Abstract
Human immunodeficiency virus type 1 (HIV-1) infects and induces syncytium formation in microglial cells from the central nervous system (CNS). A primary isolate (HIV-1BORI) was sequentially passaged in cultured microglia, and the isolate recovered (HIV-1BORI-15) showed high levels of fusion and replicated more efficiently in microglia (J. M. Strizki, A. V. Albright, H. Sheng, M. O'Connor, L. Perrin, and F. González-Scarano, J. Virol. 70:7654–7662, 1996). The parent and adapted viruses used CCR5 as coreceptor. Recombinant viruses demonstrated that the syncytium-inducing phenotype was associated with four amino acid differences in the V1/V2 region of the viral gp120 (J. T. C. Shieh, J. Martin, G. Baltuch, M. H. Malim, and F. González-Scarano, J. Virol. 74:693–701, 2000). We produced luciferase-reporter, env-pseudotyped viruses using plasmids containing env sequences from HIV-1BORI, HIV-1BORI-15, and the V1/V2 region of HIV-1BORI-15 in the context of HIV-1BORI env (named rBORI, rB15, and rV1V2, respectively). The pseudotypes were used to infect cells expressing various amounts of CD4 and CCR5 on the surface. In contrast to the parent recombinant, the rB15 and rV1V2 pseudotypes retained their infectability in cells expressing low levels of CD4 independent of the levels of CCR5, and they infected cells expressing CD4 with a chimeric coreceptor containing the third extracellular loop of CCR2b in the context of CCR5 or a CCR5 Δ4 amino-terminal deletion mutant. The VH-rB15 and VH-rV1V2 recombinant viruses were more sensitive to neutralization by a panel of HIV-positive sera than was VH-rBORI. Interestingly, the CD4-induced 17b epitope on gp120 was more accessible in the rB15 and rV1V2 pseudotypes than in rBORI, even before CD4 binding, and concomitantly, the rB15 and rV1V2 pseudotypes were more sensitive to neutralization with the human 17b monoclonal antibody. Adaptation to growth in microglia—cells that have reduced expression of CD4 in comparison with other cell types—appears to be associated with changes in gp120 that modify its ability to utilize CD4 and CCR5. Changes in the availability of the 17b epitope indicate that these affect conformation. These results imply that the process of adaptation to certain tissue types such as the CNS directly affects the interaction of HIV-1 envelope glycoproteins with cell surface components and with humoral immune responses.
Human immunodeficiency virus type 1 (HIV-1) penetrates the central nervous system (CNS) during primary infection, and a subset of HIV-1-infected individuals develops a neurological syndrome known as HIV-dementia (HIVD) or AIDS-dementia complex (16, 42, 62, 65, 82, 105). The principal neuropathological finding related to HIVD is the formation of multinucleated giant cells or syncytia, which are the end product of the fusion between infected and uninfected cells (7, 91, 106). Since within the CNS HIV-1 infects mainly microglia or brain macrophages (7, 48, 91, 106), syncytia formation is thought to be the result of fusion of microglia mediated by HIV-1 glycoproteins. Furthermore, microglia can be infected in vitro with certain HIV-1 strains (41, 43, 46, 57, 92) and, depending on the isolate, this infection induces syncytia (95, 103).
HIV-1 infection of the CNS itself is primarily due to R5- or macrophage-tropic HIV-1 isolates (9, 15, 19, 22, 27, 60, 79), which use CD4 (26, 47, 64) and the seven-transmembrane-domain, G-protein-coupled chemokine receptor molecule CCR5 as coreceptors (4, 23, 28, 30, 32, 101, 109). Binding to CD4 induces conformational changes in gp120 that are postulated to promote subsequent steps in the fusion process, such as coreceptor binding (89, 90, 96, 97, 99, 101, 109, 114). The gp120 glycoprotein itself is heavily glycosylated (58, 59, 61) and contains variable loops that are exposed in the native state as well as more conserved regions folded into a core structure (52, 70, 85, 113, 115). Among the variable loops, V1 and V2, but also V3, are thought to change conformation following CD4 binding (88–90, 97, 114), resulting in the exposure of conserved, discontinuous structures recognized by the 17b and 48d monoclonal antibodies (MAbs) (99, 114). The close relationship between the 17b and 48d epitopes and the gp120 structures important for CCR5 binding (85) supports a model in which a conformational change in the V1/V2 region induced by CD4 binding allows the exposure of high-affinity binding sites for CCR5 (49, 50). Although microglial cells express low levels of CD4 (29), they also express both CXCR4 and CCR5, as well as other potential HIV-1 coreceptors like CCR3 (1, 40, 43, 55). Among these, CCR5 is the most important coreceptor for adult microglial cells (1, 92).
Analysis of HIV-1 sequences derived from the CNS as well as other organs has demonstrated the existence of some degree of tissue compartmentalization (37, 51, 80, 107). In addition, some investigators have proposed that certain HIV-1 sequences—and presumably isolates—might be associated with the development of HIVD in HIV-1-infected individuals (80, 81). In order to investigate whether adaptation to replication in CNS cells, and specifically microglia, could be reproduced in vitro, a primary, nonsyncytium-inducing blood-derived isolate, HIV-1BORI (25), was passaged sequentially in cultured microglia (95). The isolate recovered after 15 passages, HIV-1BORI-15, replicates to a higher titer than the parental virus in microglia and monocyte-derived macrophages in comparison with peripheral blood mononuclear cells and also induces prominent syncytia, particularly in microglia (95). Since the envelope glycoproteins are responsible for binding to receptors and fusion of viral envelope and cell membrane, it can be hypothesized that the envelope of the microglia-adapted virus is involved in the acquisition of this particular phenotype. In this regard, while Shieh et al. (93) previously reported that both parental and microglia-adapted isolates use CD4 and CCR5 for infection and fusion, he and collaborators also demonstrated, using chimeric viruses in which env genes were introduced into a common virus background, that the HIV-1BORI-15 envelope glycoproteins are responsible for the syncytium-inducing phenotype (93). Sequence analysis indicated that the HIV-1BORI and HIV-1BORI-15 envelopes differ by eight amino acids; four of them are located in the V1/V2 region of gp120 and two of these eliminate potential N-linked glycosylation sites. Analysis of syncytium formation in microglia infected by viruses containing the chimeric envelopes indicated that the effects of amino acid alterations are context dependent, although those in the V1/V2 region were primarily responsible for the syncytium-forming phenotype.
Since the acquisition of this phenotype was not the result of a switch in the specific coreceptor use, we have studied possible differences in the interaction with CD4 and CCR5 by the envelope glycoproteins of these HIV-1 isolates. Using env-pseudotyped viruses expressing a luciferase reporter gene construct, we determined that the HIV-1BORI-15 envelope (and, in particular, the V1/V2 region of gp120) mediated infection of cells expressing low levels of CD4 and/or CCR5 on the membrane with greater efficiency than those of HIV-1BORI. In addition, we noted differences in their ability to use CCR5 chimeric constructions and mutants. The BORI-15 and V1V2 recombinant viruses were more easily neutralized by a panel of sera from HIV-1-infected patients than the parental BORI virus. Finally, the 17b epitope was more accessible on the gp120 from HIV-1BORI-15, and concurrently, these pseudotypes were more sensitive to neutralization by this MAb.
Microglia or CNS macrophages have low levels of CD4 in comparison with other cells, such as peripheral blood mononuclear cells and adaptation to growth in these cells appears to be to be associated with changes in gp120 that modify its ability to utilize CD4 and CCR5, perhaps by affecting the induction of conformational changes in V1/V2. These results imply that the process of adaptation to certain tissue types such as the CNS directly affects the interaction with cell surface components and renders tissue-defined compartmentalization theoretically possible from a phenotypic as well as a genetic standpoint.
MATERIALS AND METHODS
Cells.
Microglial cultures were prepared as previously described from fresh adult human brain tissue obtained during temporal lobectomy for medication-resistant epilepsy (1, 2, 95, 116). Microglia were cultured in Dulbecco's modified Eagle's medium (DMEM) (GibcoBRL, Gaithersburg, Md.) supplemented with 5% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, Ga.), 5% giant cell tumor conditioned medium (Igen, Gaithersburg, Md.), gentamicin (50 μg/ml; GibcoBRL), and 1 mM sodium pyruvate. 293T and U87 cells were cultured in DMEM supplemented with 10% FBS. U87 cells stably transfected for the expression of human CD4 and CCR5 molecules (U87-CD4-CCR5) were cultured in DMEM supplemented with 10% FBS, puromycin (1 μg/ml; Sigma, St. Louis, Mo.) and 300 μg of G418 (Geneticin; GibcoBRL) per ml. Human osteosarcoma (HOS)-pBABEpuro and HOS-CCR5 (stably transfected for the expression of CCR5) cells were cultured in DMEM supplemented with 10% FBS and puromycin (1 μg/ml); HOS-CD4-pBABEpuro (stably transfected for the expression of CD4) and HOS-CD4-CCR5 (stably transfected for the expression of both CD4 and CCR5) were cultured in DMEM supplemented with 10% FBS, mycophenolic acid (40 μg/ml), xanthine (250 μg/ml), hypoxanthine (13.5 μg/ml), 10 mM HEPES, and puromycin (1 μg/ml). U87-CD4-CCR5 (11) and all HOS cell lines (28, 54) were obtained through the AIDS Research and Reference Reagent Program, National Institutes of Health, from H. K. Deng and D. Littman and from N. R. Landau, respectively. MAGI-CCR5 cells—a generous gift from Julie Overbaugh (20)—were cultured in DMEM containing 10% FBS, G418 (200 μg/ml), hygromycin (100 μg/ml), and puromycin (1 μg/ml).
Transfections.
To obtain cells with differing levels of CD4 and CCR5, 3 × 105 U87 cells were placed in each well of six-well plates (Costar, Corning, N.Y.), incubated at 37°C for 16 to 18 h, and cotransfected using the calcium phosphate method (Eppendorf-5 Prime, Boulder, Co.) with 2, 0.2, or 0.02 μg of CD4 expression plasmid and 3, 0.3, or 0.03 μg of CCR5 expression plasmid, adding pcDNA3.1 (Invitrogen, San Diego, Calif.) when necessary for a total amount of 5 μg of DNA per transfection. After 6 h of incubation at 37°C, the DNA-containing medium was removed, and the cells were washed and harvested with 0.5 mM EDTA, suspended by adding fresh medium, plated in 96-well plates at a density of 2 × 104 cells/well, and incubated for 16 to 18 h before further experimentation.
For other experiments, U87 cells were also cotransfected with 2 μg of CD4 expression plasmid and 3 μg of plasmids encoding CCR5, CCR2b, or CCR5/CCR2b chimeric coreceptors or amino-terminal deletion mutants of CCR5 (87) (all of the chimeric constructions were gifts from J. Rucker and R. W. Doms, University of Pennsylvania). The CCR5/CCR2b chimeras used here are those containing a single extracellular domain of CCR5 in the context of CCR2b (named 5222, 2522, 2252, and 2225, where each number designates in order the amino-terminal domain and the extracellular loops [ECL] 1, 2, and 3 of the coreceptor molecule); a single extracellular domain of CCR2b in the context of CCR5 (named 2555, 5255, 5525, and 5552); and the amino-terminal domain and ECL2 of CCR5 together with the ECL1 and ECL3 of CCR2b (named 5252). The CCR5 amino-terminal deletion mutants are designated Δ4, Δ8, Δ12, and Δ16 and contain 4, 8, 12, and 16 amino acid deletions, respectively, in the amino-terminal domain of the CCR5 molecule. After transfection, the cells were prepared for infection as described above.
Cell surface expression of CD4, CCR5, CCR2b, CCR5/CCR2b chimeras, and CCR5 amino-terminal deletion mutants in transiently transfected cells was determined by flow cytometric analysis, as previously described (1). Cells were stained with 5 μg/ml of anti-CD4 antibody (Ab) 21 (a gift from J. A. Hoxie, University of Pennsylvania) or anti-CCR5 Abs 45502.111 and 2D7 (28, 30, 32, 111) directed against the amino-terminal and ECL2 regions, respectively (both obtained through the AIDS Reagent Program), or anti-CCR2 Ab 48607.121 (MAB150; R&D Systems, Minneapolis, Minn.). Mouse immunoglobulin G (IgG) of irrelevant specificity was used as negative control. Fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Pierce, Rockford, Ill.) was used as secondary antibody and, after fixing with 2% paraformaldehyde in phosphate-buffered saline (PBS), the cells were analyzed on a Becton Dickinson FACScan (Cancer Center Core, University of Pennsylvania) with the CellQuest flow cytometry software.
HIV-1 isolates and env clones.
HIV-1BORI, which was obtained from an individual with primary HIV-1 infection, was a gift from G. M. Shaw (University of Alabama). HIV-1BORI-15 has been described (95). The env genes were cloned (93) and then introduced into a shuttle vector which was directly used for the production of pseudotypes as described below. The following constructions were used (Table 1): BORI and B15, containing the HIV-1BORI and HIV-1BORI-15 env sequences, respectively, with the exception of the first 39 and the last 131 amino acids of env, which originate from pIIIB (a derivative of HXB3) (45); rBORI and rB15, which contain the full-length HIV-1BORI and HIV-1BORI-15 env sequences, respectively; rV1V2, which contains the V1/V2 loops of HIV-1BORI-15 env in the context of the full-length HIV-1BORI env (both differ by only four amino acids in V1/V2); and rE153G and rE153G,T162A, which contain the indicated point mutations present in HIV-1BORI-15 env in the context of full-length HIV-1BORI env (93). The production of recombinant viruses with these envelopes has been previously described (93), and their fusion phenotypes in microglial cultures are shown on Table 1.
TABLE 1.
Amino acid differences and syncytium-inducing phenotype, in the context of whole or recombinant viruses, of env used for the production of pseudotypes
| env-pseudotyped virus | Amino acid differences with respect to BORI or rBORI
|
No. of potential N-linked glycosylation sites lost | Syncytium-inducing phenotype | |
|---|---|---|---|---|
| No. | Amino acid change(s) and positiona | |||
| BORI | 0 | 0 | Noninducing | |
| rBORI | 0 | 0 | Noninducing | |
| B15 | 8 | E153G, T162Ab, R166G, S190Rb, K229N, T281I, A336T, V532M | 2 | High |
| rB15 | 8 | E153G, T162Ab, R166G, S190Rb, K229N, T281I, A336T, V532M | 2 | High |
| rV1V2 | 4 | E153G, T162Ab, R166G, S190Rb | 2 | High |
| rE153G | 1 | E153G | 0 | High |
| rE153G,T162A | 2 | E153G, T162Ab | 1 | Noninducing |
Numbers are based on the HXB2 sequence. BORI and B15 contain HXB3 sequences at both the carboxy and amino termini, as discussed in Materials and Methods and by Shieh et al. (93).
Change results in the loss of a potential N-linked glycosylation site.
Production of pseudotypes.
One-round infectious env-pseudotyped luciferase-expressing reporter viruses were produced using the calcium phosphate transfection method. 293T cells were plated at 8 × 105 cells/well in six-well plates; incubated at 37°C for 16 to 18 h; and cotransfected with 2μg of the envelope-deficient HIV-1 NL-4-3 vector (pNL-4-3-LucR+E−; a gift of N. R. Landau, The Salk Institute for Biological Studies), which carries the luciferase reporter gene; and 3 μg of envelope-expressing vectors (93). After 6 h of incubation at 37°C, the DNA-containing medium was removed and fresh medium was added. Supernatants containing the env-pseudotyped viruses were collected 48 h later, clarified by centrifugation at 1,500 × g for 15 min, aliquoted, and stored at −80°C until use.
For several experiments, supernatants containing the env-pseudotyped viruses were clarified by centrifugation at 3,000 rpm for 15 min and concentrated by ultracentrifugation over 20% sucrose in a Sorvall Ultra 80, using an SW41Ti rotor (Beckman, Palo Alto, Calif.), at 100,000 × g (29,000 rpm) for 2 h at 4°C. The pellet was suspended in DMEM, aliquoted, and stored at −80°C until use. Pseudotype stocks and ultracentrifugation pellets were quantified by p24gag content (NEN, Brussels, Belgium).
Infection with env-pseudotyped luciferase viruses.
All cells were infected with pseudotype viruses by exposing them to inocula (200 μl/well) containing p24gag (5 ng/ml) prepared by dilution of the stocks with DMEM. After 6 h of incubation at 37°C, the virus-containing medium was removed and fresh medium was added. Forty-eight to 62 h after infection the cells were washed with phosphate-buffered saline (PBS) and lysed with luciferase assay buffer (60 μl/well; Promega, Madison, Wis.). Luciferase activity was determined by adding 50 μl of freshly prepared luciferase assay substrate (Promega) to 50 μl of lysate and measuring the intensity of chemiluminescence in a LumiCount microplate luminometer (Packard, Meriden, Conn.). All experiments were performed at least in triplicate. Results are expressed as relative light units (RLU) per second.
In some experiments, the inocula were incubated prior to infection with soluble CD4 (sCD4) (AIDS Reagent Program and NEN Life Science Products, Inc.) (5 μg/ml) for 1 h at 37°C and then added to the cells.
Analysis of 17b epitope.
An enzyme-linked immunosorbent assay (ELISA) was used to test the exposure of the CD4-induced 17b epitope (52, 96, 97, 99, 101, 113–115) in either the gp120 present in supernatants containing the viral stocks (soluble and viral-particle associated), or in particle-associated gp120 obtained by ultracentrifugation. Briefly, 96-well plates (Costar) were coated with 100 μl of sheep antibody D7324 (which was raised to the conserved gp120 carboxy-terminal sequence APTKAKRRVVQREKR; International Enzymes, Fallbrook, Calif.) per well in carbonate buffer (pH 8.6) at 10 μg/ml. After overnight incubation at 4°C with continuous shaking, the plates were washed twice with wash buffer (0.05% Tween 20 in PBS), blocked with wash buffer (200 μl/well) plus 10% FBS for 1 h at 37°C, washed twice again, and incubated for 2 h at 37°C with pseudotype stocks (100 μl/well) containing 2 ng of p24gag diluted in wash buffer. After five washes, 100 μl of the human MAb 17b (obtained through the AIDS Reagent Program, as well as directly from J. E. Robinson, Tulane University) in wash buffer was added per well (0.1 μg/ml) either in the absence or in the presence of sCD4, and incubated for 1 h at 37°C. Plates were then washed five times and incubated with a 1:5,000 dilution of horseradish peroxidase conjugated, goat anti-human IgG (100 μl/well; Pierce) in wash buffer for 1 h at 37°C. Finally, after washing again five times, 150 μl of substrate (1-step ABTS; Pierce) per well was added and the optical density at 405 nm was measured after 30 min of incubation at room temperature.
Virus neutralization.
The sensitivity of recombinant viruses to neutralization by HIV-1-positive human sera was assessed using a MAGI-CCR5 cell-based, single-cycle infection assay and carefully calibrated virus stocks. Briefly, 48-well plates were seeded with 2.5 × 104 MAGI-CCR5 cells per well in 200 μl of medium without selective antibiotics but containing DEAE-dextran (20 μg/ml). Twenty to 24 h later, serial dilutions of virus were added to the cells in 200 μl of medium containing DEAE-dextran (20 μg/ml). Eighteen to 24 h after addition of virus, an HIV-1 inhibitory peptide (DP178) (104) was added at a concentration of 5 μg/ml to prevent cell-to-cell virus spread and syncytium formation. Three days after infection, the cells were fixed with paraformaldehyde and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and the number of blue nuclei (infected centers) were enumerated using an automated optical imager (Georgia Instruments, Atlanta, Ga.). The dilution of virus producing 600 to 1,200 infected centers was identified for use in the neutralization assays. Neutralization of virus by HIV-1-positive human serum was determined by preincubating virus at the predetermined dilution with serial dilutions of serum for 1 h at 37°C before inoculating MAGI-CCR5 cells. Culture conditions, fixing, staining, and counting of infected centers were performed as described above. The results were expressed as percentage of infectivity with respect to that observed when the virus was incubated with a 1/10 dilution of normal human serum (NHS).
To test neutralization with the 17b MAb, env-pseudotyped viruses equalized to contain 5 ng of p24gag per ml were incubated with the Ab (20 μg/ml) for 1 h at 37°C, and the mixture was then added to the cells. In addition, the anti-CD4 Ab 21, the anti-CCR5 MAb 2D7, and the recently described CCR5 inhibitor TAK-779 (6, 34) (kindly provided by J. Strizki, Schering-Plough) were used as controls. Cells were incubated with antibodies, TAK-779, or medium alone (100 μl/well) for 1 h at 4°C, prior to addition of virus inocula (100 μl/well) containing p24gag (10 ng/ml). After 6 h of incubation at 37°C, the medium was removed and fresh medium was added. Luciferase activity was then measured as described above.
Statistical analysis.
Data corresponding to the percentage of infection for each experiment and virus with respect to the positive control in each case were analyzed by the nonparametric Wilcoxon's rank-sum and signed-rank tests (for two independent samples and two paired samples, respectively). Data from the 17b ELISA were analyzed by Student's t test for independent samples. All tests were performed using the SPSS statistical package.
RESULTS
Infection of different cell types with env-pseudotyped luciferase viruses.
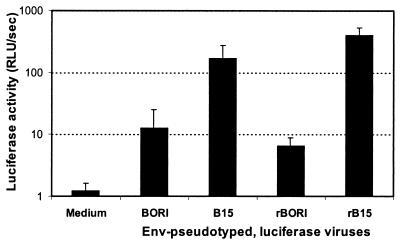
In comparison with the parental HIV-1BORI isolate, the virus derived by serial passage in human adult microglia cultures (HIV-1BORI-15) replicated to a greater extent in microglia (95). However, the recombinant viruses containing the HIV-1BORI and HIV-1BORI-15 envelopes on a common pIIIB virus backbone showed no such differences in replication, although they differed in their ability to induce syncytia (93). We tested the infectability of microglial cultures by BORI, B15, rBORI, and rB15 env-pseudotyped, luciferase viruses in order to isolate the entry process from other stages of retroviral replication. The results of a representative experiment are shown in Fig. 1. Both B15 and rB15 infected microglia up to 13 and 61 times more efficiently than did BORI and rBORI, respectively.
FIG. 1.
Microglial infection. Human adult microglial cultures were established as previously described (1, 2, 95, 116) and infected with 5 ng/ml p24gag of supernatants containing env-pseudotyped, luciferase viruses. After 48 to 62 h, cells were lysed and the extent of infection was measured by the intensity of chemiluminescence when mixing equal volumes of substrate and cell lysate. A representative experiment (performed in triplicate) is shown, and the results are expressed as the mean + standard deviation (error bars).
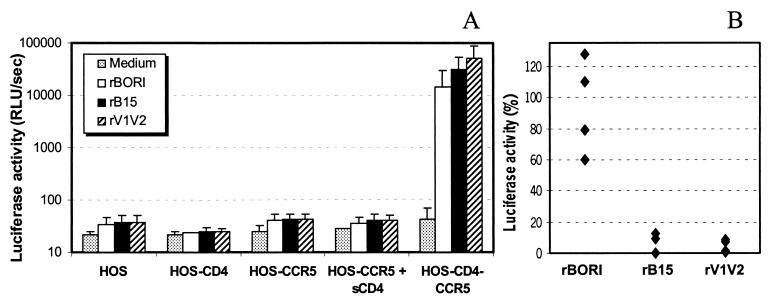
It has been recently reported that certain alterations in the glycosylation sites in the V1/V2 region are associated with CD4-independent infection of CCR5-expressing cells (50). Therefore, we investigated the infectability of a panel of HOS cells lines (HOS, HOS-CD4, HOS-CCR5, and HOS-CD4-CCR5) with the same pseudotypes, detecting infection only when both CD4 and CCR5 were present (Fig. 2A), consistent with previous cell-to-cell fusion experiments (93), and indicating that in our system the loss of glycosylation sites in V1/V2 is not associated with CD4 independence. Similar experiments were performed with U87 cells, confirming the CD4 dependence (data not shown). To then determine whether presenting CD4 in trans would allow these pseudotypes to infect cells expressing CCR5 only, we preincubated the inocula containing pseudotypes with sCD4 (5 μg/ml) (Fig. 2A). There was no infection of these cells. In contrast, the same concentration of sCD4 was able to inhibit the infection of HOS-CD4-CCR5 cells by rB15 and rV1V2 pseudotypes, while it had little if any effect on the infection by rBORI pseudotypes. In Fig. 2B, infection of HOS-CD4-CCR5 cells in the presence of sCD4 is expressed as a percentage of luciferase activity observed absent sCD4. Each set of conditions was replicated four times, and the values were compared using nonparametric statistics (Wilcoxon's rank-sum test). The differences in inhibition by sCD4 between rBORI and rB15 or rV1V2 were statistically significant (P < 0.05 in both comparisons).
FIG. 2.
Effect of sCD4 on infection of cells expressing CCR5 alone or with CD4. (A) HOS cells stably transfected for the expression of CD4 (HOS-CD4), CCR5 (HOS-CCR5), or both (HOS-CD4-CCR5) were infected with supernatants containing env-pseudotyped, luciferase viruses (p24gag [5 ng/ml]). The results are expressed as the mean + standard deviation (error bars) from four independent experiments each performed in triplicate. (B) Treatment with sCD4 (5 μg/ml) inhibited infection of HOS-CD4-CCR5 cells by rB15 and rV1V2 but not by rBORI pseudotypes. Results are shown as percent luciferase activity relative to the value in the absence of sCD4 treatment, in four independent experiments each performed in triplicate. The differences in values between rBORI and rB15 or rV1V2 were statistically significant (Wilcoxon's rank-sum test: P < 0.05 in both cases).
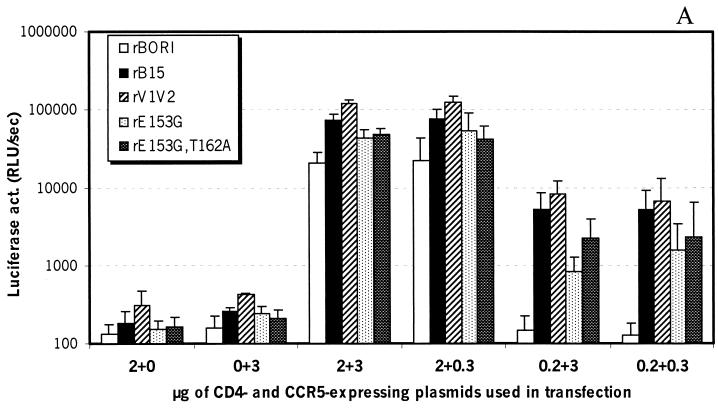
Effect of CD4-CCR5 levels on infection by pseudotypes.
Since microglia express low levels of CD4 as well as variable levels of CCR5 on the cell surface (1, 29, 40, 43, 55; A. V. Albright and F. González-Scarano, unpublished results), we performed experiments to determine whether the HIV-1BORI-15 envelope could utilize lower levels of each of these receptors. Experiments published previously demonstrated that rBORI and rB15 had varying abilities to infect 293T cells expressing low levels of CD4 (93). We extended these experiments using the full complement of recombinant viruses and decreasing both CD4 and CCR5 in the transfected cells (U87) by transfecting smaller quantities of the respective expression plasmids. As shown in Fig. 3A, infection of cells expressing high levels of CD4/CCR5 by rB15 and rV1V2 pseudotypes was 3.5 and 5.7 times higher, respectively, than infection by rBORI pseudotypes. All pseudotypes tested were able to infect cells expressing low or very low levels of CCR5 together with high levels of CD4 almost as efficiently as cells with high CD4/CCR5 levels. In contrast, infection by rBORI pseudotypes, but not by rB15 or rV1V2, was abolished by a decrease in the amount of CD4, independent of the levels of CCR5, on the target cell membrane (Fig. 3A). Flow cytometric analysis was performed in order to test cell surface expression of CD4 and CCR5 by transiently transfected U87 cells, and a very close relationship was found between the levels of CD4 and CCR5 expression and the amount of CD4- and CCR5-expressing plasmids used in the transfection step (data not shown).
FIG. 3.
Infection of cells expressing decreasing amounts of CD4 and/or CCR5. U87 cells were transiently transfected with plasmids expressing CD4 and CCR5 and subsequently infected with supernatants containing env-pseudotyped, luciferase viruses (p24gag [5 ng/ml]). (A) rBORI pseudotype did not infect cells expressing low CD4 and high CCR5 or low CD4/CCR5, while rB15 and rV1V2 did. Results are expressed as the mean + standard deviation (error bars) from seven independent experiments each performed in triplicate. (B) The luciferase activity measured following infection of low-CD4 and high-CCR5 or low-CD4/CCR5 cells was expressed as a percentage of the activity induced in the high-CD4/CCR5 cells. We performed seven independent experiments with the rBORI, rB15, and rV1V2 pseudotypes. The differences in the values between rBORI and rB15 or rV1V2 were statistically significant (Wilcoxon's rank-sum test: P < 0.01 in both comparisons for low CD4 and high CCR5 and for low CD4/CCR5).
To analyze these differences statistically, we calculated the proportion of luciferase activity resulting from infection under various conditions of cell surface receptor expression as a percentage of the activity noted with the highest expression of CD4/CCR5 tested (Fig. 3B). These results indicated that infection by rBORI was significantly lower than that by rB15 or rV1V2 pseudotypes (P < 0.01 with Wilcoxon's rank-sum test). Other differences (i.e., between rB15 and rV1V2 or between rBORI, rB15, or rV1V2 and point mutants) were not significant.
Utilization of CCR5/CCR2b chimeric coreceptors.
To determine whether the interaction of these envelope glycoproteins with CCR5 involved the same domains, we tested the ability of the rBORI, rB15, rV1V2, rE153G, and rE153G,T162A pseudotypes to use several chimeric coreceptors as well as CCR5 amino-terminal deletion mutants. U87 cells were transfected with plasmids expressing CD4 alone, CD4 and CCR5, CD4 and CCR2b, or CD4 and chimeric coreceptors prepared by combining regions of CCR5 and CCR2b (see Materials and Methods) (87). Cell surface expression of wild-type and chimeric coreceptors was confirmed by flow cytometric analysis (data not shown).
As shown in Table 2, none of the pseudotypes was able to infect either cells expressing CD4 alone or those expressing CCR2b as a coreceptor. Similarly, chimeras containing a single extracellular domain of CCR5 in the context of the CCR2b molecule did not support infection by any pseudotype. The single exception was the 5222 chimera (amino-terminal domain of CCR5 and the rest of the coreceptor of CCR2b), which all pseudotypes utilized to approximately the same low extent (between 2.0 and 3.3% of the respective infections when using wild-type CCR5, as calculated using the means of 5 to 17 independent experiments). This result confirmed the relative importance of the amino terminus of CCR5 in mediating HIV-1 entry.
TABLE 2.
Infection of U87 cells expressing CD4 and the indicated chimeric coreceptor by env-pseudotyped, luciferase viruses
| Coreceptor | Mean luciferase activity ± SD (RLU/s)a
|
||||
|---|---|---|---|---|---|
| rBORI | rB15 | RV1V2 | rE153G | rE153G, T162A | |
| None | 132 ± 68 | 195 ± 112 | 281 ± 161 | 141 ± 64 | 149 ± 59 |
| CCR5b | 24,611 ± 11,755 | 75,090 ± 15,932 | 108,548 ± 25,902 | 42,948 ± 16,214 | 38,309 ± 15,001 |
| CCR2b | 98 ± 35 | 187 ± 137 | 250 ± 70 | 192 ± 84 | 171 ± 64 |
| 5222 | 643 ± 590 | 1,509 ± 1,225 | 2,413 ± 2,356 | 1,414 ± 769 | 938 ± 1,356 |
| 2522 | 74 ± 39 | 79 ± 38 | 119 ± 55 | 60 ± 48 | 72 ± 51 |
| 2252 | 71 ± 33 | 75 ± 41 | 79 ± 11 | 54 ± 22 | 72 ± 33 |
| 2225 | 101 ± 61 | 107 ± 55 | 269 ± 216 | 86 ± 37 | 77 ± 21 |
| 2555 | 735 ± 904 | 614 ± 513 | 792 ± 573 | 208 ± 226 | 361 ± 356 |
| 5255 | 8,604 ± 4,539 | 28,211 ± 13,617 | 49,532 ± 14,623 | 13,489 ± 4,553 | 12,803 ± 6,802 |
| 5525 | 587 ± 399 | 2,284 ± 2,169 | 4,519 ± 2,191 | 2,101 ± 1,485 | 688 ± 593 |
| 5552 | 1,071 ± 814 | 15,960 ± 6,144 | 26,984 ± 8,637 | 5,845 ± 2,832 | 4,231 ± 3,273 |
| 5252 | 2,912 ± 1,269 | 9,104 ± 3,599 | 19,053 ± 11,073 | 3,892 ± 1,620 | 7,640 ± 4,246 |
| Δ4 | 1,734 ± 1,025 | 12,501 ± 8,077 | 18,904 ± 11,742 | 2,731 ± 3,092 | 4,250 ± 3,338 |
| Δ8 | 1,545 ± 1,023 | 2,442 ± 1,990 | 3,780 ± 2,227 | 3,362 ± 1,849 | 1,435 ± 1,603 |
| Δ12 | 134 ± 112 | 91 ± 38 | 63 ± 18 | 62 ± 17 | 59 ± 21 |
| Δ16 | 122 ± 108 | 89 ± 41 | 49 ± 22 | 47 ± 26 | 65 ± 26 |
Luciferase activity is calculated from 5 to 17 independent experiments performed at least in triplicate.
Values in bold represent statistical significant changes in comparison with wild-type CCR5, also in bold, as indicated in the text.
In contrast, all of the pseudotypes were able to use those chimeric molecules that contained a single extracellular domain of CCR2b in the context of CCR5, albeit with some differences (Table 2). Thus, infection of cells expressing the 2555 and 5525 chimeras was greatly reduced compared to wild-type CCR5 in all viruses (0.5 to 3.0% for 2555 and 1.8 to 4.9% for 5525), confirming that both the amino-terminal domain and the ECL2 of the chemokine receptor molecule are very important for the interactions leading to infection. In the same sense, the 5255 and 5252 chimeras supported infection by all five pseudotypes (31.4 to 45.6% for 5255 and 9.1 to 19.9% for 5252, compared with wild-type CCR5) without major differences between them.
Remarkably, when the percentages of infection with respect to CCR5 in every experiment were compared, there was a statistically significant difference in the extent of infection induced by all viruses using the 5252 chimera, compared with the level observed with 5255 (Wilcoxon's signed-rank test: P < 0.01 for rB15, P < 0.02 for rBORI, and P < 0.05 for the other three pseudotypes), although the extent of this difference did not vary between viruses (data not shown). Since the only distinction between these two chimeras is the replacement of ECL3 of CCR5 by the corresponding of CCR2b, it is likely that the ECL3 of the CCR5 molecule is also needed by these pseudotypes in order to induce high levels of infection.
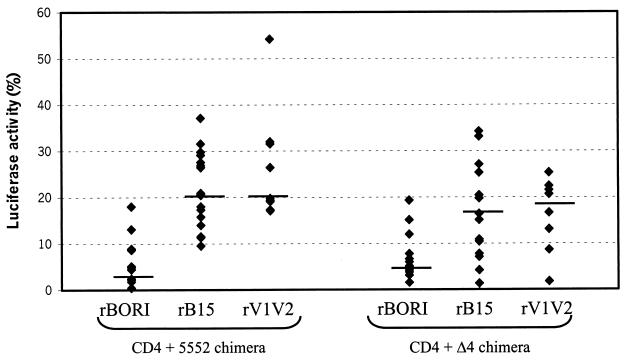
Finally, the use of the 5552 chimera was also studied and, in this case, we did observe that the levels of infection induced by rBORI (4.4% of wild-type CCR5) were markedly lower that those of rB15 or rV1V2 (21.3 and 24.9%, respectively), with the rE153G and rE153G,T162A pseudotypes resulting in an intermediate level of activity in comparison to the wild type (13.6% for rE153G and 11.0% for rE153G,T162A) (Table 2). Comparison of the percentages of infection for each virus and experiment with respect to wild type CCR5 (Fig. 4) demonstrated that rBORI infected significantly less than rB15, rV1V2, and rE153G (Wilcoxon's rank-sum test: P < 0.001, P < 0.001, and P < 0.02, respectively). Furthermore, rB15 and rV1V2 infected significantly more than rE153G,T162A (P < 0.02 and P < 0.01, respectively), but not compared with rE153G (data not shown). In summary, it is likely that although all pseudotypes somewhat require the presence of the ECL3 of CCR5 to induce high levels of infection, the gp120s of rB15, rV1V2, and rE153G seem to confer a tolerance to these pseudotypes, allowing the efficient use for infection of particular chimeric coreceptors.
FIG. 4.
Infection of cells expressing CD4 and the 5552 or Δ4 chimeric coreceptors. U87 cells were transiently transfected with plasmids expressing CD4 and the chimeric coreceptors 5552 (amino terminus, ECL1 and ECL2 of CCR5, and ECL3 of CCR2b) or Δ4 (deletion of the first four amino acids in the amino terminus of CCR5), and subsequently infected with supernatants containing env-pseudotyped, luciferase viruses (p24gag [5 ng/ml]). Luciferase activity is shown as the percentage with respect to wild-type CCR5 for each virus and experiment. Horizontal lines represent the median of each group of data. For the 5552 chimera, rBORI infections were significantly less efficient than rB15 and rV1V2 infections (Wilcoxon's rank-sum test: P < 0.001 in both cases). For the Δ4 mutant, rBORI infected significantly less than rB15 and rV1V2 (P < 0.01 and P < 0.02, respectively).
CCR5 amino-terminal deletion mutants.
Deletion of the amino-terminal 12 or 16 amino acids (Δ12 and Δ16, respectively) abolished the coreceptor function of CCR5 (Table 2), whereas the mutant coreceptor containing a deletion of the last eight amino acids (Δ8) supported a low level infection by all pseudotypes. However, when only four amino acids were deleted from the amino terminus, there were statistically significant differences in the use of the mutant molecule by the envelopes. The rBORI pseudotype gave a less robust signal than rB15 or rV1V2 (Wilcoxon's rank-sum test: P < 0.01 and P < 0.02, respectively) (Fig. 4). The statistical significance of the difference was confirmed using Wilcoxon's matched-pairs signed-rank test and Student's t tests for independent and paired samples.
Neutralization with HIV-1-positive human sera.
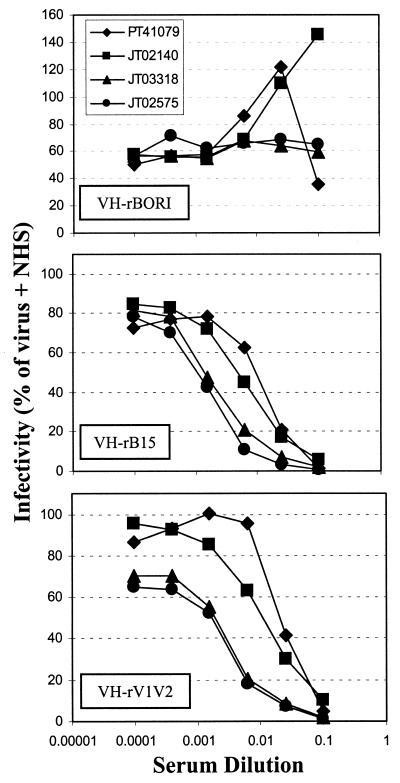
As mutations in the V1/V2 region of gp120, including those that delete potential N-linked glycosylation sites, have been previously shown to expose neutralization-sensitive epitopes (21, 66, 84, 112), the relative sensitivity of the recombinant BORI viruses to neutralization by a panel of sera from HIV-1-infected individuals was determined using a MAGI-CCR5-based single-cycle infection assay. The antisera were chosen for their ability to neutralize HIV-1IIIB at high titers. As it has been found for many primary isolates (67, 68, 108), the parental VH-rBORI recombinant virus was quite resistant to neutralization by all the sera tested (Fig. 5). In marked contrast, VH-rB15 was neutralized by all of the sera, with 90% inhibitory concentrations similar to those for neutralization of the laboratory-adapted HIV-1IIIB virus (data not shown). The VH-rV1V2 chimeric virus was equally neutralization-sensitive (Fig. 5), indicating that the determinants of neutralization sensitivity in the microglia-adapted HIV-1BORI-15 reside in this region of env. In addition, the two recombinant viruses containing point mutations present in HIV-1BORI-15 in the context of HIV-1BORI env showed a different behavior, since VH-rE153G was neutralization resistant like VH-rBORI, while VH-rE153G,T162A was neutralization sensitive like VH-B15 and VH-rV1V2 (data not shown). This result indicates the importance of the glycosylation status in the V1/V2 region of gp120 for the neutralization phenotype of the viral isolate.
FIG. 5.
Neutralization with HIV-1-positive human sera. A MAGI-CCR5 cell-based, single-cycle infection assay was performed as described in Materials and Methods, to test the neutralization sensitivity of VH-rBORI, VH-rB15, and VH-rV1V2 recombinant viruses (93) either in the presence of a 1/10 dilution of NHS or in the presence of increasing dilutions of serum from four HIV-1-infected individuals. Results were calculated as percentage of infection with respect to the extent observed with virus plus NHS, and the values shown represent the average of two to three independent experiments.
Exposure of 17b epitope.
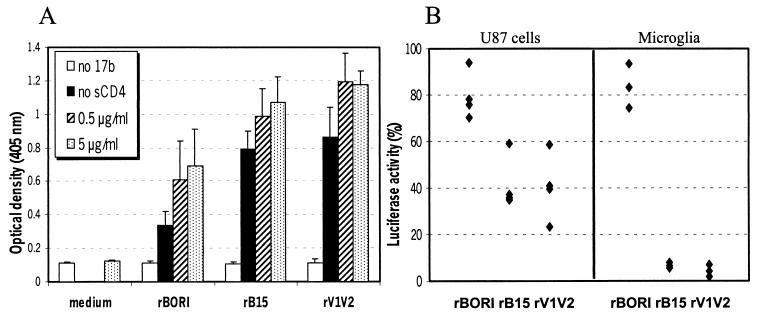
The human MAb 17b recognizes a CD4-induced conformational epitope that partially colocalizes with the chemokine receptor binding site. As described in Materials and Methods, we used an ELISA to detect the availability of this epitope on pseudotyped viruses, which presumably have a trimeric envelope on their surface. The 17b binding was tested with or without sCD4. As shown in Fig. 6A, absent sCD4, reactivity with the 17b MAb was greater in rB15 and rV1V2 than in rBORI pseudotypes (Student's t test: P < 0.02 and P < 0.05, respectively). As expected, when the 17b MAb binding was performed in the presence of sCD4 at either 0.5 or 5 μg/ml, there was a clear increase in the optical density with all pseudotypes. However, the 17b epitope exposure remained higher in rB15 and rV1V2 than in rBORI pseudotypes, although this result did not reach statistical significance. Taken together, these results support our concept that there are conformational differences between the gp120 molecules of these viruses.
FIG. 6.
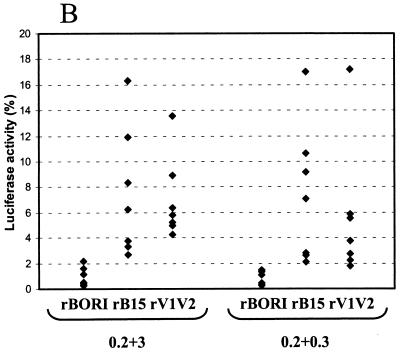
17b ELISA and neutralization assays. (A) An ELISA was performed using the 17b MAb with supernatants containing env pseudotypes, either with or without sCD4. Results are shown as mean optical density at 405 nm + standard error of the mean (error bars) from four independent experiments using different pseudotype stocks. Absent sCD4, reactivity with the 17b antibody was greater in rB15 and rV1V2 than in rBORI pseudotypes (Student's t test: P < 0.02 and P < 0.05, respectively). (B) 17b MAb neutralization was performed in U87 cells transiently transfected with CD4-CCR5 (left; four independent experiments) and in microglial cultures (right; three independent experiments with two microglial preparations). The pseudotypes were incubated with 17b MAb (20 μg/ml) at 37°C for 1 h prior to inoculation. Data are shown as percentage of luciferase activity with respect to untreated controls for each pseudotype. In both cell types, 17b inhibited infection by rB15 and rV1V2 pseudotypes but not by rBORI.
The same experiment was also performed on viral pellets obtained following ultracentrifugation of pseudotype-containing supernatants, in order to confirm these findings on gp120 molecules that were known to be attached to viral particles. Although there was distinct binding over background in all pseudotypes (data not shown), we were not able to detect significant differences among them, possibly because overall the signal levels were much lower than in the experiment performed on supernatant stocks.
Neutralization studies with 17b MAb.
We then used the 17b MAb to test if the greater exposure of the 17b epitope on gp120 correlated with a higher sensitivity to neutralization. To this end, we preincubated the pseudotypes with 17b MAb (20 μg/ml) at 37°C for 1 h prior to adding the inocula to U87 cells transiently transfected with CD4 and CCR5. The 17b MAb treatment inhibited infection by those pseudotypes that demonstrated reactivity prior to incubation with sCD4 (60% inhibition of rB15 and rV1V2, yet only 20% inhibition of rBORI [Fig. 6B]). Analysis of the proportion of infectivity with or without 17b MAb in four independent experiments demonstrated that the difference was statistically significant (Wilcoxon's rank-sum test: P < 0.05 in both comparisons). We performed the same inhibition experiments using human adult microglia cultures as target cells, and the results of three independent experiments with two different microglia preparations are shown in Fig. 6B. The 17b MAb inhibited 95% of luciferase activity with rB15 and rV1V2 pseudotypes, while inhibition with rBORI pseudotypes was about 20%. The difference observed in the 17b neutralization efficiency of rB15 and rV1V2 pseudotypes when infecting U87 cells and microglia may be related with the levels of expression of CD4 and CCR5 in these cells.
DISCUSSION
We have studied the interactions between the envelope glycoproteins of a microglia-adapted HIV-1 strain and those of its parental isolate and the CD4 and CCR5 cellular receptors for the virus. Previous studies demonstrated that in comparison with its blood-derived parental virus (HIV-1BORI), the isolate recovered after 15 sequential passages in microglial cultures (HIV-1BORI-15) was highly fusogenic, and it replicated to higher levels in microglia (95). Both viruses use CCR5 as a coreceptor in conjunction with CD4, and genetic analysis indicated that the syncytium-inducing phenotype was mainly due to four discrete amino acid changes in the V1/V2 region of the viral gp120 (93). We hypothesized that there would be differences in the interactions between the gp120s from HIV-1BORI and HIV-1BORI-15 and the cellular receptors, particularly since the level of expression of these receptors differs between microglia and other cells. The genetic changes also pointed towards the virus-cell surface interaction. For example, the V1/V2 region of HIV-1 gp120 seems to play a role (i) in determining cellular tropisms and virus spread (14, 18, 49, 66, 72, 74, 98, 100, 112); (ii) in shielding the conserved, discontinuous structures that form the coreceptor binding site on gp120 until CD4 binding has taken place (49, 50, 85, 99, 114); and (iii) in protecting the virus from neutralization by Abs (17, 21, 112) perhaps because it contains several potential glycosylation sites.
Confirming previous results, pseudotypes containing either the entire HIV-1BORI-15 envelope or its VI/V2 loops infected microglia and other cells tested better than those containing the HIV-1BORI envelope (rBORI). Furthermore, infection was only detected when both CD4 and CCR5 were present. This result is further evidence that phenotypic changes resulting from alterations in the glycosylation pattern in V1/V2 are clearly context dependent (35, 50, 53, 63). For example, one group of investigators derived a CD4-independent virus whose genetic analysis ascribed this phenotype to the loss of a single glycosylation site in V1/V2 (50). On the other hand, Ly et al. (63) found that glycosylation facilitated the interaction with CD4 and CCR5 of a different R5 isolate. These investigators also suggested that mutant viruses without glycosylation sites in V2 replicate with markedly reduced efficiency in cells expressing small numbers of receptor molecules (63).
Since we did not observe CD4-independent infection, we analyzed the relative CD4 and CCR5 dependencies by infecting cells with different amounts of CD4 or CCR5 on the surface. When the expression levels of CD4 were high, all pseudotypes infected with high efficiency even when the cells were transfected with a 100-fold-lower amount of CCR5-expressing plasmid (data not shown), similarly to what has been previously reported (78). By contrast, when the expression levels of CD4 were low, independent of the levels of expression of CCR5, the rB15 and rV1V2 pseudotypes retained their infectivity whereas the rBORI pseudotypes did not. We also studied the effects of soluble CD4 treatment of the pseudotypes prior to infection. While preincubation with sCD4 had no effect in mediating the infection of CD4− CCR5+ cells, the same concentration of sCD4 inhibited infection by rB15 and rV1V2 pseudotypes, but not rBORI, in CD4+ CCR5+ cells. Sensitivity to sCD4 treatment is typical of HIV-1 laboratory-adapted isolates. Although it has been reported that the relative resistance to sCD4 is not related to lower intrinsic affinities of their envelopes for sCD4 or a lower capacity for sCD4 binding (68, 69, 73, 102), there is some evidence that the affinities for sCD4 of the virion-associated gp120s from primary viruses are much lower than those of laboratory-adapted strains (69). Taken together, the ability to use small amounts of CD4 on the cell surface and the greater sensitivity to sCD4 treatment lead to the speculation that the four amino acid changes in the V1/V2 region of gp120 of the microglia-adapted virus are increasing the binding of CD4. However, this remains to be formally proven.
A second line of experimentation looked at the interaction between gp120 and CCR5 through the use of chimeric coreceptors containing elements of CCR2b and CCR5. These have been useful in several other contexts (3, 5, 11, 13, 30, 31, 36, 56, 75, 86, 94, 110). In agreement with the general impression that the amino-terminal domain is the most important determinant of CCR5 coreceptor function, followed by ECL2, we noted that chimeras containing a single extracellular domain of CCR5 in the context of CCR2b did not mediate infection by any pseudotype. Additionally, infection was greatly reduced by substituting the amino terminus of CCR2b in the context of CCR5.
However, the most interesting findings related to differences among pseudotypes prepared with the two glycoproteins are the effects of ECL3, which has been noted by other investigators to also play a role in entry (3, 31). The rB15 and rV1V2, but not rBORI, pseudotypes infected cells expressing CD4 with the 5552 chimeric coreceptor (containing the ECL3 of CCR2b in the context of CCR5). Replacement of the CCR5 ECL3 decreased infection by all pseudotypes (see, for example, the results comparing 5252 with 5255), and infection with rBORI was particularly sensitive to this change. It should be noted that in comparing the extracellular domains of CCR5 and CCR2b, the ECL3 is one of the most conserved because only 6 of the 23 residues differ between them, but this seems to be enough for the functional differences reported previously (3, 31) and in this work. In addition, the small number of amino acid differences in the envelope glycoproteins between rB15 or rV1V2 and rBORI (eight and four, respectively) are sufficient to modify the use of this particular chimera for infection. Since interactions between HIV-1 and entry cofactors are conformationally complex, it may be concluded that, at least for some macrophage-tropic HIV-1 isolates, the ECL3 of CCR5 plays an important role in its coreceptor function.
We found similar differences when we analyzed the use of CCR5 amino-terminal deletion mutants for infection. Previous studies have shown that the region spanning amino acids 2 to 18 of the CCR5 amino-terminal domain contains all of the residues important for viral entry (30, 31, 33, 38, 83). Accordingly, we observed that Δ12 and Δ16 CCR5 amino-terminal deletion mutants did not support infection by any pseudotype, while the Δ8 mutant supported a limited infection. However, using the CCR5 Δ4 amino-terminal deletion mutant as coreceptor, we again noted that only the rB15 and rV1V2, but not rBORI, pseudotypes were able to induce infection. Thus, it seems that the relationship between the gp120 and the amino-terminal domain of CCR5 differ in rB15 or rV1V2 pseudotypes with respect to rBORI.
In summary, it is likely that, due to the amino acid changes located in the V1/V2 region of gp120, the microglia-adapted virus HIV-1BORI-15 is intrinsically more efficient in the use of low levels of CD4 for infection than the parental virus. It also seems to be able to establish slightly different interactions with the coreceptor molecule. Binding studies using recombinant soluble gp120s should establish whether these hypotheses are indeed correct (44, 118).
Finally, since the results discussed above indicated that the use of CD4 and CCR5 differed among the two strains, we examined whether this could be related to a different conformation of the gp120 molecules. An ELISA measuring the exposure of the 17b epitope in the gp120 present in the pseudotype-containing supernatants was used to demonstrate that this epitope was indeed more exposed in rB15 and rV1V2 pseudotypes than in rBORI pseudotypes. This was evident with or without sCD4. Interestingly, the greatest difference was noted absent sCD4, confirming an intrinsic dissimilarity in the conformation between the envelopes of HIV-1BORI-15 and its parental isolate. Exposure of the 17b epitope correlated with sensitivity to neutralization by the human 17b MAb in both transiently transfected U87 cells and primary human adult microglial cells. The difference between the two viruses was much more evident in microglial cells. Based on these results, it is likely that the V1/V2 loop of HIV-1BORI-15 gp120 favors the conformation triggered by CD4 binding, allowing a higher accesibility of the high-affinity chemokine receptor binding site. Since microglial cells contain few CD4 molecules, this difference can be critical for their infection, because it has been recently reported that the low levels of CD4 on primary macrophages may be the limiting factor for infection by some M-tropic primary HIV-1 and simian immunodeficiency virus isolates (8, 71) as well as for infection of leukemic T-cell lines by some T-tropic HIV-1 isolates (77). Increased sensitivity to neutralization by HIV-1-positive human sera also correlated with the exposure of the 17b binding site in the rB15 and rV1/V2 envelope glycoproteins. In the periphery, such sensitivity to neutralization by serum antibodies would likely be lethal to an adapting virus. However, as the CNS is an immunologically privileged site, the mutations required for infection of cells with low CD4 levels may persist even if they expose neutralization-sensitive epitopes.
In addition to the neurological symptoms associated with HIV-1 infection of the CNS, the brain is one of the potential reservoirs where the virus could persist even after long periods of treatment with highly active antiretroviral therapy; this persistence is a major barrier to virus eradication. As in the CNS and microglia, latent infection of resting T cells occurs soon after infection (24, 39, 117), suggesting the involvement of R5 viruses (reviewed in reference 10). Recently it has been demonstrated that the vast majority of viruses isolated from latently infected, resting CD4+ T cells are R5 (76). However, only a subset of highly purified resting CD4+ T cells expressing low levels of CCR5 and markers of immunologic memory could be infected in vitro by an R5 isolate, HIV-1Ba-L (76). Thus, it may be that low levels of CD4 and CCR5 actually select for viruses that infect long-lived cells, populating the type of cells that are likely to survive throughout long periods of highly active antiretroviral therapy.
ACKNOWLEDGMENTS
This work was supported by Public Heath Service grants NS-27405, NS-35734, and MH-58958.
We are grateful to J. Rucker, R. W. Doms, and J. A. Hoxie (University of Pennsylvania), M. Parmentier (Université Libre de Bruxelles), G. M. Shaw (University of Alabama), N. R. Landau (The Salk Institute for Biological Studies), J. E. Robinson (Tulane University), and J. Strizki (Schering Plough), as well as to the National Institutes of Health AIDS Research Reagent and Reference Program, for providing us with excellent reagents. We also thank A. Albright, E. Ryzhova, R. Vos, and R. Doms for their helpful comments and W. Cao and L. Shawver for their technical assistance.
REFERENCES
- 1.Albright A V, Shieh J T C, Itoh T, Lee B, Pleasure D, O'Connor M J, Doms R W, González-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright A V, Strizki J, Harouse J M, Lavi E, O'Connor M, González-Scarano F. HIV-1 infection of cultured human adult oligodendrocytes. Virology. 1996;217:211–219. doi: 10.1006/viro.1996.0108. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Ahuja S S, Light D, Mummidi S, Berger E A, Ahuja S K. CC chemokine receptor 5-mediated signaling and HIV-1 co-receptor activity share common structural determinants: critical residues in the third extracellular loop support HIV-1 fusion. J Biol Chem. 1997;272:19771–19776. doi: 10.1074/jbc.272.32.19771. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC-CKR5: a RANTES, MIP-1α and MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 6.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagasara O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bannert N, Schenten D, Craig S, Sodroski J. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol. 2000;74:10984–10993. doi: 10.1128/jvi.74.23.10984-10993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger E A, Doms R W, Fenyö E-M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:340. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 10.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 11.Bieniasz D, Fridell R A, Aramori I, Ferguson S S G, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpain C, Doranz B J, Vakili J, Rucker J, Govaerts C, Baik S S W, Lorthioir O, Migeotte I, Libert F, Baleux F, Vassart G, Doms R W, Parmentier M. Multiple charged and aromatic residues in CCR5 amino-terminal domain are involved in high affinity binding of both chemokines and HIV-1 env protein. J Biol Chem. 1999;274:34719–34727. doi: 10.1074/jbc.274.49.34719. [DOI] [PubMed] [Google Scholar]
- 14.Boyd M T, Simpson G R, Cann A J, Johnson M A, Weiss R A. A single amino acid substitution in the V1 loop of human immunodeficiency virus type 1 gp120 alters cellular tropism. J Virol. 1993;67:3649–3652. doi: 10.1128/jvi.67.6.3649-3652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brew B J, Evans L, Byrne C, Pemberton L, Hurren L. The relationship between AIDS dementia complex and the presence of macrophage tropic and non syncytium inducing isolates of human immunodeficiency virus type 1 in the cerebrospinal fluid. J Neurovirol. 1996;2:152–157. doi: 10.3109/13550289609146877. [DOI] [PubMed] [Google Scholar]
- 16.Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carillo A, Ratner L. Cooperative effects of the human immunodeficiency virus type 1 envelope variable loops V1 and V3 in mediating infectivity for T cells. J Virol. 1996;70:1310–1316. doi: 10.1128/jvi.70.2.1310-1316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carne C A, Tedder R S, Smith A, Sutherland S, Elkington S G, Daly H M, Preston F E, Craske J. Acute encephalopathy coincident with seroconversion for anti-HTLV-III. Lancet. 1985;ii:1206–1208. doi: 10.1016/s0140-6736(85)90740-8. [DOI] [PubMed] [Google Scholar]
- 20.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng-Mayer C, Brown A, Harouse J, Luciw P A, Mayer A J. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J Virol. 1999;73:5294–5300. doi: 10.1128/jvi.73.7.5294-5300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng-Mayer C, Levy J A. Distinct biological and serological properties of human immunodeficiency virus from the brain. Ann Neurol. 1988;23(Suppl.):S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 23.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu J, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The b-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 24.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark S J, Saag M S, Decker W D, Campbell-Hill S, Roberson J L, Veldkamp P J, Kappes J C, Hahn B H, Shaw G M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324:954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 26.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 27.Davis L E, Hjelle B L, Miller V E, Palmer D L, Llewellyn A L, Merlin T L, Young S A, Mills R G, Wachsman W, Wiley C A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 28.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 29.Dick A D, Pell M, Brew B J, Foulcher E, Sedgwick J D. Direct ex vivo flow cytometric analysis of human microglial cell CD4 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients and patients with other neurological disease. AIDS. 1997;11:1699–1708. doi: 10.1097/00002030-199714000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Doranz B, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusin cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 31.Doranz B J, Lu Z H, Rucker J, Zhang T Y, Sharron M, Cen Y H, Wang Z X, Guo H H, Du J G, Accavitti M A, Doms R W, Peiper S C. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J Virol. 1997;71:6305–6314. doi: 10.1128/jvi.71.9.6305-6314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 33.Dragic T, Trkola A, Lin S W, Nagashima K A, Kajumo F, Zhao L, Olson W C, Wu L, Mackay C R, Allaway G P, Sakmar T P, Moore J P, Maddon P J. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72:279–285. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dragic T, Trokla A, Thompson D A D, Cormier E G, Kajumo F A, Maxwell E, Lin S W, Ying W, Smith S O, Sakmar T P, Moore J P. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the env gene of human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edinger A L, Blanpain C, Kunstman K J, Wolinsky S M, Parmentier M, Doms R W. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J Virol. 1999;73:4062–4073. doi: 10.1128/jvi.73.5.4062-4073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein L G, Kuiken C, Blumberg B M, Hartman S, Sharer L R, Clement M, Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991;180:583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 38.Farzan M, Choe H, Vaca L, Martin K, Sun Y, Desjardins E, Ruffing N, Wu L, Wyatt R, Gerard N, Gerard C, Sodroski J. A tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J Virol. 1998;72:1160–1164. doi: 10.1128/jvi.72.2.1160-1164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for livelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 40.Gabuzda D, He J, Ohagen A, Vallat A V. Chemokine receptors in HIV-1 infection of the central nervous system. Semin Immunol. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- 41.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the beta-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glass J D, Wesselingh S L, Selnes O A, McArthur J C. Clinical-neuropathological correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- 43.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman T L, LaBranche C C, Zhang W, Canziani G, Robinson J, Chaiken I, Hoxie J A, Doms R W. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci USA. 1999;96:6359–6364. doi: 10.1073/pnas.96.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 46.Ioannidis J P A, Reichlin S, Skolnik P R. Long-term productive human immunodeficiency virus-1 infection in human infant microglia. Am J Pathol. 1995;147:1200–1206. [PMC free article] [PubMed] [Google Scholar]
- 47.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 48.Koenig S, Gendelman H E, Orenstein J M, dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 49.Koito A, Harrowe G, Levy J A, Cheng-Mayer C. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein in infection of primary macrophages and soluble CD4 neutralization. J Virol. 1994;68:2253–2259. doi: 10.1128/jvi.68.4.2253-2259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney L J, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. doi: 10.1128/jvi.73.10.8120-8126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korber B T, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaBranche C C, Hoffman T L, Romano J, Haggarty B S, Edwards T G, Matthews T J, Doms R W, Hoxie J A. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landau N L, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavi E, Kolson D L, Ulrich A M, Fu L, González-Scarano F. Chemokine receptors in the human brain and their relationship to HIV infection. J Neurovirol. 1998;4:301–311. doi: 10.3109/13550289809114531. [DOI] [PubMed] [Google Scholar]
- 56.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Drell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 57.Lee S C, Hatch C, Liu W, Kress Y, Lyman B D, Dickson D W. Productive infection of human fetal microglia by HIV-1. Am J Pathol. 1993;143:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- 58.Lee W R, Syu W J, Du B, Matsuda M, Tan S, Wolf A, Essex M, Lee T H. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:2213–2217. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonard C, Spellman M, Riddle L, Harris R, Thomas J, Gregory T. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 60.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured brain tissue: identification of replication-competent and defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Luo L, Rasool N, Kang C Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993;67:584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lipton S A, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 63.Ly A, Stamatatos L. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J Virol. 2000;74:6769–6776. doi: 10.1128/jvi.74.15.6769-6776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 65.McArthur J C, Becker P S, Parisi J E, Trapp B, Selnes O A, Cornblath D R, Balakrishnan J, Griffin J W, Price D. Neuropathological changes in early HIV-1 dementia. Ann Neurol. 1989;26:681–684. doi: 10.1002/ana.410260516. [DOI] [PubMed] [Google Scholar]
- 66.Mondor I, Moulard M, Ugolini S, Klasse P J, Hoxie J, Amara A, Delaunay T, Wyatt R, Sodroski J, Sattentau Q J. Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression levels, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology. 1998;248:394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 67.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore J P, McKeating J A, Huang Y, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mori K, Rosenzweig M, Desrosiers R C. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol. 2000;74:10852–10859. doi: 10.1128/jvi.74.22.10852-10859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morikita T, Maeda Y, Fujii S, Matsushita S, Obaru K, Takatsuki K. The V1/V2 region of human immunodeficiency virus type 1 modulates the sensitivity to neutralization by soluble CD4 and cellular tropism. AIDS Res Hum Retrovir. 1997;13:1291–1299. doi: 10.1089/aid.1997.13.1291. [DOI] [PubMed] [Google Scholar]
- 73.Orloff S L, Kennedy M S, Belperron A A, Maddon P J, McDougal J S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993;67:1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer C, Balfe P, Fox D, May J C, Frederiksson R, Fenyo E-M, McKeating J A. Functional characterization of the V1V2 region of human immunodeficiency virus type 1. Virology. 1996;220:436–449. doi: 10.1006/viro.1996.0331. [DOI] [PubMed] [Google Scholar]
- 75.Picard L, Simmons G, Power C A, Meyer A, Weiss R A, Clapham P R. Multiple extracellular domains of CCR-5 contribute to human immunodeficiency virus type 1 entry and fusion. J Virol. 1997;71:5003–5011. doi: 10.1128/jvi.71.7.5003-5011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pierson T, Hoffman T L, Blankson J, Finzi D, Chadwick K, Margolick J B, Buck C, Siliciano J D, Doms R W, Siliciano R F. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J Virol. 2000;74:7824–7833. doi: 10.1128/jvi.74.17.7824-7833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Platt E J, Kozak S L, Kabat D. Critical role of enhanced CD4 affinity in laboratory adaptation of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 2000;16:871–882. doi: 10.1089/08892220050042819. [DOI] [PubMed] [Google Scholar]
- 78.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Dewey R, Chesebro B. Distinct HIV-1 env sequences are associated with neurotropism and neurovirulence. Curr Top Microbiol Immunol. 1995;202:89–104. doi: 10.1007/978-3-642-79657-9_7. [DOI] [PubMed] [Google Scholar]
- 80.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Power C, McArthur J C, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson R T, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differ between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Price R W, Brew B, Sidtis J, Rosenblum J, Scheck A C, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–591. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 83.Rabut G E E, Konner J A, Kajumo F, Moore J P, Dragic T. Alanine substitutions of polar and nonpolar residues in the amino-terminal domain of CCR5 differently impair entry of macrophage- and dualtropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:3464–3468. doi: 10.1128/jvi.72.4.3464-3468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 85.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 86.Ross T M, Bieniasz P D, Cullen B R. Multiple residues contribute to the inability of murine CCR-5 to function as a coreceptor for macrophage-tropic human immunodeficiency virus type 1 isolates. J Virol. 1998;72:1918–1924. doi: 10.1128/jvi.72.3.1918-1924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 88.Salzwedel K, Smith E D, Dey B, Berger E A. Sequential CD4-coreceptor interactions in human immunodeficiency virus type 1 Env function: soluble CD4 activates Env for coreceptor-dependent fusion and reveals blocking activities of antibodies against cryptic conserved epitopes on gp120. J Virol. 2000;74:326–333. doi: 10.1128/jvi.74.1.326-333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharer L R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992;51:3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 92.Shieh J T C, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, González-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shieh J T C, Martin J, Baltuch G, Malim M H, González-Scarano F. Determinants of syncytium formation in microglia by human immunodeficiency virus type 1: role of the V1/V2 domains. J Virol. 2000;74:693–701. doi: 10.1128/jvi.74.2.693-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siciliano S J, Kuhmann S E, Weng Y, Madani N, Springer M S, Lineberger J E, Danzeisen R, Miller M D, Kavanaugh M P, DeMartino J A, Kabat D. A critical site in the core of the CCR5 chemokine receptor required for binding and infectivity of human immunodeficiency virus type 1. J Biol Chem. 1999;274:1905–1913. doi: 10.1074/jbc.274.4.1905. [DOI] [PubMed] [Google Scholar]
- 95.Strizki J M, Albright A V, Sheng H, O'Connor M, Perrin L, González-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan N, Sun Y, Binley J, Lee J, Barbas III C F, Parren P W H I, Burton D R, Sodroski J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan N, Thali M, Furman C, Ho D D, Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993;67:3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology. 1995;213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- 101.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 102.Turner S, Tizard R, DeMarinis J, Pepinsky R B, Zullo J, Schooley R, Fischer R. Resistance of primary isolates of human immunodeficiency virus type 1 to neutralization by soluble CD4 is not due to lower affinity with the viral envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1992;89:1335–1339. doi: 10.1073/pnas.89.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watkins B A, Dorn H H, Kelly W B, Armstrong R C, Potts B J, Michaels F, Kufta C V, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–552. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- 104.Wild C T, Greenwell T K, Matthews T J. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retrovir. 1993;9:1051–1053. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 105.Wiley C A, Achim C L. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- 106.Wiley C A, Schrier R D, Nelson J A, Lamper P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong J K, Ignacio C C, Torriani F, Havlir D, Fitch N J S, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growh in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 110.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu Z, Kayman S C, Honnen W, Revesz K, Chen H, Vijh-Warrier S, Tilley S A, McKeating J, Shotton C, Pinter A. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J Virol. 1995;69:2271–2278. doi: 10.1128/jvi.69.4.2271-2278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 114.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 116.Yong V W, Antel J P. Culture of glial cells from human brain biopsies. In: Fedoroff S, Richardson A, editors. Protocols for neural cell culture. Totowa, N.J: Humana Press; 1992. pp. 81–96. [Google Scholar]
- 117.Zhang L, Ramratnam B, Tenner R K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 118.Zhang W, Canziani G, Plugariu C, Wyatt R, Sodroski J, Sweet R, Kwong P, Hendrickson W, Chaiken I. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry. 1999;38:9405–9416. doi: 10.1021/bi990654o. [DOI] [PubMed] [Google Scholar]