Abstract
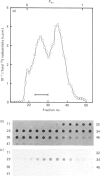
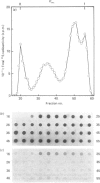
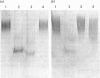
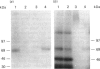
Human lung fibroblasts produce heparan sulphate proteoglycans (HSPG) that are associated with the plasma membrane. A monoclonal-antibody (Mab)-secreting hybridoma, S1, was produced by fusion of SP 2/0-AG 14 mouse myeloma cells with spleen cells from mice immunized with partially purified cellular HSPG fractions. The HSPG character of the material carrying the epitope recognized by Mab S1 was demonstrated by: (i) the co-purification of the S1 epitope with the membrane HSPG of human lung fibroblasts; (ii) the decrease in size of the material carrying the S1 epitope upon treatment with heparinase or heparitinase, and the resistance of this material to heparinase treatment after N-desulphation. The S1 epitope appears to be part of the core protein, since it was destroyed by proteinase treatment and by disulphide-bond reduction, but not by treatments that depolymerize the glycosaminoglycan chains and N-linked oligosaccharide chains. Polyacrylamide-gel electrophoresis of non-reduced heparitinase-digested membrane HSPG followed by Western blotting and immunostaining with Mab S1 revealed a single band with apparent molecular mass of 64 kDa. Membrane proteoglycans isolated from detergent extracts or from 4 M-guanidinium chloride extracts of the cells yielded similar results. Additional digestion with N-glycanase lowered the apparent molecular mass of the immunoreactive material to 56 kDa, suggesting that the core protein also carries N-linked oligosaccharides. Fractionation of 125I-labelled membrane HSPG by immuno-affinity chromatography on immobilized Mab S1, followed by heparitinase digestion and polyacrylamide-gel electrophoresis of the bound material, yielded a single labelled band with apparent molecular mass 64 kDa. Treatment with dithiothreitol caused a slight increase in apparent molecular mass, suggesting that the core protein of this membrane proteoglycan of a single subunit containing (an) intrachain disulphide bond(s).
Full text
PDF

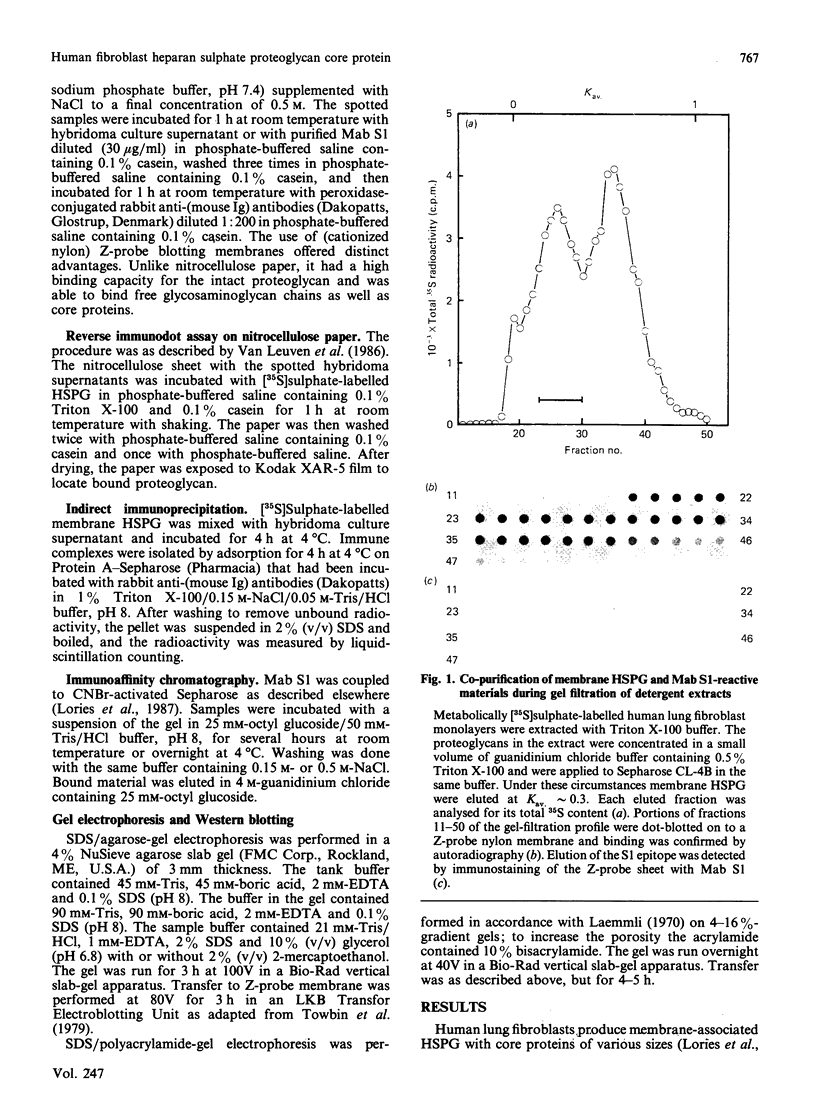
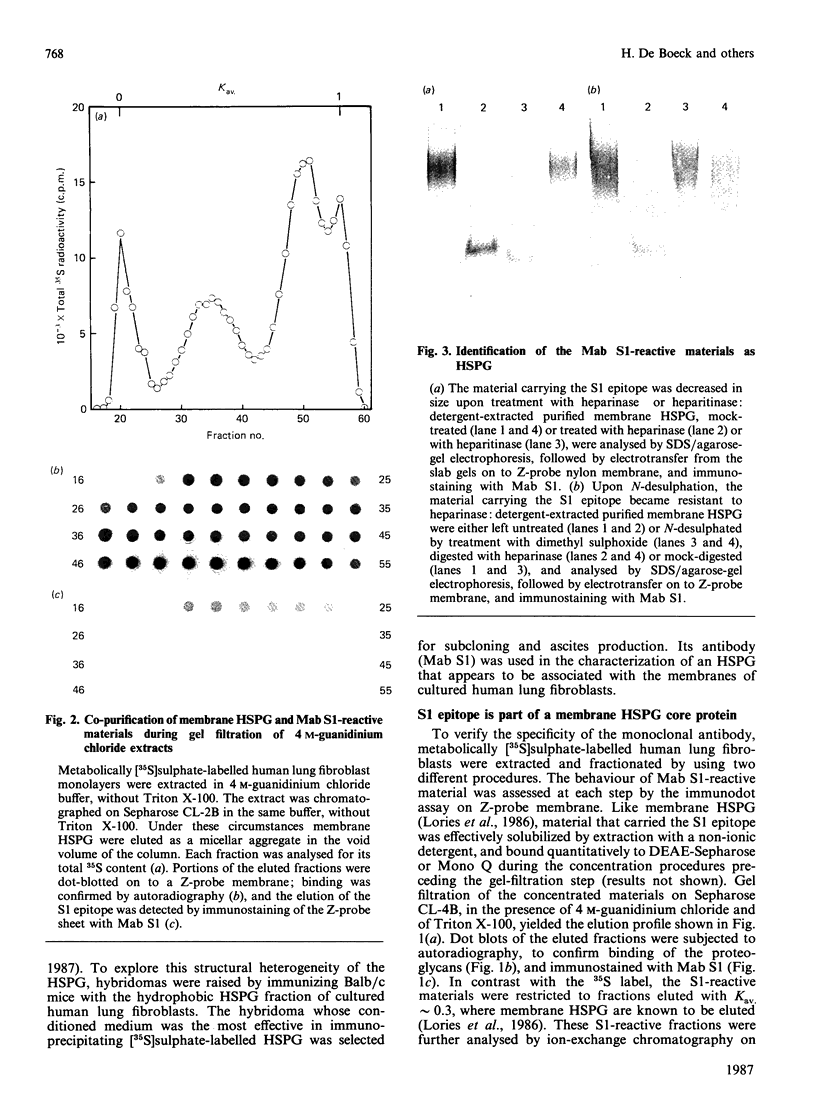
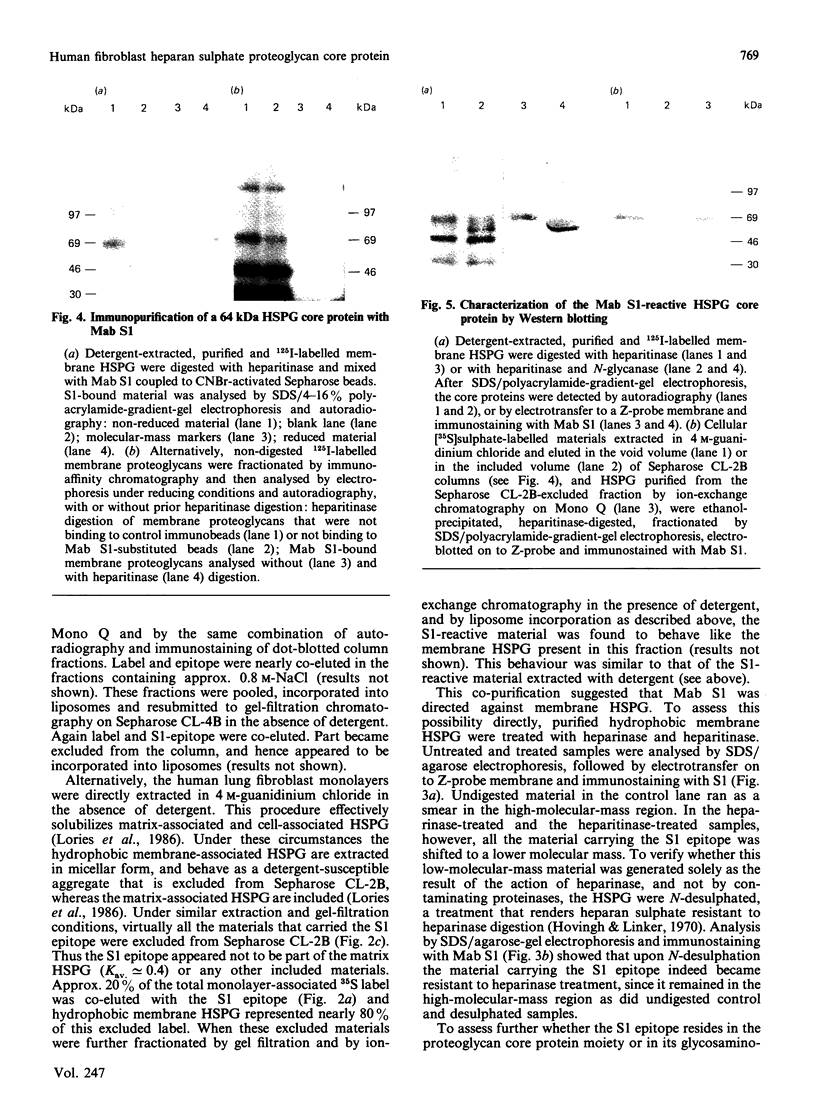


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng C. F., Oosta G. M., Bensadoun A., Rosenberg R. D. Binding of lipoprotein lipase to endothelial cells in culture. J Biol Chem. 1981 Dec 25;256(24):12893–12898. [PubMed] [Google Scholar]
- Cole G. J., Schubert D., Glaser L. Cell-substratum adhesion in chick neural retina depends upon protein-heparan sulfate interactions. J Cell Biol. 1985 Apr;100(4):1192–1199. doi: 10.1083/jcb.100.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Carlstedt I., Kendall S., Malmström A., Schmidtchen A., Fransson L. A. Structure of proteoheparan sulfates from fibroblasts. Confluent and proliferating fibroblasts produce at least three types of proteoheparan sulfates with functionally different core proteins. J Biol Chem. 1986 Sep 15;261(26):12079–12088. [PubMed] [Google Scholar]
- Cöster L., Malström A., Carlstedt I., Fransson L. A. The core protein of fibroblast proteoheparan sulphate consists of disulphide-bonded subunits. Biochem J. 1983 Nov 1;215(2):417–419. doi: 10.1042/bj2150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Van Den Berghe H. Transformed mouse mammary epithelial cells synthesize undersulfated basement membrane proteoglycan. J Biol Chem. 1983 Jun 25;258(12):7338–7344. [PubMed] [Google Scholar]
- David G., Van den Berghe H. Heparan sulfate-chondroitin sulfate hybrid proteoglycan of the cell surface and basement membrane of mouse mammary epithelial cells. J Biol Chem. 1985 Sep 15;260(20):11067–11074. [PubMed] [Google Scholar]
- Farrell D. H., Cunningham D. D. Human fibroblasts accelerate the inhibition of thrombin by protease nexin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6858–6862. doi: 10.1073/pnas.83.18.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I., Cöster L., Malmström A. Binding of transferrin to the core protein of fibroblast proteoheparan sulfate. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5657–5661. doi: 10.1073/pnas.81.18.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. J., Silbert C. K., Silbert J. E. Effects of heparan sulfate removal on attachment and reattachment of fibroblasts and endothelial cells. Biochemistry. 1986 Jan 28;25(2):405–410. doi: 10.1021/bi00350a020. [DOI] [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Kawakami H., Terayama H. Liver plasma membranes and proteoglycan prepared therefrom inhibit the growth of hepatoma cells in vitro. Biochim Biophys Acta. 1981 Aug 6;646(1):161–168. doi: 10.1016/0005-2736(81)90283-2. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laterra J., Silbert J. E., Culp L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983 Jan;96(1):112–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories V., David G., Cassiman J. J., Van den Berghe H. Heparan sulfate proteoglycans of human lung fibroblasts. Occurrence of distinct membrane, matrix and secreted forms. Eur J Biochem. 1986 Jul 15;158(2):351–359. doi: 10.1111/j.1432-1033.1986.tb09758.x. [DOI] [PubMed] [Google Scholar]
- Lories V., De Boeck H., David G., Cassiman J. J., Van den Berghe H. Heparan sulfate proteoglycans of human lung fibroblasts. Structural heterogeneity of the core proteins of the hydrophobic cell-associated forms. J Biol Chem. 1987 Jan 15;262(2):854–859. [PubMed] [Google Scholar]
- Marcum J. A., Conway E. M., Youssoufian H., Rosenberg R. D. Anticoagulantly active heparin-like molecules from cultured fibroblasts. Exp Cell Res. 1986 Sep;166(1):253–258. doi: 10.1016/0014-4827(86)90525-2. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Marynen P., Van Leuven F., Cassiman J. J., Van den Berghe H. A monoclonal antibody to a neo-antigen on alpha 2-macroglobulin complexes inhibits receptor-mediated endocytosis. J Immunol. 1981 Nov;127(5):1782–1786. [PubMed] [Google Scholar]
- Norling B., Glimelius B., Wasteson A. Heparan sulfate proteoglycan of cultured cells: demonstration of a lipid- and a matrix-associated form. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1265–1272. doi: 10.1016/0006-291x(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983 Mar 25;258(6):3632–3636. [PubMed] [Google Scholar]
- Ratner N., Bunge R. P., Glaser L. A neuronal cell surface heparan sulfate proteoglycan is required for dorsal root ganglion neuron stimulation of Schwann cell proliferation. J Cell Biol. 1985 Sep;101(3):744–754. doi: 10.1083/jcb.101.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M. Isolation of a cell-surface receptor for chick neural retina adherons. J Cell Biol. 1985 Jan;100(1):56–63. doi: 10.1083/jcb.100.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Gill P. J., Silbert J. E., Douglas W. H., Fanburg B. L. Involvement of cell surface heparin sulfate in the binding of lipoprotein lipase to cultured bovine endothelial cells. J Clin Invest. 1981 Oct;68(4):995–1002. doi: 10.1172/JCI110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuven F., Marynen P., Cassiman J. J., Van den Berghe H. Reversed dot-blotting in hybridoma screening and epitope mapping. A model study with human alpha 2-macroglobulin to select complex-specific monoclonal antibodies. J Immunol Methods. 1986 Jun 10;90(1):125–130. doi: 10.1016/0022-1759(86)90392-3. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Hök M. Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J Biol Chem. 1985 Sep 5;260(19):10872–10879. [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Johansson S., Hök M. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 1986 Apr;5(4):665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]