Abstract
Tandem autologous stem cell transplantation can improve the prognosis of patients with multiple myeloma. However, the precise role of tandem transplantation remains debatable. We evaluated the clinical benefits of tandem transplantation retrospectively. Of the 655 included patients, 117 underwent tandem transplantation; the remaining were assigned to the control group. After a single transplantation, the tandem group achieved a complete remission (CR) rate of 24.8%, which increased to 46.2% after a second transplantation. The tandem group had a significantly longer median PFS than the control group in patients with International Staging System (ISS) III and high-risk cytogenetics (23.1 vs. 14.7 months, p = 0.007 for ISS III; 21.7 vs. 13.2 months, p = 0.042 for high-risk cytogenetics). The tandem group exhibited significantly superior PFS to the control group (20.3 vs. 12.6 months, p = 0.003) among patients who failed to achieve CR after a single transplantation. Tandem transplantation was associated with significantly improved PFS after adjusting for maintenance therapy in patients with ISS III, those with high-risk cytogenetics, and those who did not achieve CR after a single transplantation. Following propensity score matching, the tandem group exhibited significantly longer PFS than the control group (30.3 vs. 13.5 months, p = 0.028). Tandem transplantation should be considered in high-risk patients.
Keywords: Multiple myeloma, Tandem, Autologous, Stem cell transplantation, High risk
Subject terms: Cancer, Diseases, Oncology
Introduction
Despite treatment advances, multiple myeloma (MM) remains incurable1. High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is considered the standard treatment for eligible patients2,3. Moreover, ASCT is reportedly effective and safe in older adults, thus expanding its applicability4. Novel agents have also been found to improve clinical outcomes in transplant-eligible and -ineligible patients.
Tandem transplantation was proposed in the late 1980s to improve the prognosis of patients with MM and involves sequential transplantation. However, the results of several studies conducted to evaluate the clinical benefits of tandem transplantation are contradictory5–8. For instance, previous studies with various clinical backgrounds have demonstrated survival benefits; however, a meta-analysis of six randomised studies found no differences in overall survival (OS) or event-free survival9. Following the development of novel agents, the BMT CTN 0702 trial revealed that tandem ASCT followed by lenalidomide maintenance could substantially improve progression-free survival (PFS) compared to consolidation chemotherapy with lenalidomide maintenance10. According to the ENM02/HO95 study, tandem transplantation was associated with significantly longer survival outcomes than single transplantation11. Patients were randomised to receive bortezomib, melphalan, or prednisolone chemotherapy or ASCT after induction therapy. ASCT was performed using either a single or double dose.
Although novel agents and intensified regimens have improved clinical outcomes, these benefits have mostly been observed in low-risk patients. Thus, the treatment of high-risk patients remains challenging. Alternative strategies, such as a triplet regimen of bortezomib, lenalidomide, and dexamethasone (VRd), have recently been introduced and have shown improved clinical outcomes in all patients. However, most studies reported no improvement in outcomes of high-risk patients12. According to previous studies, tandem transplantation can improve the prognosis of high-risk patients, including those with extramedullary disease and high-risk cytogenetics13. In the ENM02/HO95 study, patients with del17p who underwent tandem transplantation experienced longer PFS than those who did not undergo tandem transplantation11. In the BMT CTN 0702 trial, tandem transplantation reportedly improved prognosis in high-risk patients when compared with consolidation or maintenance chemotherapy10.
Accordingly, in the current study, we aimed to evaluate the clinical benefits of tandem transplantation and identify the patient population for whom tandem transplantation may be beneficial as a post-transplantation treatment.
Materials and methods
Patient eligibility
We retrospectively collected the medical records of 655 patients with symptomatic MM who were treated between 2005 and 2020 in Korean institutions affiliated with the Korean Multiple Myeloma Working Party. In total, 117 patients underwent tandem transplantation in 10 institutions, and 538 patients served as the control group and underwent a single transplantation in three institutions (Fig S1). The response to therapy was evaluated using the International Myeloma Working Group consensus guidelines14. Patients with primary amyloidosis were excluded.
The decision to proceed with tandem transplantation was primarily based on the criteria approved by the Korean insurance system and the preferences of individual physician and patients. According to these criteria, tandem transplantation is authorised within 6 months following the first transplantation if the patient demonstrates a response to treatment but does not achieve a very good partial response (VGPR). To accommodate this, stem cell collection was performed before the first transplantation, aiming to collect a sufficient cell volume for two transplantation procedures for all eligible patients. However, even if the above criteria were met, the actual implementation of tandem transplantation was determined by the patient’s physical performance status, preferences, and the discretion of the attending physician. The choice of conditioning regimen was also left to the physician’s preference.
Objectives and definitions
The primary endpoints were PFS and OS. PFS was defined as the time between transplantation and either disease progression or death. OS was defined as the time between transplantation and death or the last follow-up. The secondary endpoint was improvements in pre- and post-ASCT responses. The overall response rate (ORR) was defined as a partial response (PR). Haematological and non-haematological toxicities during ASCT were examined using the CTCAE v5.0. At diagnosis, the prognosis of each patient was evaluated using the International Staging System (ISS)15. Cytogenetic risks were stratified into high-risk (del(17p), t(4;14), or t(14;16) via fluorescence in situ hybridisation (FISH)) and standard-risk groups16.
Statistical analysis
Continuous variables were compared between patients who underwent tandem ASCT and those assigned to the control group using the t-test or Mann–Whitney U test. Categorical variables were analysed using the chi-square (χ2) or Fisher’s exact test. Survival outcomes were assessed using the Kaplan–Meier method, log-rank test, and Cox proportional hazard models. Univariate and multivariate Cox analyses yielded hazard ratios (HRs) and 95% confidence intervals (CIs). Additionally, propensity score matching with a 1:1 matching ratio of single to tandem patients was performed to adjust for potential bias owing to the retrospective nature of the study. Owing to incomplete data, the list of confounding or prognostic variables for matching was limited to patient age, serum lactate dehydrogenase (LDH) level, FISH risk stratification, maintenance therapy, and response status after the first transplantation. The matching quality was judged based on the standardised differences in the listed confounding variables within ± 0.1. R (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria) was used to perform statistical analyses, and the R package MatchIt was used for propensity score matching. Statistical significance was defined as a two-sided p-value < 0.05.
Results
Patient demographics
Table 1 presents a comparison between both patient groups. Patients who underwent tandem ASCT were significantly younger (54 vs. 57 years), were diagnosed with MM earlier, and underwent ASCT earlier than those in the control group. At diagnosis, the tandem group showed higher rates of high-risk FISH and elevated LDH levels than the control group. Moreover, the tandem group received fewer novel agent-based therapies for induction than the control group. A higher number of patients in the tandem group received high-dose melphalan than in the control group. The proportion of patients who received maintenance therapy following ASCT was consistent across groups, with approximately two-thirds receiving thalidomide-based therapy.
Table 1.
Patients’ characteristics.
| Overall | Single | Tandem | p | |
|---|---|---|---|---|
| Number | 655 | 538 | 117 | |
| Age* | 56 (28–69) | 57 (28–69) | 54 (31–67) | < 0.001 |
| Year of diagnosis* | 2013 (2004–2020) | 2013 (2004–2020) | 2008 (2004–2019) | < 0.001 |
| Year of ASCT* | 2013 (2005–2020) | 2014 (2005–2020) | 2008 (2005–2020) | < 0.001 |
| Male | 370 (56.5%) | 306 (56.9%) | 64 (54.7%) | 0.682 |
| Secretory | 634 (96.8%) | 520 (96.7%) | 114 (97.4%) | 1.000 |
| Non-secretory | 21 (3.2%) | 18 (3.3%) | 3 (2.6%) | |
| Heavy chain | 0.077 | |||
| IgG | 348 (54.0%) | 278 (52.7%) | 70 (59.8%) | |
| lgA | 116 (18.0%) | 100 (18.9%) | 16 (13.7%) | |
| IgM | 3 (0.5%) | 2 (0.4%) | 1 (0.9%) | |
| IgD | 20 (3.1%) | 17 (3.2%) | 3 (2.6%) | |
| IgE | 1 (0.2%) | 0 (0.0%) | 1 (0.9%) | |
| Light chain only | 156 (24.1%) | 131 (24.8%) | 25 (21.4%) | |
| Unknown | 1 (0.2%) | 0 (0.0%) | 1 (0.9%) | |
| Light chain | 0.149 | |||
| Kappa | 347 (53.8%) | 278 (52.7%) | 69 (59.0%) | |
| Lambda | 295 (45.7%) | 248 (47.0%) | 47 (40.2%) | |
| No light chain | 2 (0.3%) | 2 (0.4%) | 0 (0.0%) | |
| Unknown | 1 (0.2%) | 0 (0.0%) | 1 (0.9%) | |
| ECOG PS > 1 | 110 (17.3%) | 90 (17.2%) | 20 (17.7%) | 0.891 |
| ISS | 0.106 | |||
| Stage I | 217/616 (35.2%) | 187/506 (37.0%) | 30/110 (27.3%) | |
| Stage II | 199/616 (32.3%) | 156/506 (30.8%) | 43/110 (39.1%) | |
| Stage III | 200/616 (32.5%) | 163/506 (32.2%) | 37/110 (33.6%) | |
| Karyotype | 0.931 | |||
| Normal | 339/561 (60.4%) | 283/466 (60.7%) | 56/95 (58.9%) | |
| Non-complex | 17/561 (3.0%) | 14/466 (3.0%) | 3/95 (3.2%) | |
| Complex | 205/561 (36.5%) | 169/466 (36.3%) | 36/95 (37.9%) | |
| FISH | 0.002 | |||
| Standard risk | 366/455 (80.4%) | 319/384 (83.1%) | 47/71 (66.2%) | |
| High risk† | 89/455 (19.6%) | 65/384 (16.9%) | 24/71 (33.8%) | |
| 17p del | 33/455 (7.3%) | 25/384 (6.5%) | 8/71 (11.3%) | 0.156 |
| t(4;14) | 67/455 (14.7%) | 52/384 (13.5%) | 15/71 (21.1%) | 0.098 |
| t(14;16) | 11/455 (2.4%) | 6/384 (1.6%) | 5/71 (7.0%) | 0.017 |
| 1q gain/amp | 143/443 (32.3%) | 122/377 (32.4%) | 21/66 (31.8%) | 0.931 |
| EMP (present) | 201/653 (30.9%) | 174/537 (32.5%) | 27/116 (23.3%) | 0.059 |
| LDH (elevated) | 141/578 (24.5%) | 102/481 (21.4%) | 39/97 (40.2%) | < 0.001 |
| Induction regimen | < 0.001 | |||
| CTD | 268 (40.9%) | 235 (43.7%) | 33 (28.2%) | |
| VTD | 216 (33.0%) | 186 (34.6%) | 30 (25.6%) | |
| VAD | 108 (16.5%) | 61 (11.3%) | 47 (40.2%) | |
| Others | 63 (9.6%) | 56 (10.4%) | 7 (6.0%) | |
| Novel agent in induction‡ | < 0.001 | |||
| Novel agent ( +) | 505 (77.1%) | 436 (81.0%) | 69 (59.0%) | |
| Novel agent (−) | 150 (22.9%) | 102 (19.0%) | 48 (41.0%) | |
| Conditioning regimen | 0.014 | |||
| HD MEL | 588 (89.8%) | 474 (88.1%) | 114 (97.4%) | |
| BUMEL | 37 (5.6%) | 34 (6.3%) | 3 (2.6%) | |
| Other | 19 (2.9%) | 19 (3.5%) | 0 (0.0%) | |
| Unknown | 11 (1.7%) | 11 (2.0%) | 0 (0.0%) | |
| Maintenance (done) | 159 (24.2%) | 128 (23.7%) | 31 (26.5%) | 0.552 |
| Thalidomide | 99 (62.3%) | 79 (61.7%) | 20 (64.5%) | |
| Lenalidomide | 23 (14.5%) | 19 (14.8%) | 4 (12.9%) | |
| Bortezomib | 15 (9.4%) | 15 (11.7%) | 0 | |
| Steroid | 14 (8.8%) | 7 (5.5%) | 7 (22.6%) | |
| Other | 8 (5.0%) | 8 (6.3%) | 0 | |
*Presented as median (range), †defined as del(17p), t(4;14), and/or t(14;16), ‡defined as bortezomib, thalidomide, and/or lenalidomide.
ASCT, autologous stem cell transplantation; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; FISH, fluorescence in situ hybridisation; EMP, extramedullary plasmacytoma; LDH, lactate dehydrogenase; CTD, cyclophosphamide, thalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone; VAD, vincristine, adriamycin, and dexamethasone; HD MEL, high-dose melphalan; BUMEL, busulfan and melphalan.
Response status and survival outcomes in all patients
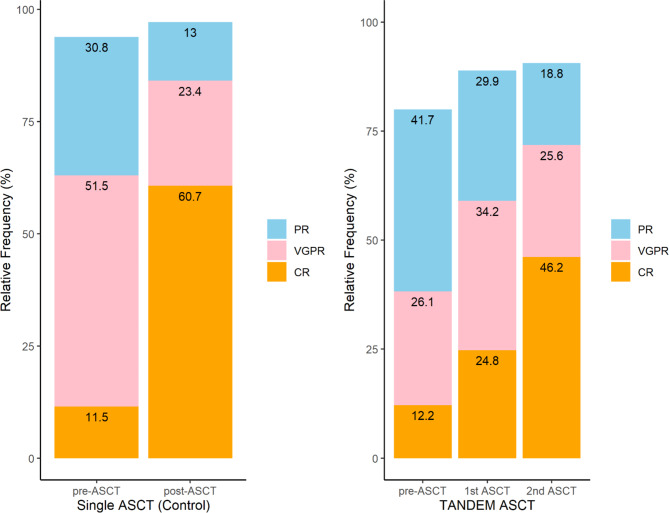
Complete response (CR) rates and ORR were compared before and after ASCT (Fig. 1). After the first transplantation, the CR rate in the tandem group increased from 12.2 to 24.8%, whereas the CR rate increased from 11.5 to 60.7% in the control group. After the second transplantation, the CR rate in the tandem group almost doubled to 46.2%. After the first transplantation, 59.0 and 84.1% of patients in the tandem and control groups, respectively, showed VGPR or better; however, after the second transplantation, the rate of VGPR or better in the tandem group increased to 71.8%. After planned transplantation, the ORR was similar in both groups (97.1% in the control group vs. 90.6% in the tandem group).
Fig. 1.
Response status before and after stem cell transplantation in patients with multiple myeloma who underwent single transplantation and those who underwent tandem transplantation.
The median follow-up durations for the entire population, the control group, and the tandem group were 74, 70, and 136 months, respectively. Figure S2 shows the median PFS and OS of all patients, with no significant differences between groups.
Cox regression analyses of other prognostic factors were performed to analyse the effects of tandem transplantation. In the univariate Cox analysis, ISS III, both abnormal and complex karyotypes (defined as having three or more chromosomal abnormalities), high-risk FISH, 1q gain/amp( +), elevated LDH levels at diagnosis, response status before 1st transplantation, response status after 1st transplantation, and maintenance therapy were all significant factors associated with PFS. ISS III, abnormal and complex karyotypes, high-risk FISH, 1q gain/amp( +), elevated LDH, response status after 1st transplantation and maintenance therapy were significantly associated with OS. However, tandem transplantation was not significantly associated with PFS or OS (Table S1). Multivariate Cox analysis of the above risk factors revealed that cytogenetics at diagnosis, response status following the first ASCT, and the administration of maintenance therapy were significant factors influencing survival outcomes (Table S2). The multivariate Cox regression analysis of the two variables, tandem transplantation and maintenance therapy—both of which are comparable treatments following the first transplant—revealed that maintenance therapy was a significant factor for both PFS and OS in the entire patient population (Table 2). Comparison of the maintenance regimens administered to patients included in this study—thalidomide (n = 99), lenalidomide (n = 23), and bortezomib (n = 15)—revealed no significant differences in the duration of maintenance therapy. While patients receiving bortezomib showed numerically the best survival outcomes, these differences were not statistically significant (Table S3).
Table 2.
Multivariate Cox analyses for progression-free survival and overall survival in whole patients.
| Category | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Tandem | 0.88 | 0.70–1.11 | 0.268 | 1.06 | 0.79–1.41 | 0.713 |
| Maintenance | 0.74 | 0.60–0.91 | 0.005 | 0.72 | 0.54–0.96 | 0.023 |
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; OS, overall survival.
Survival outcomes in subgroup populations
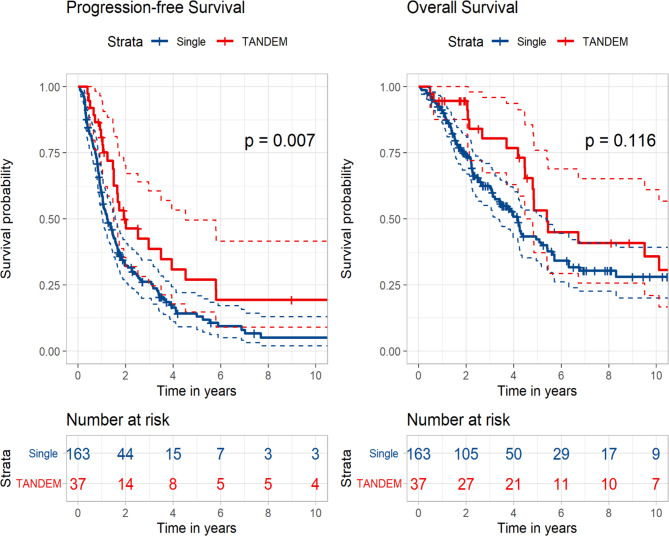
Subgroup analyses were performed to identify the benefits of tandem transplantation in specific subgroups. Among patients who presented with ISS III at diagnosis, the median PFS of the tandem group was significantly longer than that of the control group (23.1 vs. 14.7 months, p = 0.007). The tandem group also exhibited better OS than the control group, although the difference was not statistically significant (64.7 vs. 50.0 months, p = 0.116) (Fig. 2).
Fig. 2.
Kaplan–Meier curves according to single versus tandem autologous stem cell transplantation in patients with International Staging System III at diagnosis.
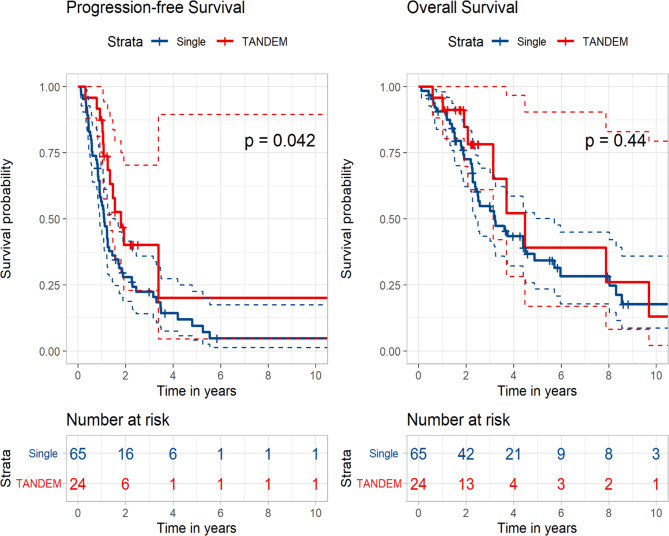
Among patients with high-risk FISH at diagnosis, the tandem group showed significantly superior PFS to that of the control group (21.7 vs. 13.2 months, p = 0.042). The median OS was longer in the tandem group than that in the control group; however, this difference was not statistically significant (53.7 vs. 38.6 months, p = 0.440) (Fig. 3). Upon analysing the subgroups of high-risk FISH, patients with t(4;14) at diagnosis demonstrated a significantly better PFS in the tandem group compared to the control group (13.5 vs. 23.1 months, p = 0.013). However, in other subgroups, although the PFS generally favoured the tandem group, no statistically significant difference was observed. (Fig S3).
Fig. 3.
Kaplan–Meier curves according to single versus tandem autologous stem cell transplantation in patients with high-risk cytogenetics at diagnosis.
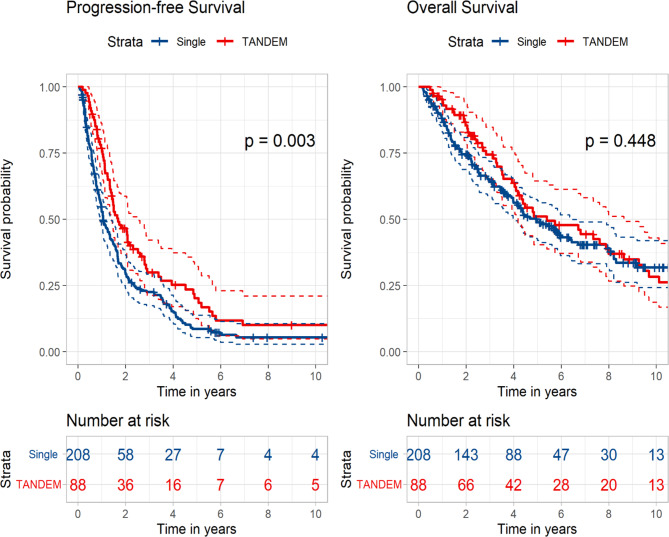
The potential clinical benefits of tandem transplantation were assessed based on response status after the first transplantation. Patients who did not achieve CR after their first transplantation were analysed. The patients in the tandem group had significantly longer PFS than those in the control group (20.3 vs. 12.6 months, p = 0.003), although the result of OS did not reveal any statistically significant difference (64.7 vs. 59.4 months, p = 0.448) (Fig. 4). Further analyses were performed to identify patients who benefited from tandem transplantation despite not achieving CR. Patients who achieved a PR after the first transplantation did not show any clinical benefit from tandem transplantation (Fig. S4), and in this group, where maintenance therapy was less frequently administered in the tandem group (49.3% vs. 34.3%), it was associated with a significant improvement in OS (49.2 months vs. median not reached, p = 0.002). However, upon analysing patients who presented with VGPR after the first transplantation, the tandem group had significantly improved PFS when compared with the control group (18.6 vs. 11.8 months, p = 0.008) (Fig. S5). Further analysis of this subgroup revealed that the tandem group had a significantly higher number of patients with high-risk FISH results than the control group (56.5% vs. 19.1%, p < 0.001). The analysis of patients who achieved CR after the first transplantation showed that both PFS (28.8 vs. 35.7 months, p = 0.288) and OS (89.1 vs. 91.5 months, p = 0.892) were numerically better in the tandem group, although these differences were not statistically significant.
Fig. 4.
Kaplan–Meier curves according to single versus tandem autologous stem cell transplantation in patients who did not achieve complete response after the first transplantation.
When the analysis was restricted to patients who had received novel agents prior to the first transplantation, no significant differences in survival rates were observed in the overall cohort (23.4 vs. 24.6 months, p = 0.927 for PFS; 87.0 vs. 57.9 months, p = 0.339 for OS). However, in key subgroups as analysed above, including patients with high-risk FISH (55 in the control group, 20 in the tandem group) and patients with ISS III (134 in the control group, 26 in the tandem group), tandem transplantation showed a tendency towards better PFS (12.9 vs. 17.5 months, p = 0.054, for high-risk FISH; 14.9 vs. 20.0 months, p = 0.090, for ISS-III), although this did not reach statistical significance. In patients who did not achieve CR after the first transplantation, the results still favoured tandem transplantation (13.5 vs. 20.2 months, p = 0.104). (Fig S6) When comparing patients diagnosed before and after 2013, those diagnosed prior to 2013 underwent tandem transplantation more frequently, had a relatively lower proportion of bortezomib and immunomodulatory drug administration, and showed a significantly higher use of thalidomide as maintenance therapy. When examining the impact of tandem transplantation and PFS based on the time of diagnosis, patients diagnosed before 2013 who were classified as ISS-III, those who did not achieve CR after the first transplantation, and those who attained VGPR after the first transplantation all demonstrated significantly improved PFS. Patients diagnosed after 2013 also showed numerically improved PFS in all these subgroups, but these improvements did not reach statistical significance (Table S4).
Multivariate Cox analyses were performed to compare the effects of tandem transplantation and maintenance therapy between subgroups. After adjusting for maintenance therapy, tandem transplantation was associated with improved PFS in patients who presented with ISS III, as well as in those who had high-risk FISH at diagnosis (Table 3). Tandem transplantation and maintenance therapy were significant factors in patients who did not achieve CR and in those who presented with VGPR after the first transplantation. Maintenance therapy was also significantly associated with improved OS in patients who did not achieve CR before the first transplantation and in those who did not achieve CR after the first transplantation (Table S5). Upon classifying all patients into four groups (S, single transplantation without maintenance therapy; T, tandem transplantation without maintenance therapy; M, single transplantation and maintenance therapy; and B, both tandem transplantation and maintenance therapy), patients in the B subgroup achieved the longest median PFS (27.8 months) when compared with those in the other subgroups (27.3 months for M, 22.9 months for T, and 21.7 months for S). In terms of OS, patients in the M subgroup had the best clinical results among all subgroups (Fig. S7).
Table 3.
Multivariate Cox analyses for progression-free survival and overall survival in subgroups according to post-1st transplantation treatment (tandem transplantation vs. maintenance therapy) according to risk factors at diagnosis.
| Risk factors at diagnosis | Category | PFS | OS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| ISS-1 | Tandem | 1.04 | 0.67–1.61 | 0.858 | 1.58 | 0.92–2.71 | 0.101 |
| Maintenance | 0.65 | 0.44–0.94 | 0.023 | 0.69 | 0.41–1.11 | 0.172 | |
| ISS-2 | Tandem | 1.04 | 0.70–1.53 | 0.861 | 1.24 | 0.76–2.03 | 0.391 |
| Maintenance | 0.78 | 0.54–1.13 | 0.188 | 0.67 | 0.41–1.11 | 0.122 | |
| ISS-3 | Tandem | 0.53 | 0.34–0.81 | 0.004 | 0.63 | 0.38–1.06 | 0.079 |
| Maintenance | 0.72 | 0.50–1.05 | 0.086 | 0.66 | 0.41–1.07 | 0.089 | |
| Standard risk (FISH) | Tandem | 0.87 | 0.61–1.22 | 0.414 | 0.93 | 0.59–1.45 | 0.741 |
| Maintenance | 0.72 | 0.55–0.94 | 0.015 | 0.64 | 0.44–0.93 | 0.020 | |
| High risk (FISH) | Tandem | 0.54 | 0.29–0.99 | 0.046 | 0.69 | 0.33–1.44 | 0.323 |
| Maintenance | 0.73 | 0.41–1.32 | 0.301 | 0.58 | 0.29–1.18 | 0.136 | |
| Normal karyotype | Tandem | 0.97 | 0.70–1.34 | 0.842 | 1.23 | 0.82–1.86 | 0.315 |
| Maintenance | 0.74 | 0.56–0.98 | 0.038 | 0.74 | 0.50–1.09 | 0.132 | |
| Abnormal karyotype | Tandem | 0.79 | 0.52–1.21 | 0.281 | 1.05 | 0.64–1.72 | 0.850 |
| Maintenance | 0.95 | 0.64–1.40 | 0.799 | 0.80 | 0.49–1.32 | 0.383 | |
| Complex karyotype | Tandem | 0.82 | 0.52–1.27 | 0.370 | 1.06 | 0.62–1.81 | 0.822 |
| Maintenance | 0.85 | 0.55–1.31 | 0.461 | 0.71 | 0.41–1.23 | 0.224 | |
| Normal LDH | Tandem | 0.73 | 0.51–1.03 | 0.074 | 1.02 | 0.66–1.56 | 0.938 |
| Maintenance | 0.88 | 0.68–1.14 | 0.340 | 0.80 | 0.56–1.14 | 0.220 | |
| Elevated LDH | Tandem | 0.68 | 0.45–1.02 | 0.061 | 0.89 | 0.55–1.44 | 0.629 |
| Maintenance | 0.38 | 0.24–0.61 | < 0.001 | 0.51 | 0.29–0.89 | 0.018 |
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; OS, overall survival; ISS, International Staging System; FISH, fluorescence in situ hybridisation; LDH, lactate dehydrogenase.
Survival outcomes with propensity score matching method
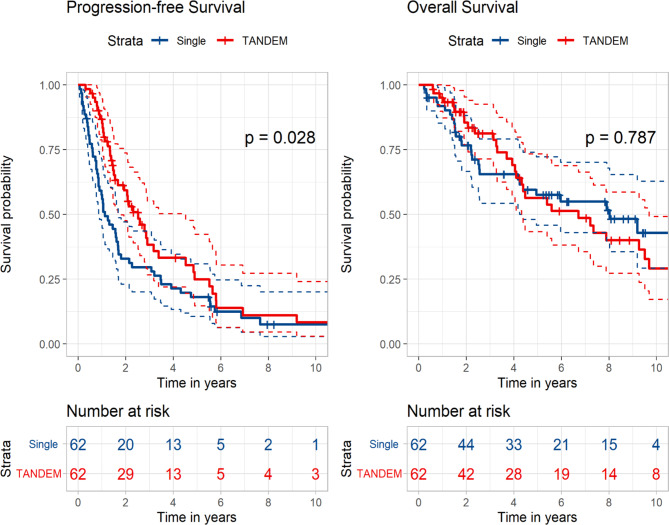
Considering that physicians may have performed tandem transplantation in selected populations, we applied the propensity score matching method to adjust for potential biases owing to the retrospective nature of the study. The statistical details are described in Table S6, in which we assumed that patients with older age (> 55 years), elevated LDH levels, high-risk FISH at diagnosis, and response status after the first transplantation underwent tandem transplantation disproportionately. As maintenance therapy may have introduced bias in evaluating the exact role of tandem transplantation, we included maintenance therapy in the propensity score matching. As shown in Fig. 5, patients who underwent tandem transplantation had significantly longer PFS than those in the control group (30.3 vs. 13.5 months, p = 0.028), although there was no statically significant difference in OS (80.6 vs. 96.3 months, p = 0.787). In the Cox analysis, tandem transplantation was significantly associated with improved PFS (HR 0.65, 95% CI 0.45–0.94, p = 0.023) but not with OS (HR 1.07, 95% CI 0.64–1.79, p = 0.788).
Fig. 5.
Kaplan–Meier curves with the propensity score matching method according to single versus tandem autologous stem cell transplantation in all patients.
Adverse events
Table 4 presents the adverse events experienced by patients in both groups. In both groups, all patients had grade ≥ 3 neutropenia and thrombocytopenia. The tandem group had more patients with grade ≥ 3 anaemia than the control group, but the incidence of anaemia did not increase after the second transplantation.
Table 4.
Adverse events during single and tandem autologous stem cell transplantation in patients with multiple myeloma.
| Single ASCT (control) | 1st ASCT (tandem) | 2nd ASCT (tandem) | ||||
|---|---|---|---|---|---|---|
| Event | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 |
| Hematologic adverse events | ||||||
| Neutropenia | 256/256 (100%) | 256/256 (100%) | 117/117 (100%) | 117/117 (100%) | 117/117 (100%) | 117/117 (100%) |
| Thrombocytopenia | 256/256 (100%) | 256/256 (100%) | 117/117 (100%) | 117/117 (100%) | 117/117 (100%) | 117/117 (100%) |
| Anaemia | 256/256 (100%) | 96/256 (37.5%) | 117/117 (100%) | 65/117 (55.6%) | 117/117 (100%) | 56/117 (47.9%) |
| Gastrointestinal adverse events | ||||||
| Nausea | 225/247 (91.1%) | 69/247 (27.9%) | 112/115 (97.4%) | 33/115 (28.7%) | 110/117 (94.0%) | 39/117 (33.3%) |
| Vomit | 117/261 (44.8%) | 17/261 (6.5%) | 70/117 (59.8%) | 3/117 (2.6%) | 75/112 (67.0%) | 5/112 (4.5%) |
| Stomatitis | 213/234 (91.0%) | 36/234 (15.4%) | 77/114 (67.5%) | 13/114 (11.4%) | 81/114 (71.1%) | 5/114 (4.4%) |
| Constipation | 8/242 (3.3%) | 0/242 (0%) | 11/115 (9.5%) | 0/115 (0%) | 15/112 (13.4%) | 0/112 (0%) |
| Diarrhoea | 226/246 (91.9%) | 97/246 (39.4%) | 100/113 (88.5%) | 22/113 (19.5%) | 91/112 (81.2%) | 21/112 (18.8%) |
| Hepatobiliary adverse events | ||||||
| Hyperbilirubinaemia | 23/256 (9.0%) | 3/256 (1.2%) | 11/116 (9.5%) | 0/116 (0%) | 14/115 (12.2%) | 0/115 (0%) |
| Elevated transaminases | 41/256 (16.0%) | 0/256 (0%) | 43/117 (36.8%) | 3/117 (2.6%) | 28/115 (24.3%) | 2/115 (1.7%) |
| Nephrologic adverse events | ||||||
| Azotaemia | 16/256 (6.1%) | 4/256 (1.6%) | 10/116 (8.6%) | 2/116 (1.7%) | 9/115 (7.8%) | 0/115 (0%) |
| Febrile neutropenia | 145/256 (56.7%) | 145/256 (56.7%) | 66/117 (56.4%) | 66/117 (56.4%) | 59/117 (50.4%) | 59/117 (50.4%) |
| Sinusoidal obstruction syndrome | 0/256 (0%) | 0/256 (0%) | 0/117 (0%) | 0/117 (0%) | 1/117 (0.9%) | 0/117 (0%) |
ASCT, autologous stem cell transplantation.
Non-haematological toxicities were similar in the tandem and control groups, with nausea being the most common side effect. Both groups exhibited similar rates of febrile neutropenia. There was no difference in the toxicity between the first and second tandem transplantations.
Six patients in the control group died within 100 days of transplantation when compared with 11 patients in the tandem group. Four patients in the control group died from infectious causes, and two died due to disease progression. In the tandem group, 10 patients died following disease progression, and one died due to an infection.
Discussion
This multicentre study investigated the clinical role of tandem transplantation in patients with MM, with emphasis on the potential role of tandem transplantation in patients with high-risk features defined as ISS III, elevated LDH levels, high-risk FISH, and/or extramedullary disease12. Herein, we found that tandem transplantation significantly improved the response status and almost doubled the CR rate when compared with the response status after the first transplantation. Although tandem transplantation was equivalent to single transplantation in the entire population, tandem transplantation was associated with significantly improved PFS in patients with ISS III and high-risk FISH when compared with those receiving maintenance therapy. Additionally, we identified the clinical benefits of tandem transplantation in patients who did not achieve a CR after the first transplantation. To overcome the limitations of the retrospective nature of this study, we performed propensity score matching, which revealed that tandem transplantation was significantly associated with longer PFS than single transplantation.
However, identifying and treating patients with high-risk MM can be challenging12. First, a high risk of MM could be stratified by ISS, with approximately one-third of the patients in previous studies and our cohort classified as stage III15. Extramedullary disease has also been identified as a prognostic factor17. Prognostic value can also be determined by genomic characterisation, such as metaphase karyotyping and FISH. Various cytogenetic abnormalities have been investigated; patients with high-risk cytogenetic abnormalities account for approximately one-quarter of all MM cases and have relatively poor survival outcomes18. Despite the promising results with novel agents in triplet or quadruplet induction regimens, high-risk patients continue to experience poor outcomes. For instance, the SWOG S0777 study compared triplet VRd with lenalidomide and dexamethasone (Rd)19. Despite demonstrating the efficacy of VRd in the overall population, high-risk patients (as defined in our study) showed no significant clinical benefits. Moreover, patients who received intensified induction regimens were found to exhibit significantly improved overall outcomes20–23, although none of these strategies could overcome the adverse prognostic impact of high-risk cytogenetics. However, tandem transplantation was found to be efficacious in improving poor prognoses in high-risk patients. Gagelmann et al. studied 488 patients with extramedullary disease and found that patients with varying cytogenetic risk factors who underwent single transplantation had significantly different survival rates, whereas patients with high-risk FISH who underwent tandem transplantation showed clinical outcomes similar to those in the single transplantation group13. According to the policies of each institution, 210 patients in the ENM02/HO95 study underwent tandem transplantation, whereas 492 underwent a single ASCT11. Patients who underwent tandem transplantation had significantly longer PFS and OS than those who underwent single transplantation. Notably, patients with high-risk cytogenetics who underwent tandem transplantation had a higher chance of survival than those who did not undergo tandem transplantation. In the STaMINA study, tandem transplantation followed by lenalidomide maintenance was associated with a significantly longer PFS than single transplantation followed by consolidation with a maintenance strategy or single transplantation followed by maintenance therapy in the high-risk group10. In our study, tandem transplantation was significantly associated with improved PFS in patients with ISS III and high-risk FISH in the subgroup analysis. In a recent phase 2 study conducted by a French group, which included patients with high-risk cytogenetics defined similarly to our own research, the efficacy of quadruple induction therapy, consolidation therapy, and daratumumab/lenalidomide maintenance therapy combined with tandem transplantation was assessed. The study reported a CR rate of 81%, with 30-month PFS and OS rates of 80% and 91%, respectively24. In our study, among the 24 patients with high-risk cytogenetics who underwent tandem transplantation, the CR rate was 70%, and the 30-month PFS and OS were 40% and 60%, respectively. We believe these differences can be attributed to variations in the induction regimen and maintenance therapy. Among these patients, seven who received maintenance therapy following tandem transplantation exhibited 30-month PFS and OS rates of 69% and 83%, respectively. This suggests that combining tandem transplantation with maintenance therapy may maximise the clinical benefit for patients with high-risk cytogenetics. Therefore, our results are consistent with those of previous studies and support the use of tandem transplantation in selected high-risk populations.
Tandem transplantation is recommended in patients who experience partial improvements after the first ASCT25. The IFM92 study randomised 399 patients to receive single or tandem transplantation after induction therapy5. The researchers found that patients who did not achieve VGPR after the first ASCT benefited from tandem transplantation. Single and tandem transplantation outcomes did not differ between patients who achieved VGPR or higher. It should be noted that the IFM92 study was conducted before the introduction of novel agents, and most of our patients received novel agent-based induction therapy. In contrast to IFM92, tandem transplantation improved PFS in patients with VGPR after their first ASCT. Our results highlighted the benefits of developing a new response adaptation strategy using these agents. Consolidation and maintenance therapies according to the response status after induction therapy are examples of potential novel approaches26,27. With newly developed agents, such as bispecific antibodies and chimeric antigen receptor T-cell therapy, the role of tandem transplantation should be further investigated in future trials to develop highly accurate risk- and response-adapted strategies28.
Although our study focused on tandem transplantation, maintenance therapy should also be emphasised. Maintenance therapy significantly improved the OS and PFS in all patients after adjusting for tandem transplantation. In the subgroup analysis, maintenance therapy showed a significant survival benefit in patients who did not achieve CR after the first ASCT. Although maintenance therapy did not demonstrate any clinical benefit in patients with high-risk FISH, it elicited favourable OS results in a subgroup of patients with standard-risk FISH. Patients who underwent tandem transplantation followed by maintenance therapy had the longest median PFS, although the sample size was small. Therefore, appropriate post-ASCT strategies to achieve the best outcome should be assessed based on available drugs, targeted patients, schedule and duration, long-term toxicity, and cost-to-benefit ratio29. Compared with maintenance therapy, tandem transplantation has a relatively shorter duration and improved cost-effectiveness. Although 11 patients died within 100 days of tandem transplantation in our study, only one died because of infection, while the remaining deaths occurred due to disease progression. The relatively high mortality rate reflects the refractory nature of high-risk diseases rather than tandem transplantation-related effects. The toxicity profiles of single and tandem transplantations were comparable. Therefore, identifying the most appropriate candidates for tandem transplantation and maintenance therapy after ASCT is necessary. The frailty score, application of an adjusted dose of the conditioning regimen, and patient preference may be important, along with risk stratification and response status30.
Our study had several limitations. First, the patients were heterogeneous in age at diagnosis, treatment period, and induction regimen, owing to the retrospective nature of the study. When divided based on the approval of the VTD regimen as a first-line therapy in Korea in 2013, our analysis showed that even among patients diagnosed after 2013, tandem transplantation was favoured for PFS as well. However, this result did not reach statistical significance, likely due to the limitation in sample size. Further ongoing research is warranted to explore the clinical significance of tandem transplantation following treatment with more recently introduced induction regimens. We performed propensity score matching to overcome the possible bias introduced by these factors, given that these factors may affect the treatment offered by physicians. We reported a significant clinical benefit of tandem transplantation, prolonging the median PFS from 13.5 to 30.3 months when compared with single transplantation. Second, we evaluated the response-adapted approach according to response status after the first ASCT. Our results suggest that patients with VGPR may benefit from tandem transplantation after the first ASCT scan. However, we were unable to include the minimal residual disease (MRD) status, which has recently become a key endpoint in determining therapeutic responses. In this study, we included and analysed patients diagnosed up until 2020 to ensure an adequate follow-up period. However, as MRD testing only became covered by the national insurance in Korea after 2022, we were unable to perform MRD analysis on the patients included in this study. Future studies assessing the MRD status may further refine the optimal criteria for tandem transplantation.
In conclusion, tandem transplantation was beneficial in high-risk patients with MM and improved the clinical outcomes in those who did not achieve CR, particularly in those who presented with VGPR after the first ASCT. Further studies are needed to evaluate the clinical role of tandem transplantation in patients with MM.
Supplementary Information
Acknowledgements
The abstract was presented orally at the 2022 Korean Society of Hematology International Conference and the 63rd Annual Meeting.
Author contributions
JJ and SJ collected the data and wrote the article, JP statistically analysed the data, JL, YRD, KK, JLL, SY, CM, HJK, and JHL collected the data, KK and HE conceived the idea and critically appraised the paper. The authors provide consent for the publication of the manuscript
Data availability
Data are available upon request from the corresponding author
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board (IRB) (National Cancer Center 2021-0304) and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Informed consent was waived by the IRB owing because of the retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jongheon Jung and Sung-Hoon Jung.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Kihyun Kim, Email: kihyunkimk@gmail.com.
Hyeon-Seok Eom, Email: hseom@ncc.re.kr.
The Korean Multiple Myeloma Working Party (KMMWP):
Jongheon Jung, Sung-Hoon Jung, Je-Jung Lee, Young Rok Do, Ka-Won Kang, Jung Lim Lee, Sung-Soo Yoon, Chang-Ki Min, Hye Jin Kang, Ji Hyun Lee, Kihyun Kim, and Hyeon-Seok Eom
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74625-9.
References
- 1.Rodriguez-Otero, P., Paiva, B. & San-Miguel, J. F. Roadmap to cure multiple myeloma. Cancer Treat Rev 100, 102284. 10.1016/j.ctrv.2021.102284 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Cunningham, D. et al. High-dose melphalan for multiple myeloma: long-term follow-up data. J. Clin. Oncol. 12, 764–768. 10.1200/JCO.1994.12.4.764 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Jung, S. H. et al. Frontline therapy for newly diagnosed patients with multiple myeloma. Blood Res 55, S37–S42. 10.5045/br.2020.S007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung, J. et al. Autologous stem cell transplantation in elderly patients with multiple myeloma in Korea: the KMM1807 study. Int. J. Hematol. 112, 84–95. 10.1007/s12185-020-02869-y (2020). [DOI] [PubMed] [Google Scholar]
- 5.Attal, M. et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 349, 2495–2502. 10.1056/NEJMoa032290 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Mai, E. K. et al. Single versus tandem high-dose melphalan followed by autologous blood stem cell transplantation in multiple myeloma: Long-term results from the phase III GMMG-HD2 trial. Br. J. Haematol. 173, 731–741. 10.1111/bjh.13994 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Cavo, M. et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J. Clin. Oncol. 25, 2434–2441. 10.1200/JCO.2006.10.2509 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Sonneveld, P. et al. Intermediate-dose melphalan compared with myeloablative treatment in multiple myeloma: Long-term follow-up of the Dutch Cooperative Group HOVON 24 trial. Haematologica 92, 928–935. 10.3324/haematol.11168 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Kumar, A., Kharfan-Dabaja, M. A., Glasmacher, A. & Djulbegovic, B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: A systematic review and meta-analysis. J. Natl. Cancer Inst. 101, 100–106. 10.1093/jnci/djn439 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Stadtmauer, E. A. et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J. Clin. Oncol. 37, 589–597. 10.1200/JCO.18.00685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavo, M. et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 7, e456–e468. 10.1016/S2352-3026(20)30099-5 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Caro, J. et al. How to treat high-risk myeloma at diagnosis and relapse. Am. Soc. Clin. Oncol. Educ. Book 41, 291–309. 10.1200/EDBK_320105 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Gagelmann, N. et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: A study from the chronic malignancies working party of the European Society for blood and marrow transplantation. Biol. Blood Marrow Transplant. 25, 2134–2142. 10.1016/j.bbmt.2019.07.004 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet. Oncol. 17, e328–e346. 10.1016/S1470-2045(16)30206-6 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Greipp, P. R. et al. International staging system for multiple myeloma. J. Clin. Oncol. 23, 3412–3420. 10.1200/JCO.2005.04.242 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Kumar, S. K. et al. Multiple myeloma, version 3.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 15, 230–269 10.6004/jnccn.2017.0023 (2017). [DOI] [PubMed]
- 17.Bhutani, M., Foureau, D. M., Atrash, S., Voorhees, P. M. & Usmani, S. Z. Extramedullary multiple myeloma. Leukemia 34, 1–20. 10.1038/s41375-019-0660-0 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Sonneveld, P. et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 127, 2955–2962. 10.1182/blood-2016-01-631200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durie, B. G. M. et al. Longer term follow-up of the randomized phase III trial SWOG S0777: Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 10, 53 10.1038/s41408-020-0311-8 (2020). [DOI] [PMC free article] [PubMed]
- 20.Moreau, P. et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394, 29–38. 10.1016/S0140-6736(19)31240-1 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Mateos, M. V. et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for untreated myeloma. N. England journal of medicine 378, 518–528. 10.1056/NEJMoa1714678 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Facon, T. et al. Daratumumab plus Lenalidomide and Dexamethasone for untreated myeloma. N. Engl. J. Med. 380, 2104–2115. 10.1056/NEJMoa1817249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voorhees, P. M. et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 136, 936–945. 10.1182/blood.2020005288 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touzeau, C. et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with tandem transplant for high-risk newly diagnosed myeloma. Blood 143, 2029–2036. 10.1182/blood.2023023597 (2024). [DOI] [PubMed] [Google Scholar]
- 25.Cavo, M. et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 117, 6063–6073. 10.1182/blood-2011-02-297325 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, G. H. et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 20, 57–73. 10.1016/S1470-2045(18)30687-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldschmidt, H. et al. Response-adapted lenalidomide maintenance in newly diagnosed myeloma: Results from the phase III GMMG-MM5 trial. Leukemia 34, 1853–1865. 10.1038/s41375-020-0724-1 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Cazaubiel, T. et al. Risk and response-adapted treatment in multiple myeloma. Cancers 12 10.3390/cancers12123497 (2020). [DOI] [PMC free article] [PubMed]
- 29.More, S. et al. Developments in consolidation and maintenance strategies in post-remission multiple myeloma. Expert Rev. Hematol. 13, 351–362. 10.1080/17474086.2020.1739517 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Nampoothiri, R. V. et al. Impact of frailty, melphalan pharmacokinetics, and pharmacogenetics on outcomes post autologous hematopoietic cell transplantation for multiple myeloma. Bone Marrow Transplantation 54, 2088–2095. 10.1038/s41409-019-0631-0 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding author