Abstract
Understanding how coral reefs respond to disturbances is fundamental to assessing their resistance and resilience, particularly in the context of climate change. Due to the escalating frequency and intensity of coral bleaching events, it is essential to evaluate spatio-temporal responses of coral reef communities to disentangle the mechanisms underlying ecological changes. Here, we used benthic data collected from 59 reefs in the Red Sea over five years (2014–2019), a period that encompasses the 2015/2016 mass bleaching event. Reefs were located within three different geographic regions with different environmental settings: north (Duba; Al Wajh), central (Jeddah; Thuwal), and south (Al Lith; Farasan Banks; Farasan Islands). Coral community responses were region-specific, with communities in the south being more promptly affected than those in the northern and central regions, with hard and soft coral cover dropping drastically in several reefs from around > 40% to < 5% two years after bleaching. Coral bleaching effects were particularly evident in the decrease of cover in branching corals. Overall, we documented a shift towards a dominance of macroalgae, turf algae, and crustose coralline algae (CCA). Using remote sensing data, we analyzed sea surface temperature (SST) regimes at the study sites to infer potential drivers of changes in benthic composition. Both SST and Degree Heating Weeks (DHW) only partially aligned with the responses of benthic communities, highlighting the need for more accurate predictors of coral bleaching in the Red Sea. In times of intense coastal development along Saudi Arabia’s Red Sea coast, our study provides crucial baseline information on developments in coral reef community composition, as well as to guide decision-making, namely restoration efforts.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74956-7.
Subject terms: Ecology, Environmental sciences
Introduction
Coral reefs are currently facing multiple stressors arising from a combination of local and global threats that have contributed to coral cover loss1–3. Increasing sea surface temperature (SST) has resulted in more frequent and intense climate-induced bleaching events, impacting coral reefs globally4–7. Thermal anomalies and coral bleaching have increased in frequency, from once every 25 to 30 years at the beginning of the 80’s, to once every 5.9 years by 20166, affecting even regions considered coral refuges8–11. Regardless of the projected future climate scenarios, coral reefs are expected to be degraded, resulting in changes in species composition12, including a general loss of biodiversity and ecosystem services13,14. Indeed, by 2050, it is predicted that coral cover will decrease globally by 5–14%, while by the end of the century more than 40% of coral may be lost from the world’s oceans15.
Coral bleaching occurs when, under sustained stress, corals expel their algal symbionts from their transparent polyp tissue, thereby exposing the white, calcareous coral skeleton16. In a zooxanthellate coral, these symbionts may provide over 90% of the energetic requirements for the host17. If the duration is long enough, bleaching can cause coral death18 and consequently reef degradation16,19, thus directly affecting reef functioning20,21. Reef degradation often manifests as a shift towards dominance by macroalgae or other non-calcifying organisms (e.g. soft corals, sponges) which are able to outcompete calcifiers (e.g. hard corals) after a disturbance3,22,23. This shift in community structure and the potential loss in coral reef-associated biodiversity has a direct effect on reef functioning, affecting diverse ecological processes such as biogeochemical fluxes, primary production, herbivory, and predator-prey interactions24–26. Ultimately, these ecological changes will be translated into negative economic effects27.
The assessment of long-term changes to coral reef benthic community composition as a response to stressors associated with climate change requires the investigation across large temporal scales, supported wherever possible by well-established and standardized monitoring programs28. Nevertheless, in many tropical countries long-term monitoring programs are scanty and rarely standardized; not only are temporal data scarce or missing, but often baseline data themselves is absent, making evaluation of the ecological effects of coral bleaching challenging29. Such lack of data hampers the ability to make projections based on future scenarios. This is particularly true coral reef communities in the Red Sea, which remain largely understudied compared to other major reef systems (e.g. the Great Barrier Reef)30. Red Sea coral reef community responses (and recovery trajectories) to bleaching are not always documented as national monitoring programs have been limited in scope and duration. Monitoring by local stakeholders often faces the same obstacles (but see PERSGA31).
Over the last decade, the Red Sea’s coral reefs have seen several heat-related coral bleaching events32–34, and more recently, seasonal bleaching due to cold stress35. Though heat-related bleaching events in the Red Sea are beginning to garner attention, Genevier et al.36 reported that thermal anomalies in the Red Sea have increased in frequency and intensity since 1998, mirroring the trend seen worldwide37,38. In the aforementioned study, the authors determined historical marine heatwave events in the region by analyzing 35 years of SST data, identifying areas at risk, and highlighting potentially undetected bleaching events. According to the study, marine heatwaves encompassed more extensive areas and occurred more frequently than previously reported, even in the non-El Niño years, suggesting that coral bleaching may have been underestimated in the past.
The Red Sea is characterized by well-known north-south gradients of salinity, SST, and primary productivity39, making the basin an ideal natural laboratory to study coral reef dynamics. Communities in the three regions of the Red Sea (northern, central, and southern) are exposed to different environmental conditions and, therefore, may respond to SST anomalies in contrasting ways due to their different environmental history.
Based on a network of reefs that have been monitored since 2014 along the Saudi Arabian Red Sea coast (revisited nearly every two years) using standardized photo-transects, we investigated spatial and temporal changes in the community composition of coral reef benthic organisms over a six-year period. This period (2014–2019) was comprised of surveys conducted before and after the third and longest global-scale coral bleaching event caused by the El Niño-Southern Oscillation (ENSO) in 2015/201619,40. This study provides insights into the dynamics of benthic community structure across regional and temporal scales in the hypersaline and naturally warm Red Sea, and lays the foundation to track future trajectories of recovery or changes in these coral reefs. The relationship between patterns in Degree Heating Weeks (DHW) and SST data was also investigated (following findings by DeCarlo et al.41), as were the reported regional differences in bleaching prevalence. We hypothesize that coral communities of the northern Red Sea will be the most resistant to marine heat waves due to their higher thermal tolerance; indeed, this region is often considered a coral refuge that could potentially help to repopulate other regions42,43.
Materials and methods
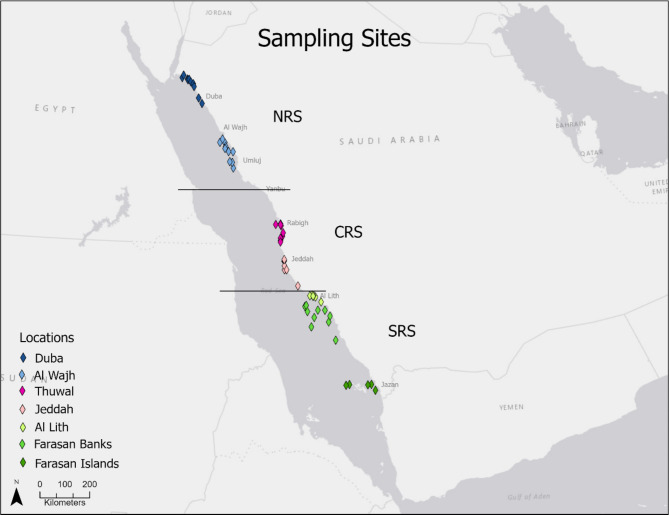
A total of 59 reefs along the Saudi Arabian Red Sea coast (28° N to 16.68° N), were sampled every two years (2014–2019) for three time points (Fig. 1, Supplementary Table S1). Sampled reefs were grouped into three regions (North, Central, South) based on observations in previous studies on spatial patterns of the coral reef cryptobiome44, spatiotemporal patterns of salinity and SST, and the distribution and abundance of surface phytoplankton biomass45. The regions and sites are as follows: Northern Red Sea (NRS) region: 20 reefs among two locations, Central Red Sea (CRS) region: 17 reefs among two locations, and the Southern Red Sea (SRS) region: 22 reefs among three locations (Fig. 1). Not all the reefs were sampled at every period due to limited human resources and complications in obtaining permits for some localities.
Fig. 1.
Location of sampling sites along the Saudi Arabian coast of the Red Sea. The 59 reefs are distributed across seven locations within three regions: North Red Sea (NRS) represented in shades of blue (Duba, Al Wajh), Central Red Sea (CRS) in shades of pink (Thuwal, Jeddah), and South Red Sea (SRS) in shades of green (Al Lith, Farasan Banks, Farasan Islands). Horizontal lines delimit the different regions. Maps throughout this paper were created using ArcGIS Pro software by Esri (software version 2.5.22; www.esri.com). ArcGIS and ArcMap are the intellectual property of Esri and are used herein under license. Copyright Esri. All rights reserved.
Sea surface temperature
To characterize thermal conditions at the study sites, we retrieved SST remote sensing data at a resolution of 4 km from the MODIS system. For this, a running 8-day mean of longwave (11 μm) night-time Level-3 data spanning seven years (June 2013 to May 2020) was obtained for each site from the Aqua satellite46 via NASA’s Ocean color group website (http://oceancolor.gsfc.nasa.gov/). Occurrence of heat stress during the same period was assessed using NOAA’s Coral Reef Watch Degree Heating Week (DHW) product47. Daily DHW data at a resolution of 5 km was downloaded from the Pacific Islands Ocean Observing System (PacIOOS) via the Environmental Research Division’s Data Access Program (ERDDAP) data server (https://pae-aha.pacioos.hawaii.edu/erddap/griddap/dhw_5km.html). According to Coral Reef Watch48, coral bleaching is likely to occur in an area when DHW reaches values of 4 and above, and severe bleaching (with subsequent mortality) is likely for DHW values of 8 and above. Given the resolution of the remote sensing products used here (i.e., 4 km for SST and 5 km for DHW data, respectively), some sites located near each other were associated with the same remote sensing data tile.
Benthic assessments
To record benthic cover at the selected reefs, semipermanent transects were surveyed at a depth of around 10 m. At each site, GPS coordinates were taken and used to deploy a transect tape along which surveys were conducted. Local observations of the reef structure were also taken to guide future assessments. In some cases, these were related to the presence of Autonomous Reef Monitoring Structures (ARMS) deployed at many of the reefs surveyed that marked the starting point of the transects. From this starting point, a series of three replicate 20 m long and 1 m wide benthic transects were conducted taking sequential photos (1 m x 1 m) for future analysis. A consistent distance to the bottom was the benthic composition (i.e. percent cover of benthic groups) for each photo was analyzed using Coral Point Count software with Microsoft Excel extensions (CPCe)49. In each 1 m2 frame, 48 points were randomly generated. This point count method has previously been applied to infer percent cover and has been shown to provide comparable results to line intercept methods50, with the added benefit of allowing for an archive of photos that can be used for future assessments and taxonomic validation. Here, we averaged the calculated percent cover of benthic categories across all photos of each transect. Subsequently, the percent cover at the reef level was calculated as the average of the three replicate transects for further analysis.
The benthic composition of each randomly generated point was assigned one of the following categories: hard corals (including calcifying hydrozoans of the genera Distichopora and Millepora), soft corals (including gorgonians), other anthozoans (zoanthids, anemones, and corallimorpharians), fleshy macroalgae, turf algae, Crustose Coralline Algae (CCA), other invertebrates (e.g., sponges, bivalves), pavement/rock, debris (plastic debris, litter, nylon nets), rubble (small hard fragments, rocks or lose coral fragments), and sand. Hard corals, soft corals, and macroalgae were recorded to the genus level whenever possible. Acropora and Porites specimens were further separated based on their growth forms into the following categories: table (Acropora), branching (Acropora, Porites), columnar (Porites), massive (Porites), and encrusting (Porites). The full list of benthic categories is shown in Supplementary Table S2.
Data analysis
The study period was divided into three different periods: period 1 (2014/2015), period 2 (2016/2017), and period 3 (2018/2019). This division was based on the occurrence of a major bleaching event in the summer of 2015 that differentially affected coral reefs along the Red Sea34,41. All surveys conducted in 2015 were carried out before the summer, therefore they were combined with the 2014 surveys and considered the reference or pre-bleaching period. For the assessment of changes in benthic community composition over time and across locations as well as regions, we performed a Non-Metric-Multidimensional Scaling (NMDS) analysis of all benthic categories. Percent cover data was square root transformed for normality, and a resemblance matrix was created using Bray-Curtis distance. A cluster analysis was conducted using the same resemblance matrix to confirm the associations of samples. We generated a heat map with the relative percent cover of the main benthic categories per site and period (i.e. any category with percent cover greater than 2%): hard corals, soft corals, algae, turf algae, CCA, rubble, pavement/rock, and sand. To visualize differences in the abundance of hard and soft corals pre- and post-bleaching, a bar graph was created using the ggplot251 package in R v4.3.052 via RStudio v2023.09.153. This graph showcases the relative cover of both hard and soft corals on the reef that were surveyed before and after the anomalous event. To investigate the coral genera (including in some cases morphotypes) that contributed to the differences among the regions within each period, a SIMPER analysis was conducted using a Bray-Curtis dissimilarity index based on square root transformed data. All analyses were conducted in PRIMER-v754 with the PERMANOVA + extension55 unless otherwise noted.
Differences in the benthic community composition were further analyzed using a four-way Permutational Multivariate Analysis of Variance (PERMANOVA)56 on the following factors: region (three levels: NRS, CRS, SRS, ), period (three levels: 2014/2015, 2016/2017, 2018/2019), location (seven levels: Duba, Al Wajh, Thuwal, Jeddah, Al Lith, Farasan Banks, Farasan Islands) and reef (59 levels), based on the same dissimilarity matrix described above. The factor ‘region’ was fixed, and the rest of the factors were considered as random; ‘location’ was nested within ‘region’, and ‘reef’ was nested within ‘location’. To investigate comparisons within the different factors, pair-wise PERMANOVA comparisons were conducted in PRIMER-v7. To assess changes in the percentage of coral cover over time, the difference in coral cover was calculated for reefs sampled in more than one period and subsequently binned in the following categories indicating percent gain or loss in coral cover: +10 to 15%, + 6 to 10%, 0 to 5%, -1 to -5%, -6 to 10%, -11 to 15%, and ≤-16%. These reefs were plotted on a map according to the geographical location of the reef using the ArcGIS Pro software (Version 2.5.22) by Esri. To analyze the changes in coral cover per genus in the three regions, the species that contributed to the dissimilarity in the regional coral assemblages were selected according to the SIMPER analysis mentioned above.
Differences in thermal regimes (i.e. SST and DHW) were determined using a non-parametric Kruskal-Wallis rank sum tests with R’s base function kruskal.test and post-hoc Dunn’s tests using rank sums with the function dunn.test from the package of the same name (v1.3.557). Tests were carried out for the following factors: ‘year’ (seven levels: 2013/14, 2014/15, 2015/16, 2016/17, 2017/18, 2018/19, 2019/2020), ‘location’ (seven levels, see above) and year-location (49 levels; no post-hoc test was carried out for this interaction factor). To reduce zero-inflation, DHW data were subset to the months June through December for statistical tests (no heat stress occurred in any year at any of the reefs during January-May). Mean and standard deviation of SST and DHW for each location and year were calculated using R base functions. Data were formatted and visualized using the R packages reshape2 v1.4.458, ggpubr v0.4.059, gridExtra v2.360 and packages from the tidyverse collection v1.3.161. All analyses and visualizations of SST and DHW data were performed in R via RStudio and the code is found in Supplementary File 3.
Results
Thermal regimes
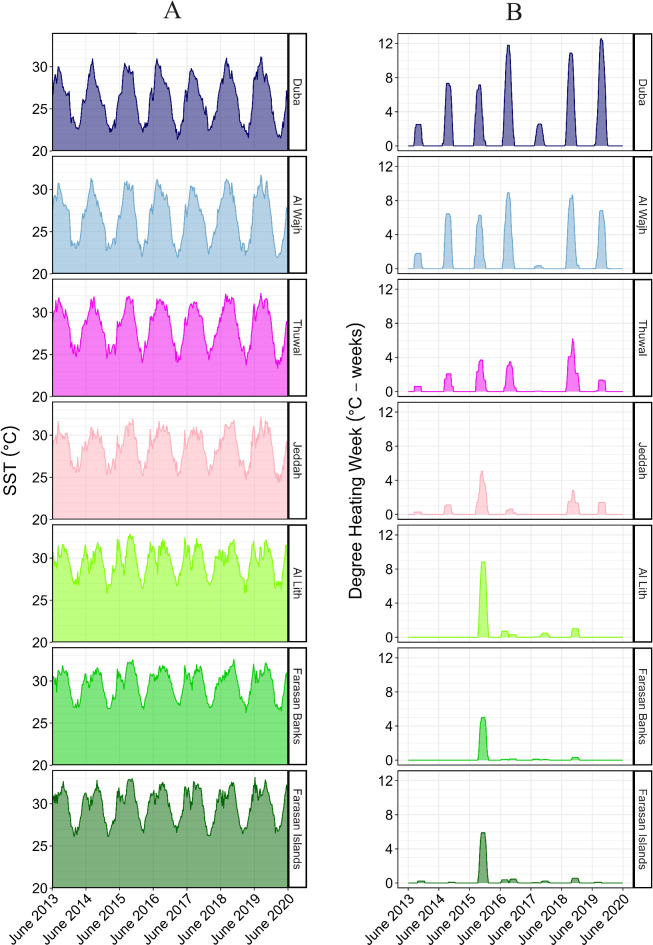
SST exhibited seasonal variability during the study period, with minima of ~ 21.5 °C in higher latitudes (i.e. in the NRS region in winter) and maxima of ~ 33.0 °C in lower latitudes (i.e. the SRS region in fall) (Fig. 2A). Annual SST variability within locations decreased from north to south, which was expected given generally more pronounced seasonality in higher latitudes62. In most years, locations towards the south showed a second minor SST peak in early summer before temperatures dropped and then increased again to the annual maximum (Fig. 2A).
Fig. 2.
Time series of Sea Surface Temperature (SST, A) and Degree Heating Weeks (DHW, B) between June 2013 and May 2020 for the seven locations examined in this study. Profiles represent the averages at each point in time for all surveyed reefs within each location.
Timing of northern-hemisphere summer fall temperature peaks showed a latitudinal trend, with northern locations exhibiting maxima in August/September and central and southern locations in October / November. Highest annual SST maxima were observed in the south (i.e., Farasan Islands and Farasan Banks). See Supplementary File 1 for the full SST data set. The year 2015/16 displayed the highest annual SST during the study period, with an average of 28.46 ± 2.72 °C. This year also represented the warmest period on average within each location, except for Thuwal and Jeddah (for which 2018/19 and 2016/17 were the warmest years, respectively). The mean SST during 2015/16 at Farasan Islands represents the sole incidence of our study locations exceeding an annual mean SST of 30 °C (30.23 ± 1.93 °C). The three SRS locations (i.e., Al Lith, Farasan Banks, Farasan Islands) exhibit a clear plateau of elevated SST in late summer/fall of 2015 (Fig. 2A). Though peaks of similar magnitude occurred in other years, they were ephemeral. The distinguishing factor in 2015 is the clear plateau of elevated temperatures lasting between one to two months occurring at all locations in the SRS (and to some extent in the CRS) (Fig. 2A; Supplementary File 1).
The SST profiles differed significantly among years (Kruskal-Wallis χ2 = 103.29, p < 0.001), and SST was significantly higher in 2015/16 than during the preceding two years and the following year (pairwise adjusted p < 0.001). Thermal conditions at the seven locations mirrored the separation of study sites into three regions. Sites at Duba and Al Wajh (i.e., NRS), Thuwal and Jeddah (i.e., CRS) and Al Lith, Farasan Banks and Farasan Islands (i.e., SRS), respectively, exhibited similar temporal trends in SST among them (Fig. 2A). This was supported by results of the statistical tests, where SST regimes displayed significant differences among locations during the entire study period (Kruskal-Wallis χ2 = 4371.60, p < 0.001). Pairwise comparisons supported the regional grouping of the study locations. Intra-regional differences in location SST were all non-significant, while inter-regional differences were significant in every case (all pairwise adjusted p < 0.001). SST profiles were significantly shaped by the interaction of the factors ‘location’ and ’year’ (p-value < 0.001) which was expected given the large sample size. See Supplementary Table S4 for detailed information on mean SST per location and year and Supplementary Material S2 for results of statistical tests.
The occurrence of heat stress measured as DHW showed marked differences among locations and years, although the general trend of DHW peaking in summer/fall at all locations was consistent (Fig. 2B). As with SST, locations in the north showed a DHW peak in August/September, while this peak shifted towards October/November with decreasing latitude (Fig. 2B). Duba exhibited the highest DHW across all locations in each summer/fall season during the study period (maxima ranged between 2.57 °C-weeks between August 31 and September 26, 2017 and 12.58 °C-weeks between September 15 and 18, 2019), with the exception of 2015/16 when Al Lith experienced the highest DHW (maximum of 8.82 °C-weeks between October 23 and December 1, 2015). Duba also showed highest overall mean DHW throughout the study period (3.07 ± 3.79 °C-weeks). Overall mean DHW consistently decreased towards the south, with the exception of the Farasan Banks which showed the lowest mean DHW (0.32 ± 1.05 °C-weeks). See Supplementary Table S5 for data on means per year and location. Total mean DHW per year across the entire Red Sea was highest in 2015/16 (2.33 ± 2.60 °C-weeks) and lowest in 2017/18 (0.22 ± 0.53 °C-weeks). All locations southwards from (and including) Jeddah experienced their highest heat stress during the study period in 2015/16. Remarkably, 2015/16 represents the only period of significant heat stress in the SRS (i.e. Al Lith, Farasan Banks and Farasan Islands) (Fig. 2B and Supplementary Table S5). See supplementary File 2 for the full DHW data set.
Benthic cover
A total of 106 living benthic categories were identified on the reefs surveyed, including 57 hard corals, 18 macroalgae, 13 soft corals, 11 other invertebrates, 5 taxa of other cnidarians, CCA and turf algae (Supplementary material Table S2). The main benthic categories with a relevant contribution in benthic percentage cover (i.e., ≥ 2%) in the reefs along the Saudi coast of the Red Sea were hard corals (17.5%±12.5), soft corals (7.58%±8.6), CCA (14.43%±13.1), turf algae (21.94%±12), macroalgae (6.61%±12.7), sand (21.01%±19.4), rubble (2.79%±5.9) and, pavement/rock (6.32%±9.9).
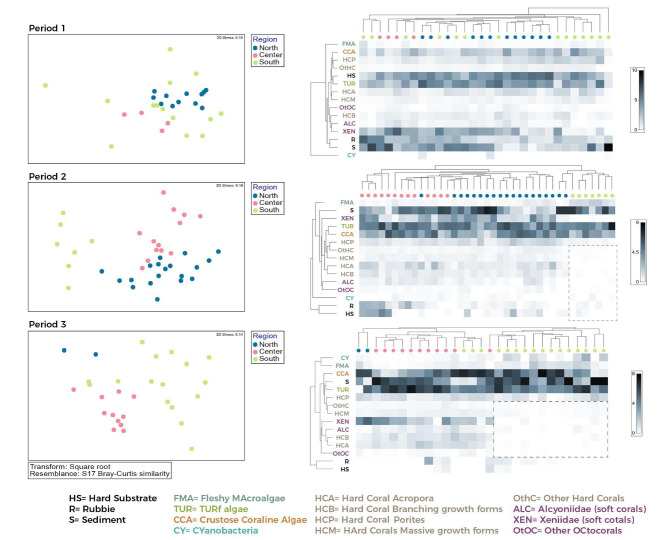
NMDS analysis showed that the benthic communities relatively homogeneous in composition and did not differ among locations before the bleaching event (Fig. 3). NRS and CRS locations generally clustered together, whereas those from the SRS showed the largest variability, with some reefs presenting high affinity to both central and northern regions. After the 2015 mass bleaching event (period 2), communities from the south had a different composition from those in the north and central regions (Table 1) and were clearly segregated as a unique region in the multivariate space based on our data (Fig. 3). However, three-four years post-bleaching (period 3), most of the reefs in the south were still generally distinct from reefs in the CRS and the NRS regions, although some southern reefs again showed affinity in composition to the northern and central locations (Fig. 3).
Table 1.
Results of four-way permutational multivariate analysis of variance (PERMANOVA) on factors region (R), period (P), locations (Lo) and reef (re), based on square-root transformed percentage benthic cover data and Bray Curtis similarity index. Asterisks indicate statistically significant values. Df = degrees of freedom, MF = mean of squares, Pseudo-F = variance ratio, P = significance level.
| Source | df | MS | Pseudo-F | P |
|---|---|---|---|---|
| Region, R | 2 | 14,519 | 2.1232 | 0.003* |
| Period, P | 2 | 22,596 | 13.232 | 0.001* |
| Locations (R), Lo | 4 | 4440.5 | 1.5244 | 0.037* |
| RxP | 4 | 5052.8 | 2.8748 | 0.006* |
| Reef (Lo(R)), Re | 52 | 2743.8 | 2.1389 | 0.001* |
| Lo (R) x P | 3 | 1656.8 | 1.2989 | 0.197 |
| Reef (Lo(R)) x P | 30 | 1263.7 | 4.5889 | 0.001* |
Fig. 3.
Non-metric multidimensional Scaling Plot (NMDS) of the coral reef benthic communities, for the three geographical regions: NRS (blue), CRS (pink) and SRS (green); (A) Period 1: 2014/2015, (B) Period 2: 2016/2017, (C) Period 3: 2018/2019. Hierarchical cluster analysis using the Bray-Curtis dissimilarity index for seven different locations (DU: Duba, AW: Al Wajh, TH: Thuwal, JD: Jeddah, AL: Al Lith, FI: Farasan Islands, FB: Farasan Banks) in three periods (Period 1: 2014/2015, Period 2: 2016/2017, Period 3: 2018/2019.). Heatmaps show the percentage cover of the benthic categories with a cover greater than 2% (hard corals, soft corals, CCA turf algae, algae sand, rubble, pavement/rock); the darker the shade, the higher the percentage cover.
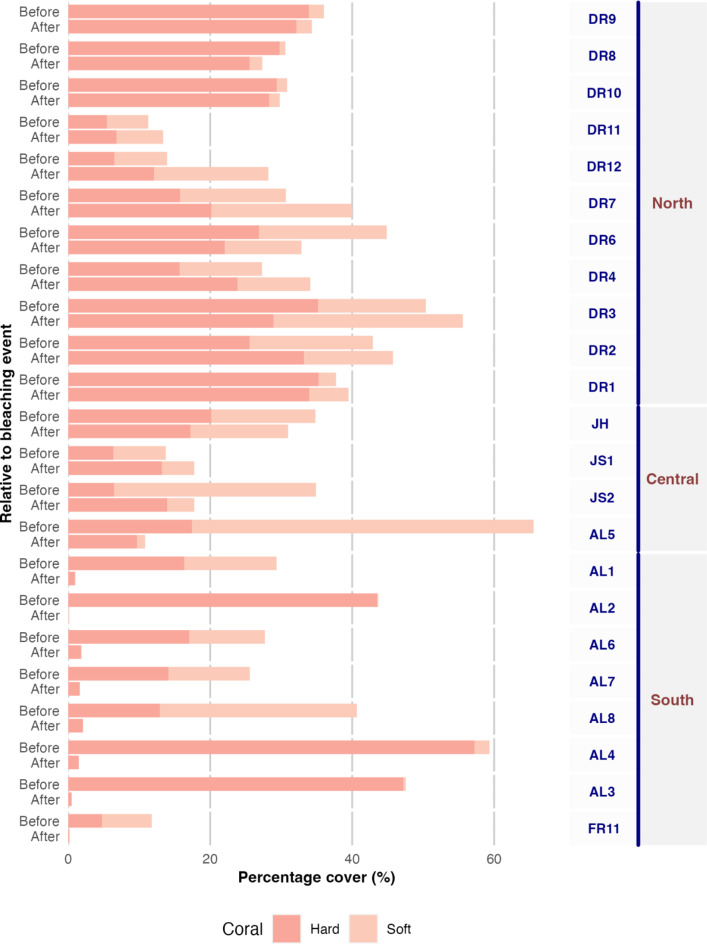
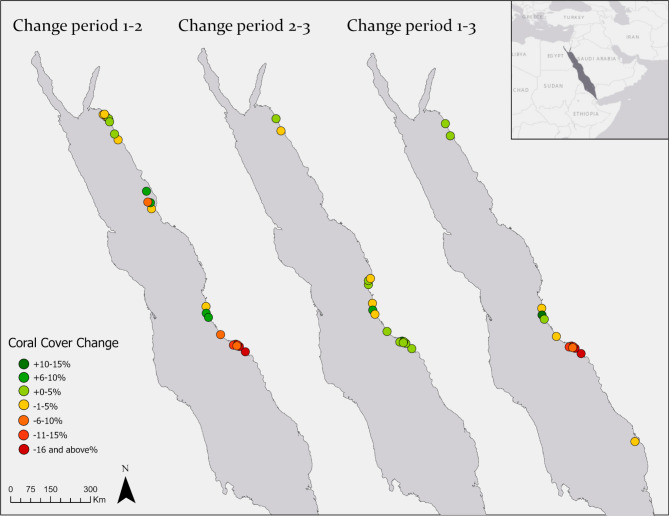
In period 1, the benthic communities in the south were dominated by hard and soft corals (Fig. 4). In period 2 (post-bleaching) the abundance and richness in corals decreased, and 15 hard coral categories disappeared completely, including Porites branching, Porites encrusting, Psammocora, Euphyllia, and Plerogyra (Fig. 3). In contrast, macroalgal percent cover increased, particularly the genera Dyctiota, Lobophora, and Peyssonnelia; turf algae also increased in percent cover, though to a lesser degree. When analyzing the magnitude of change among periods, the SRS reefs were the most affected, showing limited recovery four years after the bleaching (Fig. 5).
Fig. 4.
Soft and hard coral cover before and after the 2015–2016 bleaching event among the three regions. The bars show relative cover, and codes to the right represent the reef surveyed before and after the bleaching order latitudinally.
Fig. 5.
Changes in hard coral cover at the study locations along the Saudi Arabian Red Sea coast. Colored points indicate the locations where an increase (shades of green) or decrease (from yellow to dark red) in coral cover was recorded across the different periods. (a) From period 1 (2014/2015) to period 2 (2016/2017), (b) Form period 2 (2016/2017) to period 3 (2018/2019), and (c) from period 1(2014/2015) to period 3 (2018/2019). Created using the ArcGIS Pro software (Version 2.5.22).
Significant statistical differences in benthic composition were found among regions (Pseudo-F = 2.1216, P = 0.007), periods (Pseudo-F = 13.224, P = 0.001), and zones (Pseudo-F = 1.5249, P = 0.032) (Table 1). Interaction effects between region and period (Pseudo-F = 2.8754, P = 0.009) and between reef and period (Pseudo-F = 4.6073, P = 0.001) became apparent with the PERMANOVA analysis.
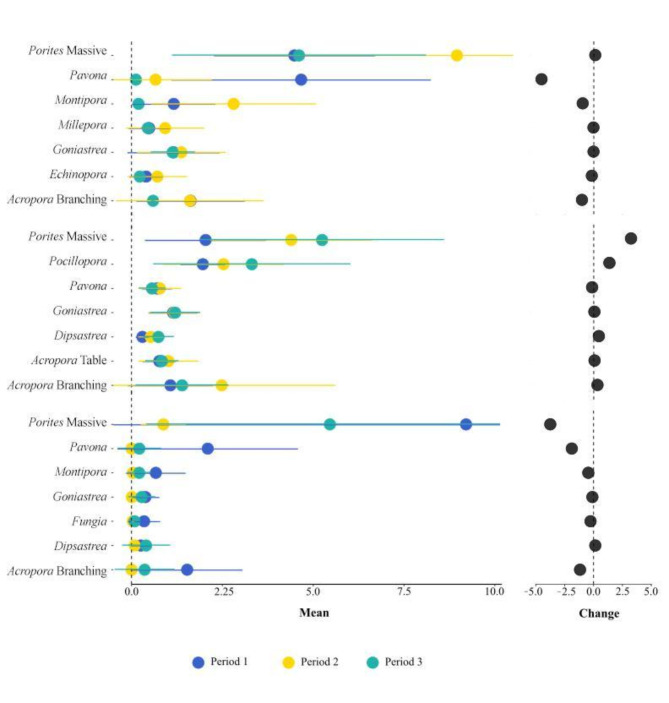
In the NRS, the most affected (i.e. biggest decrease in percent cover) genera were Pavona, Acropora branching, and Montipora (Fig. 6). In the CRS region, the genera with the highest decrease in coral cover recorded were Pavona, and Acropora table, although there was an increase in the relative proportion of Porites massive. In the SRS, Pavona, Acropora branching, and Montipora showed the largest reductions, but here we also assisted to a decrease in cover of Porites massive; they persisted in period 3, while the rest of the genera on the list disappeared in this period (Fig. 6).
Fig. 6.
Differences in percentage of cover of the coral genera per region in the three study periods represented by different colors (period 1: 2014/2015; period 2: 2016/2017; period 3: 2018/2019). Coral genera were selected based on SIMPER analysis (Supplementary Table S3). Colored dots represent the average and the lines the standard deviation. Black dots on the right represent the overall changes in percentage cover between periods 3 and 1.
Discussion
This study documented latitudinal and temporal changes in the composition and structure of coral reef benthic communities over a 5-year period encompassing the 2015–2016 mass bleaching event along the Saudi Arabian coast of the Red Sea. The results show that Red Sea coral reef benthic communities responded differently to a mass bleaching event dependent on the environmental context they are set in. This study also highlights the value of in situ biological data regards to assessing the effects and recovery trajectory of bleaching events. Indeed, we show here a strong mismatch between the predictions of bleaching using DHW metrics and observations, reinforcing the synergistic effects between SST and other variables, like nutrients, triggering bleaching as previously reported by DeCarlo et al.41 in the Red Sea.
Interestingly, responses of coral genera appear to be region-specific; although some genera seem to generally be more sensitive (e.g., Pavona, Montipora, and Acropora branching), or more resistant (e.g., Porites massive), this does not hold true across all sites or regions. The nearshore reefs of the most affected region (SRS) did not show clear signs of recovery four years after the mass bleaching event. The variability in coral bleaching across different sites, where some corals are affected while others remain unscathed, can be attributed to multiple factors. This diversity in bleaching patterns suggests that the phenomenon is influenced by a complex interplay of elements, probably including water temperature, water quality, light intensity, local adaptation of coral organisms and acclimatization, individual thermal history, and the type of symbiotic zooxanthellae present63,64.
The increased CCA cover reported in this study may signify an encouraging possibility for coral reef regeneration, as CCA plays a pivotal role in fortifying the reef substrate through the secretion of calcium carbonate, and is linked to the settlement and attachment of coral larvae65,66. This calcified deposit provides an anchor for coral development and acts as a protective shield, mitigating the effects of physical erosion67. Even so, any potential for positive outcomes are counterbalanced by the influence of human-induced pressures. Factors such as the overfishing of reef herbivores and nutrient enhancement can impede these beneficial effects, thus hindering coral reef recovery68.
Region-specific responses of Red Sea benthic communities to the 2015/2016 mass bleaching event
Investigating the patterns of variability in coral reef benthic communities through space and time, we found a region-specific response that aligned with the prevailing SST trends and the known productivity patterns in the region45. Specifically, benthic communities from the southern Red Sea were characterized by high coral cover (25.9%±19.4 in period 1), a low cover of fleshy macroalgae (3.1%± 6.0), and a substantial cover of soft corals (8.0%±8.4) before the bleaching event, but were severely affected by the 2015/2016 mass bleaching event. This result based on in situ photo transects supports the findings by DeCarlo et al.41 The authors detected a higher intensity of the 2015/2016 bleaching event in the southern Red Sea compared to previous bleaching events affecting the Red Sea region (i.e., 1982, 1995, and 2002), hence a region-specific signature of these events. Although the SRS region is known for its comparatively high primary productivity due to Gulf of Aden Intermediate Water (GAIW) intrusion69,70 and monsoon-driven upwelling71,72, corals are still very sensitive to the synergistic effects of both temperature and nutrient stressors when above certain thresholds. Indeed, it seems that the combination of thermal anomalies and an increase in nutrients from an early upwelling caused by the effects of El Niño may determine the intensity of bleaching events in the region41,72. In other words, corals in the Red Sea can resist an increase in SST if not accompanied by an increase in nutrients, as observed in 1998 or 2010 in the southern Red Sea41. Another study reported that environmental pressures like heat stress and higher levels of dissolved organic carbon (DOC) could potentially accelerate the breakdown of the coral-algae symbiotic by boosting the rates of nitrogen fixation linked to corals73.
In this study, a relatively high turf cover was also recorded in period 1 (16.6%), and it increased after the bleaching event (24.1%); this trend was strongest in the SRS which increased from 16.5% in period 1 to 28.4% in period 2). Although there are several factors related to the decline and loss of coral species, including coral disease, invasive species, and climate change, PERSGA31 reports that major threats in this region are primarily linked to human pressures such as overfishing, coastal development, population expansion, and industrial pollution. Thus, the observed increase in turf algae cover may be a part of the broader pattern of ecological shifts driven by these anthropogenic factors and not an outcome of the bleaching event. It is important to note that these factors can contribute to decrease the tolerance of coral species to additional stressors like those related to increased SST usually associated with bleaching events.
The most remarkable, yet expected, response to the 2015/2016 bleaching event was a significant decrease in coral cover. Particularly for hard corals (both branching and massive forms demonstrated clear reductions, showing a 20-fold drop in cover (from 25.8 to 1.2%). A similar, though less drastic, decrease in soft coral cover was also detected (from 8 to 0.3%). Such sharp decreases in coral cover have been reported in response to bleaching events worldwide6,74,75, followed by either an increase in turf or macroalgae (an increase from 15.3 to 25.9%, and 3.1–34.8%, respectively for the present study)76,77.
Even though Red Sea coral reef communities have widely been considered resilient, namely due to their high recruitment rates78,79, more frequent bleaching events in the region may hinder their potential for recovery, as observed elsewhere16,80. This can be particularly true for nearshore reefs that are more affected by the bleaching events due to, among other stressors, more drastic variation in SST than offshore reefs81. Indeed, considering that four years after bleaching the percent cover of hard corals was about 12% of the pre-bleaching levels ( pre-bleaching: 29.8%±18.8%; four years post-bleaching: 3.6%±3.2%), signs of solid recovery were not detected. Corals in the southern Red Sea may face a serious threat as mass bleaching events are expected to occur on average every 5.9 years globally6. Nevertheless, though slow, signs of recovery were noticed. Average hard coral cover almost doubled in two years (period 2: 1.2 ± 0.8%; period 3: 3.6%±3.2%). It is important to note that such signs of recovery ere not homogenous, as clarified by the high standard deviation.
Taxa susceptibility to bleaching: toward a prevalence of massive forms with a loss of rapid growing branching corals
During a heat stress event, coral species will respond differently to changes in environmental conditions. In some cases, high plasticity may be observed, and response patterns may not be consistent across reef habitats or across a shelf gradient82. In general, branching corals are considered more prone to bleaching and subsequent mortality83–86. A similar pattern has been detected in previous studies in the Red Sea32,34 and is supported by our findings. A previous study on the Saudi Arabian Red Sea coast showed that the coral genera more sensitive to changes in environmental conditions were indeed mainly genera with branching growth forms, such as Acropora, Stylophora, Seriatopora, and some species of Porites87. In our study, Porites, Pocillopora, Pavona, and tabular and branching Acropora were the most abundant corals in the south in the first sampling period contributing to ~ 12% total cover. After the bleaching event, these genera specifically (and all branching corals in general) were severely affected and almost disappeared, confirming the growth form’s sensitivity to bleaching. In our study, the massive Porites category was the main driver of the community composition. Consistent levels (and a general dominance) of coverage highlights its relative tolerance to global warming effects. This is supported by a previous study in the Great Barrier Reef, which found that acroporids bleach faster and present a higher whole-colony mortality rate, while species such as Platygyra daedalea and Porites lobata, both massive species, were characterized by a later onset of bleaching and partial mortality88.
Changes in benthic community composition does not necessarily correspond with heat stress metrics
It has previously been shown that the occurrence of widespread coral bleaching and heat stress in the northern Red Sea have so far been disconnected from each other43. The present study also showed a discrepancy between heat stress and observed changes in benthic reef communities. Although showing constantly high heat stress each summer during the study period, reefs in the northern Red Sea experienced changes in benthic cover on a much lower scale compared to reefs in the central and southern regions (with the exceptions of some reefs at Al Wajh). These observations are in line with the proposal of the northern Red Sea as a thermal refuge for scleractinian corals42,43. However, this region is not without its own unique set of environmental stressors. Intense coastal development in the region associated with two major development projects (NEOM, and the Red Sea Global89,90) which can affect turbidity, nutrient availability, and local hydrodynamics, all of which have been shown to influence the response of corals to bleaching91–93. Temperature variability patterns experienced by corals can, in some cases, be more relevant to the susceptibility of corals than the maximum temperature populations experience over a certain period of time92–95. Significant coral cover decline in some of the CRS and SRS locations observed here may indicate that corals in these areas are already close to their thermal tolerance thresholds, while this may not be the case yet for corals in the NRS43. However, it remains unclear for how long reefs in the NRS will be able to withstand widespread bleaching and coral cover loss in the future given the already high rates of ocean warming in the north96.
The DHW peaks particularly in the southern locations combined with the early summer upwelling / GAIW-intrusion most likely played a major part in the bleaching pattern observed in the southern Red Sea, as reported by DeCarlo et al.41,72. Upwelling in the southern Red Sea in June to August 2015 was the strongest recorded since the 1980s but ceased earlier than usual, leading to relatively low SST during the upwelling but a longer than usual subsequent warming period41,72. This strong upwelling of GAIW also led to comparatively high nutrient levels in otherwise oligotrophic waters where (sub-) tropical coral reefs thrive, another major stressor for corals41. The combination of a long and intense warming period and a strong upwelling event leading to high ambient nutrient levels are most probably what caused bleaching in southern-central and southern Saudi Arabian reefs in 2015 and potentially the subsequent coral cover decline observed at these sites.
Conclusions
This study investigated the response of benthic communities along a latitudinal gradient on the Saudi coast of the Red Sea. We observed a significant regional variation in the effects of the mass bleaching event in 2015–2016. The southern region was the most affected, showing a major decline in hard coral cover, a major loss of branching corals, and a shift in the benthic composition towards an algal (macro- and turf) community, accompanied by a loss in coral diversity. In contrast, the northern and central reefs registered a small decline in coral cover (1–5%), and in some cases coral cover remained stable or even slightly increased.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the personnel from the Coastal and Marine Resources Core Lab from King Abdullah University of Science and Technology for logistical support, as well as to the crews of RV Thuwal and Dream Divers. Special thanks go to Alexandra Dempsey and Holger Anlauf for contributing to some of the benthic identifications, Joao Curdia for helping with the data analysis and Fig. 4, and Dylan Cottrell for his assistance in editing the writing of this manuscript. We also thank everyone at KAUST who contributed to the fieldwork over the last years supporting this time-series.
Author contributions
SC planned the study and coordinated it with MB. DLC participated in field surveys. KG, FB, ND and SC conducted the data analysis. KG and SC wrote the main manuscript text. All authors reviewed and approved the final version of the manuscript.
Data availability
Data generated or analyzed during this study are included in this published article (and its Supplementary Information files). Additional data are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kutser, T., Dekker, A. G. & Skirving, W. Modeling spectral discrimination of Great Barrier Reef benthic communities by remote sensing instruments. Limnol. Oceanogr.48, 497–510 (2003). [Google Scholar]
- 2.Hughes, T. P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 265, 1547–1551. 10.1126/science.265.5178.1547 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Bellwood, D. R., Hughes, T. P., Folke, C. & Nyström, M. Confronting the coral reef crisis. Nature. 429 (6994), 827–833. 10.1038/nature02691 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science. 301, 929–933 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature. 543, 373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 359, 80–83. 10.1126/science.aan8048 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Skirving, W. J. et al. The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs. 38, 547–557. 10.1007/s00338-019-01799-4 (2019). [Google Scholar]
- 8.Caldeira, K. Coral Bleaching: Coral Refugia amid heating seas. Nat. Clim. Change. 3, 444–445. 10.1038/nclimate1888 (2013). [Google Scholar]
- 9.Chollett, I., Mumby, P. J. & Cortés, J. Upwelling areas do not guarantee refuge for coral reefs in a warming ocean. Mar. Ecol. Prog Ser.416, 47–56. 10.3354/meps08775 (2010). [Google Scholar]
- 10.Van Hooidonk, R., Maynard, J. A. & Planes, S. Temporary refugia for coral reefs in a warming world. Nat. Clim. Change. 3, 508–511. 10.1038/nclimate1829 (2013). [Google Scholar]
- 11.Kim, S. W. et al. Refugia under threat: Mass bleaching of coral assemblages in high-latitude eastern Australia. Glob Change Biol.25, 3918–3931. 10.1111/gcb.14772 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Villalobos, R. et al. Inter-annual variability patterns of reef cryptobiota in the central Red Sea across a shelf gradient. Sci. Rep.12 (1), 16944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowlton, N. The future of coral reefs. Proc. Nat. Acad. Sci.98, 5419–5425 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camp, E. F. et al. The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front. Mar. Sci.5, 1–21. 10.3389/fmars.2018.00004 (2018).29552559 [Google Scholar]
- 15.Sully, S., Hodgson, G. & van Woesik, R. Present and future bright and dark spots for coral reefs through climate change. Glob Change Biol.28, 4509–4522. 10.1111/gcb.16083 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker, A. C., Glynn, P. W. & Riegl, B. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast Shelf Sci.80, 435–471. 10.1016/j.ecss.2008.09.003 (2008). [Google Scholar]
- 17.Muscatine, L. & Porter, J. W. Reef corals: Mutualistic Symbioses adapted to nutrient-poor environments. Bioscience. 27, 454–460 (1977). [Google Scholar]
- 18.Anthony, K. R. N., Hoogenboom, M. O., Maynard, J. A., Grottoli, A. G. & Middlebrook, R. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct. Ecol.23, 539–550. 10.1111/j.1365-2435.2008.01531.x (2009). [Google Scholar]
- 19.Heron, S. F., Maynard, J. A., van Hooidonk, R. & Eakin, C. M. Warming trends and bleaching stress of the World’s Coral Reefs 1985–2012. Sci. Rep.6, 1–14. 10.1038/srep38402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuart-Smith, R. D., Brown, C. J., Ceccarelli, D. M. & Edgar, G. J. Ecosystem restructuring along the great barrier reef following mass coral bleaching. Nature. 560, 92–96. 10.1038/s41586-018-0359-9 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Pratchett, M. S., Hoey, A. S., Wilson, S. K., Messmer, V. & Graham, N. A. J. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity. 3, 424–452. 10.3390/d3030424 (2011). [Google Scholar]
- 22.McManus, J. W. & Polsenberg, J. F. Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr.60, 263–279. 10.1016/j.pocean.2004.02.014 (2004). [Google Scholar]
- 23.Smith, J. E. et al. Re-evaluating the health of coral reef communities: Baselines and evidence for human impacts across the central pacific. Proc. Royal Soc. B. 283, 20151985, (2016). 10.1098/rspb.2015.1985 [DOI] [PMC free article] [PubMed]
- 24.Bellwood, D. R., Streit, R. P., Brandl, S. J. & Tebbett, S. B. The meaning of the term ‘function’in ecology: a coral reef perspective. Funct. Ecol.33, 948–961 (2019). [Google Scholar]
- 25.Brandl, S. J. et al. Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front. Ecol. Environ.17, 445–454 (2019). [Google Scholar]
- 26.Pratchett, M. S. et al. Effects of climate-induced coral bleaching on coral-reef fishes. Ecological and economic consequences. Oceanogr. Mar. Biology: Annual Rev.46, 251–296 (2008). [Google Scholar]
- 27.Yasir Haya, L. O. M. & Fujii, M. Assessing economic values of coral reefs in the Pangkajene and Kepulauan Regency, Spermonde Archipelago, Indonesia. J. Coast Conserv.23, 699–711. 10.1007/s11852-019-00700-3 (2019). [Google Scholar]
- 28.Lin, Y. J. et al. Long-term ecological changes in fishes and macro-invertebrates in the world’s warmest coral reefs. Sci. Total Environ.750, 142254. 10.1016/j.scitotenv.2020.142254 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Carvalho, S., Kürten, B., Krokos, G., Hoteit, I. & Ellis, J. World seas: An environmental. 2nd edition. Volume II: The Indian Ocean to the Pacific. In The Red Sea. In World seas: An Environmental Evaluation (ed. Sheppard, C.) 49–74 (Elsevier, 2019).
- 30.Berumen, M. L. et al. The status of coral reef ecology research in the Red Sea. Coral Reefs. 32, 737–748 (2013). [Google Scholar]
- 31.PERSGA. The Status of Coral Reefs in the Red Sea and Gulf of Aden: 2009, Technical Series Number 16. Jeddah, p. 125. (2010).
- 32.Furby, K. A., Bouwmeester, J. & Berumen, M. L. Susceptibility of central Red Sea corals during a major bleaching event. Coral Reefs. 32, 505–513. 10.1007/s00338-012-0998-5 (2013). [Google Scholar]
- 33.Lozano-Cortes, D. et al. Coral bleaching report-Saudi Arabia (the Red Sea and the Arabian Gulf). Reef. Encount. 31, 50–52 (2016). [Google Scholar]
- 34.Monroe, A. A. et al. In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015 / 2016 global coral bleaching event. PLoS One. 13, e0195814 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich, W. A., Carvalho, S. & Berumen, M. L. Coral bleaching due to cold stress on a central Red Sea reef flat. Ecol. Evol.12, e9450. 10.1002/ece3.9450 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genevier, L. G. C., Jamil, T., Raitsos, D. E., Krokos, G. & Hoteit, I. Marine heatwaves reveal coral reef zones susceptible to bleaching in the Red Sea. Glob Change Biol.25, 2338–2351. 10.1111/gcb.14652 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Juza, M., Fernández-Mora, À. & Tintoré, J. Sub-regional marine heat waves in the Mediterranean Sea from observations: long-term surface changes, sub-surface and coastal responses. Front. Mar. Sci.9, 785771 (2022). [Google Scholar]
- 38.Oliver, E. C. J. et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun.9, 1–12. 10.1038/s41467-018-03732-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berumen, M. L. et al. Springer,. The Red Sea: Environmental Gradients Shape a Natural Laboratory in a Nascent Ocean. In Coral Reefs of the Red Sea (eds Voolstra, M.L., Berumen, M.L) 1–10 (2019).
- 40.Eakin, C. M., Sweatman, H. P. A. & Brainard, R. E. The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs. 38, 539–545. 10.1007/s00338-019-01844-2 (2019). [Google Scholar]
- 41.DeCarlo, T. M. et al. Nutrient-supplying ocean currents modulate coral bleaching susceptibility. Sci. Adv.6, 1–8. 10.1126/sciadv.abc5493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fine, M., Gildor, H. & Genin, A. A coral reef refuge in the Red Sea. Glob Change Biol.19, 3640–3647. 10.1111/gcb.12356 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Osman, E. O. et al. Thermal refugia against coral bleaching throughout the northern Red Sea. Glob Change Biol.24, e474–e484. 10.1111/gcb.13895 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Carvalho, S. et al. Beyond the visual: using metabarcoding to characterize the hidden reef cryptobiome. Proc. R Soc. B Biol. Sci.286, 20182697. 10.1098/rspb.2018.2697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raitsos, D. E., Pradhan, Y., Brewin, R. J. W., Stenchikov, G. & Hoteit, I. Remote sensing the Phytoplankton Seasonal Succession of the Red Sea. PLoS ONE. 8, e64909. 10.1371/journal.pone.0064909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NASA OPBG.MODIS Aqua Global Level 3 Mapped SST & Ver 0. Accessed on November 28, 2021. (2019). 10.5067/MODAM-8D4N9 (2020).
- 47.NOAA Coral Reef Watch (CRW). NOAA Coral Reef Watch Operational Daily Near-Real-Time Global 5-km Satellite Coral Bleaching Monitoring Products. Version 3.1. Subset 2013-06-01–2020-05-31; 12–30 °N, 32–44 °E. Distributed by the Pacific Islands Ocean Observing System (PacIOOS). Http://Pacioos.Org/Metadata. updated 2020). (2017).
- 48.Liu, G., Strong, A. E. & Skirving, W. Remote sensing of sea surface temperatures during 2002 Barrier Reef coral bleaching. EOS84, 137–141, (2003). 10.1029/2003EO150001
- 49.Kohler, K. E. & Gill, S. M. Coral Point Count with Excel extensions (CPCe): a visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci.32, 1259–1269. 10.1016/j.cageo.2005.11.009 (2006). [Google Scholar]
- 50.Urbina-Barreto, I. et al. Which method for which purpose? A comparison of line intercept transect and underwater photogrammetry methods for coral reef surveys. Front. Mar. Sci.8, 636902. 10.3389/fmars.2021.636902 (2021). [Google Scholar]
- 51.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-, 2016). https://ggplot2.tidyverse.org
- 52.R Core Team. _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria, (2023). https://www.R-project.org/
- 53.Posit team & Boston, M. A. RStudio: Integrated Development Environment for R. (Posit Software (PBC, 2023). http://www.posit.co/
- 54.Clarke, K. R. & Gorley, R. N. PRIMER version7:user manual/tutorial. (PRIMER-E192, (2015).
- 55.Anderson, M., Gorley, R. N. & Clarke, R. K. Permanova + for Primer: Guide to Software and Statistical Methods (Primer-E Ltd, 2008).
- 56.Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol.26, 32–46. 10.1111/j.1442-9993.2001.01070.pp.x (2001). [Google Scholar]
- 57.Dinno A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R package version 1.3.5. (2017). https://cran.r-project.org/package=dunn.test
- 58.Wickham, H. Reshaping data with the reshape Package. J. Stat. Softw.2110.18637/jss.v021.i12 (2007).
- 59.Kassambara, A. & ggpubr ggplot2 Based Publication Ready Plots. R package version 0.4.0. (2020). https://cran.r-project.org/package=ggpubr
- 60.Baptiste, A. gridExtra: Miscellaneous Functions for Grid Graphics. R package version 2.3. (2017). https://CRAN.R-project.org/package=gridExtra
- 61.Wickham, H. et al. Welcome to the Tidyverse. J. Open. Source Softw.4, 1686. 10.21105/joss.01686 (2019). [Google Scholar]
- 62.Shaltout, M. Recent sea surface temperature trends and future scenarios for the Red Sea. Oceanologia. 61, 484–504 (2019). [Google Scholar]
- 63.J Barshis, D. Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol. Ecol.30 (18), 4466–4480 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Tagliafico, A., Baker, P., Kelaher, B., Ellis, S. & Harrison, D. The effects of shade and light on corals in the context of coral bleaching and shading technologies. Front. Mar. Sci.9, 919382 (2022). [Google Scholar]
- 65.Mallela, J. & Perry, C. T. Calcium carbonate budgets for two coral reefs affected by different terrestrial runoff regimes, Rio Bueno, Jamaica. Coral Reefs. 26, 129–145 (2007). [Google Scholar]
- 66.Webster, N. S. et al. Metamorphosis of a scleractinian coral in response to microbial biofilms. App Environ. Microbiol.70, 1213–1221 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gove, J. M. et al. Coral reef benthic regimes exhibit non-linear threshold responses to natural physical drivers. Mar. Ecol. Prog Ser.522, 33–48 (2015). [Google Scholar]
- 68.Koester, A. et al. Early trajectories of benthic coral reef communities following the 2015/16 coral bleaching event at remote Aldabra Atoll, Seychelles. Sci. Rep.10, 17034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dreano, D., Raitsos, D. E., Gittings, J., Krokos, G. & Hoteit, I. The gulf of aden intermediate water intrusion regulates the southern Red Sea summer phytoplankton blooms. PLoS ONE. 11. 10.1371/journal.pone.0168440 (2016). [DOI] [PMC free article] [PubMed]
- 70.Sofianos, S. S. & Johns, W. E. Observations of the summer Red Sea circulation. J. Geophys. Res. Oceans. 11210.1029/2006JC003886 (2007).
- 71.Churchill, J. H., Bower, A. S., McCorkle, D. C. & Abualnaja, Y. The transport of nutrient-rich Indian Ocean water through the Red Sea and into coastal reef systems. J. Mar. Res.72, 165–181 (2014). [Google Scholar]
- 72.DeCarlo, T. M. et al. Patterns, drivers, and ecological implications of upwelling in coral reef habitats of the southern Red Sea. J. Geophys. Res. Oceans. 126, e2020JC016493 (2021). [Google Scholar]
- 73.Rädecker, N., Pogoreutz, C., Voolstra, C. R., Wiedenmann, J. & Wild, C. Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol.23, 490–497 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Miller, M. Ecology of Anguilliform Leptocephali: remarkable transparent fish larvae of the Ocean Surface Layer. Aqua-BioSci Monogr.2, 1–94. 10.5047/absm.2009.00204.0001 (2009). [Google Scholar]
- 75.Moore, J. A. Y. et al. Unprecedented Mass Bleaching and loss of Coral across 12 6 of Latitude in Western Australia in 2010–11. PLoS One. 7, e51807. 10.1371/journal.pone.0051807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paddack, M. J. & Sponaugle, S. Recruitment and habitat selection of newly settled Sparisoma viride to reefs with low coral cover. Mar. Ecol. Prog Ser.369, 205–212. 10.3354/meps07632 (2008). [Google Scholar]
- 77.Bruno, J. F., Sweatman, H., Precht, W. F., Selig, E. R. & Schutte, V. G. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology. 90, 1478–1484 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Riegl, B. M., Bruckner, A. W., Rowlands, G. P., Purkis, S. J. & Renaud, P. Red Sea coral reef trajectories over 2 decades suggest increasing community homogenization and decline in coral size. PLoS One. 7, e38396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rowlands, G. et al. Satellite imaging coral reef resilience at regional scale. A case-study from Saudi Arabia. Mar. Pollut Bull.64, 1222–1237 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Lambo, A. L. & Ormond, R. F. G.Continued post-bleaching decline and changed benthic community of a Kenyan coral reef. Mar. Pollut Bull.52, 1617–1624 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Falter, J. L. et al. Assessing the drivers of spatial variation in thermal forcing across a nearshore reef system and implications for coral bleaching. Limnol. Oceanogr.59, 1241–1255. 10.4319/lo.2014.59.4.1241 (2014). [Google Scholar]
- 82.Rich, W. A. et al. Size structure of the coral Stylophora pistillata across reef flat zones in the central Red Sea. Sci. Rep.12, 13979. 10.1038/s41598-022-17908-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burn, D., Hoey, A. S., Matthews, S., Harrison, H. B. & Pratchett, M. S. Differential bleaching susceptibility among coral taxa and colony sizes, relative to bleaching severity across Australia’s great barrier reef and Coral Sea Marine Parks. Mar. Pollut Bull.191, 114907 (2023). [DOI] [PubMed] [Google Scholar]
- 84.Loya, Y. et al. Coral bleaching: the winners and the losers. Ecol. Lett.4, 122–131. 10.1046/j.1461-0248.2001.00203.x (2001). [Google Scholar]
- 85.Matsuda, S. B. et al. Coral Bleaching susceptibility is predictive of subsequent mortality within but not between Coral species. Front. Ecol. Evol.8, 178. 10.3389/fevo.2020.00178 (2020). [Google Scholar]
- 86.van Woesik, R., Sakai, K., Ganase, A. & Loya, Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog Ser.434, 67–76. 10.3354/meps09203 (2011). [Google Scholar]
- 87.Ellis, J. I. et al. Multiple stressor effects on coral reef ecosystems. Glob Change Biol.25, 4131–4146. 10.1111/gcb.14819 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Baird, A. H. & Marshall, P. A. Mortality, growth and reproduction in scleractinian corals following bleaching on the great barrier reef. Mar. Ecol. Prog Ser.237, 133–141 (2002). [Google Scholar]
- 89.Chalastani, V. I. et al. Reconciling Tourism Development and Conservation outcomes through Marine spatial planning for a Saudi giga-project in the Red Sea (the Red Sea Project, Vision 2030). Front. Mar. Sci.7, 168. 10.3389/fmars.2020.00168 (2020). [Google Scholar]
- 90.Gajdzik, L. et al. A portfolio of climate-tailored approaches to advance the design of marine protected areas in the Red Sea. Glob Change Biol.27, 3956–3968. 10.1111/gcb.15719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maina, J., McClanahan, T. R., Venus, V., Ateweberhan, M. & Madin, J. Global gradients of coral exposure to environmental stresses and implications for local management. PLoS One. 6, e23064 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McClanahan, T. R. et al. Temperature patterns and mechanisms influencing coral bleaching during the 2016 El Niño. Nat. Clim. Chang.9, 845–851. 10.1038/s41558-019-0576-8 (2019). [Google Scholar]
- 93.McClanahan, T. R. et al. Highly variable taxa-specific coral bleaching responses to thermal stresses. Mar. Ecol. Prog Ser.648, 135–151. 10.3354/meps13402 (2020). [Google Scholar]
- 94.Safaie, A. et al. High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun.9, 1671. 10.1038/s41467-018-04074-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sully, S., Burkepile, D. E., Donovan, M. K., Hodgson, G. & van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun.10, 1264. 10.1038/s41467-019-09238-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaidez, V., Dreano, D., Agusti, S., Duarte, C. M. & Hoteit, I. Decadal trends in Red Sea maximum surface temperature. Sci. Rep.7, 8144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are included in this published article (and its Supplementary Information files). Additional data are available from the corresponding author on reasonable request.