (indicative of the lowest risk of Cholesterol <200). The specific criteria for TC risk assessment in males and females generated by the DT model can be found in Table 4. Male and female DT are shown in Figures 2 and 3.
FIGURE 2.
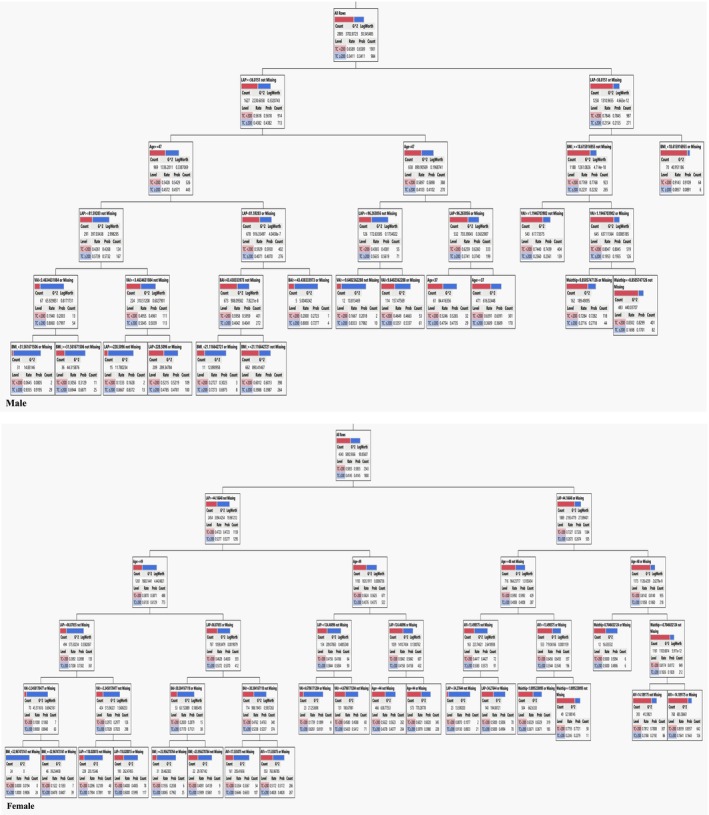
Decision tree for TC <200 and TC ≥200 in males and females.
FIGURE 3.
Result of random forest.
3.4. Random Forest Model for Males and Females
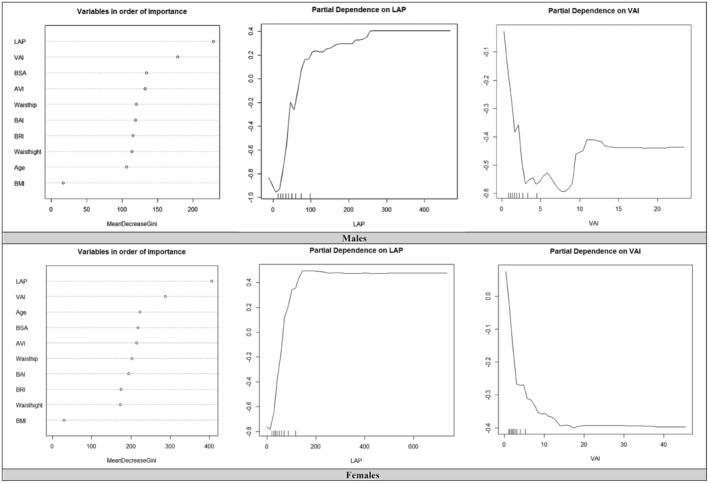
According to the results of the Random Forest model, as shown in Figure 1, the LAP and VAI were the important variables for males. It can be concluded that when the value of LAP is greater than almost 50, the likelihood of individuals developing higher TC increases, if this value exceeds 200, the likelihood of developing the disease remains constant. The VAI is the second in terms of importance in this model, when the VAI was <5, the likelihood of developing a high TC level was very low, between 5 and 10 the likelihood increased and if the VAI was above 10 the likelihood of developing is approximately constant (Table 5).
TABLE 5.
Evaluation indices for DT and RF algorithms.
|
Again, for women, the LAP and VAI were the important variables in the model. In the case of LAP exceeding 200, the likelihood of developing a high TC level increase, when this value is above 200 this likelihood is almost constant. The VAI is the second in terms of importance for females, when the VAI was <20 the likelihood was increasing and if the VAI was above 20 the likelihood of developing is constant.
4. Discussion
The Random Forest model determined that the most important variables for males were VAI and LAP. The likelihood of developing tuberculosis (TC) increases when the LAP value is greater than approximately 50, and it stays constant when the value is >200. There was a very low likelihood of developing TC when the VAI was <5, an increasing likelihood between 5 and 10, and an approximately constant likelihood above 10 in this model. The VAI is the second‐most important variable in this model. The most important variables in the model for women were LAP and VAI. The risk of developing TC increases when LAP is >200 and remains relatively constant beyond this value. The second most important factor for females is the VAI. If the VAI was <20, the likelihood of developing increased, and above 20, it was constant.
Similar findings were reported in 2015 by a study that indicated the relation between lipid profile indices and anthropometric parameters. The study stated the results show the poor health status of ethnic Italian people and these data are needed for future clinical use and screenings [44]. Another study on this matter resulted in the lipid profile indices (TC, TG, TG/HDL) being associated with similar factors such as BMI, WHR, and waist circumference [45]. The risk of cardiovascular diseases by these aerometric factors has been previously assessed in Iran and viewed as a better understanding of what the population is like [46].
When laboratory measurements are unavailable, anthropometric measures may replace total cholesterol in predicting major adverse cardiovascular events in individuals under 50 years old, according to the overall model performance. However, this might not be the case for individuals 50 years of age and older. Even when the anthropometric measures were used together, total cholesterol remained the best among participants aged 50 and above. According to several studies [47, 48, 49, 50], the predictive power of BMI for cardiovascular disease (CVD) does reduce with age. Another study recently accomplished in Iran indicates waist‐to‐hip‐to‐height ratio and waist‐to‐height ratio are more precise indices than BMI and WC for predicting distinct risk factors for cardiovascular diseases [51]. This statement is consistent with the idea that placing exclusive reliance on conventional risk factors may not be suitable for older individuals, where further improvement in discriminatory capacity can be achieved through the utilization of laboratory findings, such as novel circulating biomarkers [52, 53]. While it is generally feasible to evaluate conventional CVD risk factors in high‐income nations, this may not be the situation in resource‐constrained environments. Many nonlaboratory risk equations have been proposed, but they depend on a lot of different factors and might be hard to use in everyday clinical practice [7].
Several epidemiologic studies involving different genders and ethnic groups have conclusively shown that dyslipidemia, which is defined as high levels of LDL cholesterol, low levels of HDL cholesterol, and high levels of VLDL cholesterol and triglycerides, is positively associated with excess body fat, especially abdominal fat [54, 55]. Increasing BMI may identify prevalent dyslipidemia more accurately than other anthropometric measurements in the nonobese Chinese population aged 40 years and older [56]. An earlier study found a similar trend when looking at obese children (aged 8–18), no association between BMI, WC, TC, and a weak correlation between BMI and triglycerides. A study conducted by Sarni et al. [57] found no correlation between WC and triglyceride or TC levels in preschoolers. According to the results of the multiple regression analysis, the association between WC, TG, and HDL‐C was stronger than that between BMI and LDL‐C, while BMI was more closely related to LDL‐C [58]. The inability to differentiate between fat and muscle mass or to reveal the age‐related increase in body fat mass and decrease in muscle mass are some of the limitations of using BMI as a metric for defining obesity, a risk factor for cardiovascular diseases [59, 60]. Hence, the VAI assumes significant importance as a gender‐specific detection tool that incorporates metabolic/lipidemic parameters (HDL‐c, TG) and anthropometric measurements (BMI, WC) to identify visceral adiposity dysfunction and serve as a marker for obesity and cardiometabolic risk [61, 62].
Our study findings emphasized the significance of LAP and VAI as key variables for males. VAI exhibited a robust correlation with HDL‐C and TG as well. The potential association between dyslipidemia and these biochemical parameters (TG and HDL‐C) can be inferred due to their incorporation in the VAI formula [63].
The study carried out by Uzdil et al. [64] among patients with Metabolic Syndrome (MetS) revealed that the VAI and BRI indices could serve as valuable tools for assessing plasma lipid profiles. When examining these indices of blood lipids, VAI exhibited the strongest correlation in both genders [64]. Conversely, contrary to the results, the level of LDL‐c in men showed an increase of 5.56 mg/dL with every one‐unit rise in BRI [64]. While research on BRI is limited, available evidence suggests its potential utility in predicting the risk of conditions such as diabetes, CVD, and left ventricular hypertrophy [65, 66]. Geraci et al. [66] identified a correlation between BRI and atherosclerotic damage. Li et al. [67], after adjusting for age and gender, reported associations between BRI and TG, HDL‐c, LDL‐c, and blood pressure in the adult population.
Amato et al. [59] observed a robust correlation between VAI and CVD events in their AlkaMeSy study involving Caucasian adults. Among Asian individuals, Mohammadreza et al. [68] found that an increase in VAI elevated the risk of CVD in both males and females. A study conducted in Argentina demonstrated a positive correlation between VAI and CVD [69]. In a study by Ferraù et al. [70], HDL‐c and the Visceral Adiposity Index were found to be linked in craniofacial patients. However, there is a scarcity of prospective studies on VAI and its long‐term cardiovascular risk implications to date.
Based on our study, for men and women, the LAP and VAI were the important variables. Compared with men, women have a stronger indicator of all‐cause mortality in LAP [71]. The exact reason behind this is still unknown, but it is likely connected to the fact that lipid overaccumulation occurs differently in men and women as they age [72]. Furthermore, metabolic risk and lipid distribution may interact differently in women than in men. Women show metabolic risk factors at lower weight cutoffs than men [72]. Women are more likely than men to develop CVD if they have hypertriglyceridemia [73]. In agreement with our study, LAP is a more accurate predictor of mortality in males under the age of 50 than in men over the age of 50 [71]. This could be because men experience an increase in cardiovascular events at a younger age than women do, possibly because LAP increases more quickly for men than for women among younger adults [32].
In clinical practice, anthropometric indices (such as LAP and VAI) are a valuable part of risk assessment, especially in situations in which access to laboratory data is limited. These simple and noninvasive measures can provide additional understanding of an individual's status.
For instance, the DT analysis identified specific thresholds for LAP and VAI that could be used to divide patients into high and low‐risk categories for elevated TC. Using these thresholds, along with other factors such as age and family history, could assist physicians in providing better prevention and management of diseases.
Lastly, the stronger relation found between LAP/VAI and TC levels, compared with BMI, suggests these indices could be more sensitive factors of visceral adiposity and metabolic dysfunction. This fact may be especially associated with older patients, in which the predictive power of BMI may not be as useful.
4.1. Limitations and Strengths
There are several limitations in the study that should be taken into account. First, due to the limitation of ethnicity in our country, we were not able to evaluate these findings in different ethnicities as most of Iran's population are Persians. The extent to which the results can be generalized to other populations is therefore limited. Second, specific thresholds and risk factors that have been found in this study need further validation for being used in clinical practice. Additional work on this matter is needed to assess the utility of incorporating our findings into cholesterol management guidelines. And lastly, since this is a cross‐sectional study, we cannot establish relationships between the anthropometric measures and TC level. Longitudinal studies would be necessary for a better understanding of the temporal associations.
Some of the findings that strengthen our results should also be addressed. Our findings resulted in a broader range of anthropometric indices, including newer factors such as the Lipid LAP and VAI, which indicate the comprehensive assessment of various anthropometric indices in our study. We also utilized gender‐stratified analysis, which is a useful finding for tailoring the findings and thresholds for men and women. Our findings could be able to aid physicians in their management and monitoring of diseases, which in limited accessed areas can be helpful.
5. Conclusion
An important discovery lies in the association between anthropometric indices and total cholesterol (TC) levels, revealing substantial variations among different age and gender, specifically LAP and VAI. These insights present valuable information that can assist healthcare providers in assessing and screening risk factors for cardiovascular and metabolic diseases. Noteworthy for its simplicity, cost‐effectiveness, and ease of implementation, these data can potentially serve as a practical tool for monitoring cardiovascular and metabolic health in both adult and elderly populations.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Sahar Arab Yousefabadi, Somayeh Ghiasi Hafezi and Alireza Kooshki contributed equally to this study.
Funding: This study was supported by Mashhad University of Medical Science (MUMS), Mashhad, Iran (951214).
Contributor Information
Habibollah Esmaily, Email: esmailyh@mums.ac.ir.
Majid Ghayour‐Mobarhan, Email: ghayourm@mums.ac.ir.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Joshi S. R., Anjana R. M., Deepa M., et al., “Prevalence of Dyslipidemia in Urban and Rural India: The ICMR‐INDIAB Study,” PLoS One 9, no. 5 (2014): e96808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy S. M., “Cholesterol and Coronary Heart Disease. The 21st Century,” Archives of Internal Medicine 157, no. 11 (1997): 1177–1184. [PubMed] [Google Scholar]
- 3. Borgia M. C. and Medici F., “Perspectives in the Treatment of Dyslipidemias in the Prevention of Coronary Heart Disease,” Angiology 49, no. 5 (1998): 339–348. [DOI] [PubMed] [Google Scholar]
- 4. Kromhout D., “Epidemiology of Cardiovascular Diseases in Europe,” Public Health Nutrition 4, no. 2b (2001): 441–457. [DOI] [PubMed] [Google Scholar]
- 5. Piepoli M. F., Hoes A. W., Agewall S., et al., “2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts) Developed With the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR),” European Heart Journal 37, no. 29 (2016): 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grundy S. M., Stone N. J., Bailey A. L., et al., “2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines,” Journal of the American College of Cardiology 73, no. 24 (2019): 3168–3209. [DOI] [PubMed] [Google Scholar]
- 7. McGorrian C., Yusuf S., Islam S., et al., “Estimating Modifiable Coronary Heart Disease Risk in Multiple Regions of the World: The INTERHEART Modifiable Risk Score,” European Heart Journal 32, no. 5 (2011): 581–589. [DOI] [PubMed] [Google Scholar]
- 8. Veronesi G., Borchini R., Landsbergis P., et al., “Cardiovascular Disease Prevention at the Workplace: Assessing the Prognostic Value of Lifestyle Risk Factors and Job‐Related Conditions,” International Journal of Public Health 63, no. 6 (2018): 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD 2017 Causes of Death Collaborators , “Global, Regional, and National Age‐Sex‐Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet 392, no. 10159 (2018): 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaziano T. A., Young C. R., Fitzmaurice G., Atwood S., and Gaziano J. M., “Laboratory‐Based Versus Non‐laboratory‐Based Method for Assessment of Cardiovascular Disease Risk: The NHANES I Follow‐Up Study Cohort,” Lancet 371, no. 9616 (2008): 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bulhões K. and Araújo L., “Metabolic Syndrome in Hypertensive Patients: Correlation Between Anthropometric Data and Laboratory Findings,” Diabetes Care 30, no. 6 (2007): 1624–1626. [DOI] [PubMed] [Google Scholar]
- 12. Garcia‐D'Urso N., Climent‐Pérez P., Sánchez‐Sansegundo M., Zaragoza‐Martí A., Fuster‐Guilló A., and Azorín‐López J., “A Non‐Invasive Approach for Total Cholesterol Level Prediction Using Machine Learning,” IEEE Access 10 (2022): 58566–58577. [Google Scholar]
- 13. Elekima I. and Inokon A., “A Study of Correlation of Anthropometric Data With Atherogenic Indices of Students of Rivers State University, Port Harcourt, Nigeria,” Asian Journal of Research in Medical and Pharmaceutical Sciences 6, no. 1 (2019): 1–12. [Google Scholar]
- 14. Lampignano L., Zupo R., Donghia R., et al., “Cross‐Sectional Relationship Among Different Anthropometric Parameters and Cardio‐Metabolic Risk Factors in a Cohort of Patients With Overweight or Obesity,” PLoS One 15 (2020): e0241841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mansoori A., Hosseini Z. S., Ahari R. K., et al., “Development of Data Mining Algorithms for Identifying the Best Anthropometric Predictors for Cardiovascular Disease: MASHAD Cohort Study,” High Blood Pressure & Cardiovascular Prevention 30, no. 3 (2023): 243–253. [DOI] [PubMed] [Google Scholar]
- 16. Khader Y., Batieha A., Jaddou H., el‐Khateeb M., and Ajlouni K., “The Performance of Anthropometric Measures to Predict Diabetes Mellitus and Hypertension Among Adults in Jordan,” BMC Public Health 19, no. 1 (2019): 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saberi‐Karimian M., Mansoori A., Bajgiran M. M., et al., “Data Mining Approaches for Type 2 Diabetes Mellitus Prediction Using Anthropometric Measurements,” Journal of Clinical Laboratory Analysis 37, no. 1 (2022): e24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rativa D., Fernandes B., and Roque A., “Height and Weight Estimation From Anthropometric Measurements Using Machine Learning Regressions,” IEEE Journal of Translational Engineering in Health and Medicine 6 (2018): 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamon‐Fava S., Wilson P. W., and Schaefer E. J., “Impact of Body Mass Index on Coronary Heart Disease Risk Factors in Men and Women. The Framingham Offspring Study,” Arteriosclerosis, Thrombosis, and Vascular Biology 16, no. 12 (1996): 1509–1515. [DOI] [PubMed] [Google Scholar]
- 20. Brown C. D., Higgins M., Donato K. A., et al., “Body Mass Index and the Prevalence of Hypertension and Dyslipidemia,” Obesity Research 8, no. 9 (2000): 605–619. [DOI] [PubMed] [Google Scholar]
- 21. Alexander J. K., “Obesity and Coronary Heart Disease,” American Journal of the Medical Sciences 321, no. 4 (2001): 215–224. [DOI] [PubMed] [Google Scholar]
- 22. Walton C., Lees B., Crook D., Worthington M., Godsland I. F., and Stevenson J. C., “Body Fat Distribution, Rather Than Overall Adiposity, Influences Serum Lipids and Lipoproteins in Healthy Men Independently of Age,” American Journal of Medicine 99, no. 5 (1995): 459–464. [DOI] [PubMed] [Google Scholar]
- 23. Katzel L. I., Busby‐Whitehead M. J., and Goldberg A. P., “Adverse Effects of Abdominal Obesity on Lipoprotein Lipids in Healthy Older Men,” Experimental Gerontology 28, no. 4–5 (1993): 411–420. [DOI] [PubMed] [Google Scholar]
- 24. Zamboni M., Armellini F., Cominacini L., et al., “Obesity and Regional Body‐Fat Distribution in Men: Separate and Joint Relationships to Glucose Tolerance and Plasma Lipoproteins,” American Journal of Clinical Nutrition 60, no. 5 (1994): 682–687. [DOI] [PubMed] [Google Scholar]
- 25. Perry A. C., Applegate E. B., Allison M. L., Miller P. C., and Signorile J. F., “Relation Between Anthropometric Measures of Fat Distribution and Cardiovascular Risk Factors in Overweight Pre‐ and Postmenopausal Women,” American Journal of Clinical Nutrition 66, no. 4 (1997): 829–836. [DOI] [PubMed] [Google Scholar]
- 26. Huxley R., Mendis S., Zheleznyakov E., Reddy S., and Chan J., “Body Mass Index, Waist Circumference and Waist:Hip Ratio as Predictors of Cardiovascular Risk – A Review of the Literature,” European Journal of Clinical Nutrition 64, no. 1 (2010): 16–22. [DOI] [PubMed] [Google Scholar]
- 27. Valdez R., “A Simple Model‐Based Index of Abdominal Adiposity,” Journal of Clinical Epidemiology 44, no. 9 (1991): 955–956. [DOI] [PubMed] [Google Scholar]
- 28. Amato M. C., Giordano C., Galia M., et al., “Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated With Cardiometabolic Risk,” Diabetes Care 33, no. 4 (2010): 920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas D. M., Bredlau C., Bosy‐Westphal A., et al., “Relationships Between Body Roundness With Body Fat and Visceral Adipose Tissue Emerging From a New Geometrical Model,” Obesity 21, no. 11 (2013): 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larson‐Meyer D. E., Heilbronn L. K., Redman L. M., et al., “Effect of Calorie Restriction With or Without Exercise on Insulin Sensitivity, Beta‐Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects,” Diabetes Care 29, no. 6 (2006): 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calmarza P., Trejo J. M., Lapresta C., and Lopez P., “Lack of Association Between Carotid Intima‐Media Thickness and Apolipoprotein (a) Isoforms in a Sample of Spanish General Population,” Journal of Cardiology 61, no. 5 (2013): 372–377. [DOI] [PubMed] [Google Scholar]
- 32. Kahn H. S., “The ‘Lipid Accumulation Product’ Performs Better Than the Body Mass Index for Recognizing Cardiovascular Risk: A Population‐Based Comparison,” BMC Cardiovascular Disorders 5 (2005): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guerrero‐Romero F. and Rodríguez‐Morán M., “Abdominal Volume Index. An Anthropometry‐Based Index for Estimation of Obesity Is Strongly Related to Impaired Glucose Tolerance and Type 2 Diabetes Mellitus,” Archives of Medical Research 34, no. 5 (2003): 428–432. [DOI] [PubMed] [Google Scholar]
- 34. Khan S. H., Shahid R., Fazal N., and Ijaz A., “Comparison of Various Abdominal Obesity Measures for Predicting Metabolic Syndrome, Diabetes, Nephropathy, and Dyslipidemia,” Journal of the College of Physicians and Surgeons–Pakistan 29, no. 12 (2019): 1159–1164. [DOI] [PubMed] [Google Scholar]
- 35. Busquets‐Cortés C., López C., Paublini H., Arroyo Bote S., López‐González Á. A., and Ramírez‐Manent J. I., “Relationship Between Atherogenic Dyslipidaemia and Lipid Triad With Different Scales of Overweight and Obesity in 418,343 Spanish Workers,” Journal of Nutrition and Metabolism 2022 (2022): 9946255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergman R. N., Stefanovski D., Buchanan T. A., et al., “A Better Index of Body Adiposity,” Obesity 19, no. 5 (2011): 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verbraecken J., van de Heyning P., de Backer W., and van Gaal L., “Body Surface Area in Normal‐Weight, Overweight, and Obese Adults,” Metabolism 55, no. 4 (2006): 515–524. [DOI] [PubMed] [Google Scholar]
- 38. Ghayour‐Mobarhan M., Moohebati M., Esmaily H., et al., “Mashhad Stroke and Heart Atherosclerotic Disorder (MASHAD) Study: Design, Baseline Characteristics and 10‐Year Cardiovascular Risk Estimation,” International Journal of Public Health 60, no. 5 (2015): 561–572. [DOI] [PubMed] [Google Scholar]
- 39. Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., and Kannel W. B., “Incidence of Coronary Heart Disease and Lipoprotein Cholesterol Levels: The Framingham Study,” JAMA 256, no. 20 (1986): 2835–2838. [PubMed] [Google Scholar]
- 40. Makowski D., Ben‐Shachar M. S., and Lüdecke D., “bayestestR: Describing Effects and Their Uncertainty, Existence and Significance Within the Bayesian Framework,” Journal of Open Source Software 4, no. 40 (2019): 1541. [Google Scholar]
- 41. Aghasizadeh M., Samadi S., Sahebkar A., et al., “Serum HDL Cholesterol Uptake Capacity in Subjects From the MASHAD Cohort Study: Its Value in Determining the Risk of Cardiovascular Endpoints,” Journal of Clinical Laboratory Analysis 35, no. 6 (2021): e23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saberi‐Karimian M., Safarian‐Bana H., Mohammadzadeh E., et al., “A Pilot Study of the Effects of Crocin on High‐Density Lipoprotein Cholesterol Uptake Capacity in Patients With Metabolic Syndrome: A Randomized Clinical Trial,” BioFactors 47, no. 6 (2021): 1032–1041. [DOI] [PubMed] [Google Scholar]
- 43. Ghazizadeh H., Shakour N., Ghoflchi S., et al., “Use of Data Mining Approaches to Explore the Association Between Type 2 Diabetes Mellitus With SARS‐CoV‐2,” BMC Pulmonary Medicine 23, no. 1 (2023): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gualdi‐Russo E., Zaccagni L., Dallari G. V., and Toselli S., “Anthropometric Parameters in Relation to Glycaemic Status and Lipid Profile in a Multi‐Ethnic Sample in Italy,” Public Health Nutrition 18, no. 3 (2015): 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahraki T. and Shahraki M., “Waist Circumference: A Better Index of Fat Location Than WHR for Predicting Lipid Profile in Overweight/Obese Iranian Women,” Eastern Mediterranean Health Journal 15, no. 4 (2009): 899–905. [PubMed] [Google Scholar]
- 46. Tabary M., Cheraghian B., Mohammadi Z., et al., “Association of Anthropometric Indices With Cardiovascular Disease Risk Factors Among Adults: A Study in Iran,” European Journal of Cardiovascular Nursing 20, no. 4 (2021): 358–366. [DOI] [PubMed] [Google Scholar]
- 47. Clark K., “The Effect of Age on the Association Between Body‐Mass Index and Mortality,” Journal of Insurance Medicine 30, no. 1 (1998): 48–49. [PubMed] [Google Scholar]
- 48. Km F., “Excess Deaths Associated With Underweight, Overweight, and Obesity,” JAMA 293, no. 15 (2005): 1861–1867. [DOI] [PubMed] [Google Scholar]
- 49. Flegal K. M., Graubard B. I., Gail M. H., and Williamson D. F., “Underweight, Overweight, Obesity, and Excess Deaths—Reply,” JAMA 294, no. 5 (2005): 551–553. [DOI] [PubMed] [Google Scholar]
- 50. Dobbelsteyn C. J., Joffres M. R., MacLean D. R., and Flowerdew G., “A Comparative Evaluation of Waist Circumference, Waist‐to‐Hip Ratio and Body Mass Index as Indicators of Cardiovascular Risk Factors. The Canadian Heart Health Surveys,” International Journal of Obesity 25, no. 5 (2001): 652–661. [DOI] [PubMed] [Google Scholar]
- 51. Mahdavi‐Roshan M., Rezazadeh A., Joukar F., Naghipour M., Hassanipour S., and Mansour‐Ghanaei F., “Comparison of Anthropometric Indices as Predictors of the Risk Factors for Cardiovascular Disease in Iran: The PERSIAN Guilan Cohort Study,” Anatolian Journal of Cardiology 25, no. 2 (2021): 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pareek M., Bhatt D. L., Vaduganathan M., et al., “Single and Multiple Cardiovascular Biomarkers in Subjects Without a Previous Cardiovascular Event,” European Journal of Preventive Cardiology 24, no. 15 (2017): 1648–1659. [DOI] [PubMed] [Google Scholar]
- 53. Frary C. E., Blicher M. K., Olesen T. B., et al., “Circulating Biomarkers for Long‐Term Cardiovascular Risk Stratification in Apparently Healthy Individuals From the MONICA 10 Cohort,” European Journal of Preventive Cardiology 27, no. 6 (2020): 570–578. [DOI] [PubMed] [Google Scholar]
- 54. Thomas G., Ho S.‐Y., Lam K. S. L., et al., “Impact of Obesity and Body Fat Distribution on Cardiovascular Risk Factors in Hong Kong Chinese,” Obesity Research 12, no. 11 (2004): 1805–1813. [DOI] [PubMed] [Google Scholar]
- 55. Hu D., Hannah J., Gray R. S., et al., “Effects of Obesity and Body Fat Distribution on Lipids and Lipoproteins in Nondiabetic American Indians: The Strong Heart Study,” Obesity Research 8, no. 6 (2000): 411–421. [DOI] [PubMed] [Google Scholar]
- 56. Yang Z., Ding X., Liu J., et al., “Associations Between Anthropometric Parameters and Lipid Profiles in Chinese Individuals With Age ≥40 Years and BMI <28 kg/m2 ,” PLoS One 12, no. 6 (2017): e0178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sarni R. S., Souza F. I. S., Schoeps D. O., et al., “Relationship Between Waist Circumference and Nutritional Status, Lipid Profile and Blood Pressure in Low Socioeconomic Level Pre‐School Children,” Arquivos Brasileiros de Cardiologia 87 (2006): 153–158. [DOI] [PubMed] [Google Scholar]
- 58. Xu C., Yang X., Zu S., Han S., Zhang Z., and Zhu G., “Association Between Serum Lipids, Blood Pressure, and Simple Anthropometric Measures in an Adult Chinese Population,” Archives of Medical Research 39, no. 6 (2008): 610–617. [DOI] [PubMed] [Google Scholar]
- 59. Amato M. C., Pizzolanti G., Torregrossa V., Misiano G., Milano S., and Giordano C., “Visceral Adiposity Index (VAI) Is Predictive of an Altered Adipokine Profile in Patients With Type 2 Diabetes,” PLoS One 9, no. 3 (2014): e91969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rahman M. and Berenson A. B., “Accuracy of Current Body Mass Index Obesity Classification for White, Black and Hispanic Reproductive‐Age Women,” Obstetrics and Gynecology 115, no. 5 (2010): 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ding Y., Gu D., Zhang Y., Han W., Liu H., and Qu Q., “Significantly Increased Visceral Adiposity Index in Prehypertension,” PLoS One 10, no. 4 (2015): e0123414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang F., Wang G., Wang Z., et al., “Visceral Adiposity Index May Be a Surrogate Marker for the Assessment of the Effects of Obesity on Arterial Stiffness,” PLoS One 9, no. 8 (2014): e104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goldani H., Adami F. S., Antunes M. T., et al., “Applicatility of the Visceral Adiposity Index (VAI) in the Prediction of the Components of the Metabolic Syndrome in Elderly,” Nutrición Hospitalaria 32, no. 4 (2015): 1609–1615. [DOI] [PubMed] [Google Scholar]
- 64. Uzdil Z., Kaya S., Sökülmez Kaya P., Terzi M., and Dünder E., “The Effectiveness of New Adiposity Indices on Plasma Lipid Profile in Patients With Multiple Sclerosis: A Cross‐Sectional Study With a Body Shape Index, Body Roundness Index, and Visceral Adiposity Index,” Multiple Sclerosis and Related Disorders 43 (2020): 102214. [DOI] [PubMed] [Google Scholar]
- 65. Chang Y., Guo X., Li T., Li S., Guo J., and Sun Y., “A Body Shape Index and Body Roundness Index: Two New Body Indices to Identify Left Ventricular Hypertrophy Among Rural Populations in Northeast China,” Heart, Lung & Circulation 25, no. 4 (2016): 358–364. [DOI] [PubMed] [Google Scholar]
- 66. Geraci G., Zammuto M., Gaetani R., et al., “Relationship of a Body Shape Index and Body Roundness Index With Carotid Atherosclerosis in Arterial Hypertension,” Nutrition, Metabolism, and Cardiovascular Diseases 29, no. 8 (2019): 822–829. [DOI] [PubMed] [Google Scholar]
- 67. Li G., Wu H. K., Wu X. W., et al., “The Feasibility of Two Anthropometric Indices to Identify Metabolic Syndrome, Insulin Resistance and Inflammatory Factors in Obese and Overweight Adults,” Nutrition 57 (2019): 194–201. [DOI] [PubMed] [Google Scholar]
- 68. Mohammadreza B., Farzad H., and Davoud K., “Prognostic Significance of the Complex ‘Visceral Adiposity Index’ vs. Simple Anthropometric Measures: Tehran Lipid and Glucose Study,” Cardiovascular Diabetology 11 (2012): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salazar M. R., Carbajal H. A., Espeche W. G., Aizpurúa M., Maciel P. M., and Reaven G. M., “Identification of Cardiometabolic Risk: Visceral Adiposity Index Versus Triglyceride/HDL Cholesterol Ratio,” American Journal of Medicine 127, no. 2 (2014): 152–157. [DOI] [PubMed] [Google Scholar]
- 70. Ferraù F., Spagnolo F., Cotta O. R., et al., “Visceral Adiposity Index as an Indicator of Cardiometabolic Risk in Patients Treated for Craniopharyngioma,” Endocrine 58 (2017): 295–302. [DOI] [PubMed] [Google Scholar]
- 71. Ioachimescu A. G., Brennan D. M., Hoar B. M., and Hoogwerf B. J., “The Lipid Accumulation Product and All‐Cause Mortality in Patients at High Cardiovascular Risk: A PreCIS Database Study,” Obesity 18, no. 9 (2010): 1836–1844. [DOI] [PubMed] [Google Scholar]
- 72. Kahn H. and Cheng Y., “Longitudinal Changes in BMI and in an Index Estimating Excess Lipids Among White and Black Adults in the United States,” International Journal of Obesity 32, no. 1 (2008): 136–143. [DOI] [PubMed] [Google Scholar]
- 73. Sallam T. and Watson K. E., “Predictors of Cardiovascular Risk in Women,” Women's Health 9, no. 5 (2013): 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schlich E., Schumm M., and Schlich M., “3D‐Body‐Scan als anthropometrisches Verfahren zur Bestimmung der spezifischen Körperoberfläche,” Ernahrungs‐Umschau 57, no. 4 (2010): 178–183. [Google Scholar]
- 75. Swinburn B. A., Sacks G., Hall K. D., et al., “The Global Obesity Pandemic: Shaped by Global Drivers and Local Environments,” Lancet 378, no. 9793 (2011): 804–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


