Abstract
Genetically susceptible C57BL/6 (B6) mice that are infected with the LP-BM5 isolate of murine retroviruses develop profound splenomegaly, lymphadenopathy, hypergammaglobulinemia, terminal B-cell lymphomas, and an immunodeficiency state bearing many similarities to the pathologies seen in AIDS. Because of these similarities, this syndrome has been called murine AIDS (MAIDS). We have previously shown that CD154 (CD40 ligand)-CD40 molecular interactions are required both for the initiation and progression of MAIDS. Thus, in vivo anti-CD154 monoclonal antibody (MAb) treatment inhibited MAIDS symptoms in LP-BM5-infected wild-type mice when either a short course of anti-CD154 MAb treatment was started on the day of infection or a course was initiated 3 to 4 weeks after LP-BM5 administration, after disease was established. Here, we further characterize this required CD154-CD40 interaction by a series of adoptive transfer experiments designed to elucidate which cellular subsets must express CD154 or CD40 for LP-BM5 to induce MAIDS. Specifically with regard to CD154 expression, MAIDS-insusceptible B6 nude mice reconstituted with highly purified CD4+ T cells from wild-type, but not from CD154 knockout, B6 donors displayed clear MAIDS after LP-BM5 infection. In contrast, nude B6 recipients that received CD8+ T cells from wild-type B6 donors did not develop MAIDS after LP-BM5 infection. B6 CD40 knockout mice, which are also relatively resistant to LP-BM5-induced MAIDS, became susceptible to LP-BM5-induced disease after reconstitution with highly purified wild-type B cells but not after receiving purified wild-type dendritic cells (DC) or a combined CD40+ population composed of DC and macrophages obtained from B6 SCID mouse donors. Based on these and other experiments, we thus conclude that the cellular basis for the requirement for CD154-CD40 interactions for MAIDS induction and progression can be accounted for by CD154 expression on CD4+ T cells and CD40 expression on B cells.
The LP-BM5 murine leukemia retrovirus (MuLV) isolate causes an immunodeficiency syndrome in genetically susceptible mice such as the highly susceptible C57BL/6 (B6) strain. This MuLV mixture includes ecotropic, recombinant mink cell cytopathic focus-inducing, and replication-negative or -defective viruses, with the defective genome serving as the proximal agent causing the syndrome (3, 7, 18, 22, 36). Many of the described disease features of this LP-BM5-induced mouse immunodeficiency syndrome are similar to those seen in human immunodeficiency virus (HIV)-infected individuals, hence the designation murine AIDS (MAIDS). Noted disease similarities are activation-related parameters such as hypergammaglobulinemia (hyper-Ig), splenomegaly, and lymphadenopathy; severely dampened T- and B-cell responses; increased susceptibility to infection, disease progression, and death upon exposure to environmental pathogens that normally cause limited infections; and the development of terminal B-cell lymphomas (5, 6, 26, 27, 29, 34, 37, 40, 53).
It has been suggested that CD4+ T cells and B cells are required for LP-BM5 MAIDS induction (6, 53, 47). In these experiments, in vivo depletion of CD4+ T cells before LP-BM5 infection rendered genetically susceptible mice resistant to the development of MAIDS (53). Similarly, after in vivo depletion of B cells from birth in neonatal mice by the administration of rabbit antibody to immunoglobulin M (IgM), followed by infection with LP-BM5 virus, MAIDS failed to develop (6). These studies, however, did not exclude the required involvement of other cellular subsets in MAIDS pathogenesis and did not experimentally address the possible interaction of these subsets or the molecular basis for interaction.
We have recently examined the effect of CD154-CD40 interactions in MAIDS initiation and progression (15, 16). CD154 is transiently expressed on activated T cells, especially murine CD4+ T cells after stimulation through the T-cell receptor for antigen (2, 39), and once expressed serves as the ligand for CD40, a 50-kDa membrane signaling protein responsible for driving B-cell activation and differentiation to antibody secretion (51). In view of the fact that CD154-CD40 interactions had been shown to be important in a wide array of immunological responses (1, 23, 44), we treated LP-BM5-infected B6 mice in vivo with blocking anti-CD154 monoclonal antibody (MAb), either during the first week of infection (15) or chronically, starting at 3 to 4 weeks after LP-BM5 infection (16). Both time courses of administered anti-CD154 MAb were very effective in inhibiting MAIDS-associated splenomegaly, hyper-Ig, and immunodeficiency. The data from these two experimental approaches strongly suggested that CD154-CD40 interactions are necessary for both the initiation and the progression of LP-BM5-induced MAIDS.
In the study presented here, we further characterize the CD154-CD40 interaction in MAIDS by a series of in vivo adoptive transfer experiments designed to elucidate which cellular subsets in a susceptible B6 mouse must express CD154 or CD40 for LP-BM5 virus-induced pathogenesis. Progression to MAIDS was evaluated in reconstituted, LP-BM5-infected nude CD154 knockout (k.o.) and CD40 k.o. mice, all on the B6 genetic background, by the following standard readouts of MAIDS-associated symptoms, which we (15, 16) and others (3, 5, 6, 7, 18, 21, 36) have previously established: (i) spleen size, with enlargement indicating MAIDS-associated B- and T-cell lymphoproliferation; (ii) serum IgG2a levels, with increases due to MAIDS polyclonal B-cell activation; (iii) splenic B-cell responses to lipopolysaccharide (LPS) stimulation, with decreases indicative of B-cell-associated MAIDS-induced immunodeficiency; and (iv) T-cell responses to either concanavalin A (ConA) mitogen stimulation or allogeneic major histocompatibility complex stimulation (as determined by cytotoxic T-lymphocyte [CTL] generation), with decreases indicative of MAIDS-induced T-cell immunodeficiency.
MATERIALS AND METHODS
Mice.
Seven-week-old male B6 mice were purchased from the National Institutes of Health (Bethesda, Md.), housed in the Dartmouth Medical School Animal facility, and used when 8 to 10 weeks of age as control mice or spleen cell donors. Breeding pairs of CD154 k.o. mice (fully backcrossed to B6) were obtained from the Jackson Laboratory (Bar Harbor, Maine); the mice were originally derived by Immunex Corporation (Seattle, Wash.) as previously described (45). B6-backcrossed CD40 k.o. breeding pairs, derived as reported elsewhere (24), for initial experiments were obtained from Anthony Hayward's laboratory (University of Colorado Health Science Center, Denver); mice for later experiments were obtained from the Jackson Laboratory. Six-week-old B6 nude mice were purchased from the Jackson Laboratory. Equal numbers of nude and k.o. mice, either three or four in a given experiment, were reconstituted with various cell preparations (see below) before infection with LP-BM5 virus. B6 SCID mice, purchased from the Jackson Laboratory were used at 6 weeks of age as spleen cell donors in a reconstitution experiment.
Cell lines.
LB27.4, a murine B-cell hybridoma (H-2b,d), and P815, a murine mastocytoma (H-2d), were cultured at 37°C with 5% CO2 in RPMI 1640 with 5% fetal calf serum (FCS), l-glutamine, 2-mercaptoethanol and antibiotics (Gibco BRL, Life Technologies, Grand Island, N.Y.).
LP-BM5 virus inoculations.
LP-BM5 was prepared in our laboratory as previously described (26). G6 cells, cloned from SC-1 cells infected with the LP-BM5 virus mixture and originally provided by Janet Hartley and Herbert Morse, were cocultured with uninfected SC-1 cells. Mice were infected intraperitoneally with 0.25 ml of a virus stock which was quantitated by an XC plaque assay (46) to contain approximately 5 × 105 ecotropic PFU/ml and which postinoculation caused MAIDS-related splenomegaly, as shown by at least a doubling of spleen weight as early as 4 weeks after inoculation into B6 mice.
ELISA determinations of serum Ig.
For measuring hyper-Ig, affinity-purified goat anti-mouse IgG2a antibody (Southern Biotechnology Associates, Birmingham, Ala.), was diluted to 7 μg/ml in phosphate-buffered saline (PBS) to coat 96-well enzyme-linked immunosorbent assay (ELISA)-grade plates (Becton Dickinson, Oxford, Calif.) overnight at room temperature. The plates were then washed three times with PBS and blocked for 1 h with 5% bovine serum albumin–PBS (Sigma, St. Louis, Mo.) at 37°C. Sera obtained at the termination of all LP-BM5 infection experiments were then plated and allowed to incubate for 2 h at 37°C. The plates were washed three times with PBS, and alkaline phosphatase-labeled goat anti-mouse Ig (Southern Biotechnology Associates) was added. After a 2-h, 37°C incubation, p-nitrophenyl phosphate (Sigma) provided a colorimetric change which was then quantitated at 405 nm by an ELISA reader (Dynatech Laboratories, South Hampton, United Kingdom).
Spleen cell responses to mitogens.
Spleen cells (4 × 105) from control and experimental mice were plated in triplicate into 96-well plates with medium containing 5% FCS, l-glutamine, antibiotics, and a final concentration of 2 μg/ml for ConA or 10 μg/ml for LPS. After 72 h, all wells were pulsed with 1 μCi of [3H]thymidine (Dupont NEN, Boston, Mass.) and harvested (Packard, Meriden Conn.) 6 h later for assessment of thymidine incorporation by scintillation counting (Packard).
Generation of primary allogeneic CTL (allo-CTL) and 51Cr release assays.
Responder splenocytes (107) from experimental or control mice were mixed with irradiated (8,000 rad) LB27.4 tumor cells at a responder-to-stimulator ratio of 35:1 in RPMI 1640 containing 5% FCS, antibiotics, and l-glutamine. These cultures were maintained at 37°C in a 5% CO2 incubator for 6 days. P815 target cells were resuspended in 100 μl of FCS and labeled with 100 μl of sodium chromate (2 mCi/ml; Dupont NEN). After washing, 5 × 103 51Cr-labeled P815 target cells were plated in duplicate with various numbers of effector cells to achieve effector-to-target ratios of 100:1, 20:1, and 4:1. The plates were centrifuged briefly and incubated at 37°C in 5% CO2 for 4 h. Aliquots of cell-free supernatant (100 μl) were collected for counting on a gamma counter (Wallac, LKB Gaithersburg, Md). Percent specific lysis was defined as [(a − b)/c] × 100, where a is experimental cpm released, b is spontaneous cpm released, and c is freeze-thaw releasable (about 80% of total) cpm. The values for spontaneous target cell release were 15% or lower, with the duplicate experimental percent specific lysis values ranging ± 10% of the mean value.
Splenocyte subpopulation preparation.
Splenocyte suspensions were labeled with antibody-coupled paramagnetic beads (MACS; Miltenyi Biotec, Auburn, Calif.) and subjected to column purification according to the manufacturer's protocol. The following purified cellular subsets were used in reconstitution experiments.
(i) CD4+ T-cell preparations.
Spleen cell suspensions were incubated with anti-CD8a beads, positively selecting for CD8+ T cells. The flowthrough cellular preparation was then labeled with anti-CD4 (L3T4) beads, and positive selection yielded cell preparations which were ≥90% CD3+ CD4+, ≤7% B220+, and ≤1% CD8+ as detected by flow cytometric analysis.
(ii) CD8+ T-cell preparations.
Spleen cell suspensions were incubated with anti-CD4 (L3T4) beads, and the flowthrough of this positive selection was then incubated with anti-CD8a beads, with positive selection yielding cell preparations which were ≥89% CD8a+, ≤9% B220+, and ≤1% CD4+.
(iii) B-cell preparations.
Spleen cell suspensions were incubated with a cocktail of anti-CD43 (Ly48, leukosialin) and anti-CD11c magnetic beads. The flowthrough of this positive selection was then positively selected with anti-CD19 magnetic beads and yielded cell preparations which were ≥98% CD19+.
(iv) DC preparations.
Spleen cell suspensions were obtained from B6 mice which had received injections of 10 μg per day of purified Flt3 ligand subcutaneously for 9 days. These cell suspensions were incubated with mouse Ig as specified by the manufacturer (Miltenyi Biotech) to block Fc receptor-mediated magnetic labeling of macrophages and subsequently with anti-CDIIc paramagnetic beads. After column purification, positively selected fractions were evaluated by direct immunofluorescence for cell surface markers as described for DC subsets in Flt3 ligand-treated mice (43) and were ≥71% CDIIc+ I-Ab+, ≥18% CDIIc+ I-Ab−, and ≥68% CD40+. Based on the reported number of DC present in a collagenase-treated spleen from a non-Flt3-treated mouse (0.5% of the viable lymphocytes) (8), we reconstituted each experimental CD40 k.o. mouse with >10 DC splenic equivalents.
SCID spleen cell preparation.
A cell suspension prepared from SCID spleens contained no detectable CD4-, CD8-, or CD19-expressing cells and was 19% CD40+ CDIIc+ and 27% CD40+ CD1lb+ by flow cytometric analysis.
All of the above cell preparations were injected intravenously via the tail vein, and those recipient mice whose transfers we rated technically as suboptimal were not used in these experiments.
PCR amplification.
DNA was extracted from splenic tissues of reconstituted and nonreconstituted CD40 k.o. mice and wild-type (w.t.) B6 mice as specified by the manufacturer (Qiagen Inc., Valencia, Calif.). PCR amplification was performed with primers specific for the CD40 gene product (CD40upG, 5′-dGGCAGTAAGACGATGTGACAACAGAG-3′; CD40Holo, 5′-dGAGATGAGAAGGAAGAATGGGAAAAC-3′) according to standard procedures. The predicted 2,100-bp band was detected for all CD40 k.o. mice, and a 900-bp band indicative of w.t. CD40 was detected only from amplified DNA samples obtained from control w.t. B6 mice.
Flow cytometry.
Spleen cells were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated antibodies, and direct immunofluorescence by linear amplification (FACScan) was used to detect the expression of murine CD4 (L3T4), CD8a (Ly2), CD3e, CD40, Thyl.2 (CD90.2), B220, CD19, CD11b, CD11c, I-Ab, NK1.1 (NKR-PIC Ly-55), CD43 (Ly48, leukosialin), and CD5 (Ly-1) (Pharmingen, San Diego, Calif.). The appropriate FITC- or phycoerythrin-conjugated Ig isotype of irrelevant specificity were used to control for each antibody.
RESULTS AND DISCUSSION
Determination of which cellular subset(s) must express CD154 for MAIDS pathogenesis.
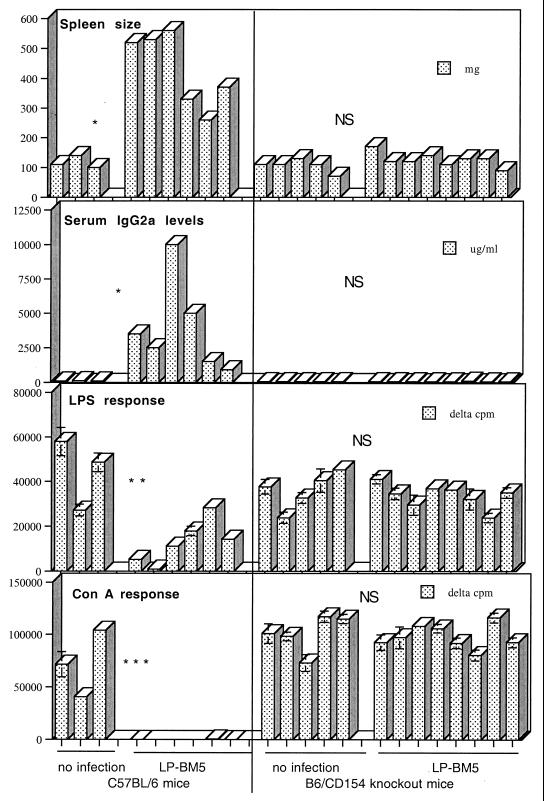
We challenged B6 CD154 k.o. mice with LP-BM5 virus and found that, as with anti-CD154 MAb-treated LP-BM5-infected B6 mice from our previous studies (15, 16), such k.o. mice are resistant to developing MAIDS. As shown in Fig. 1, LP-BM5-infected CD154 k.o. mice did not exhibit any indications of LP-BM5-induced MAIDS. LP-BM5-infected and uninfected B6 CD154 k.o. mice were essentially the same with respect to spleen weights, terminal IgG2a values, and spleen cell mitogenic responses to LPS and ConA. Positive control LPBM5-infected B6 mice, in this same experiment and consistent with our previous reports (15, 16), compared to uninfected B6 (or CD154 k.o.) mice, had approximately three- to fivefold-larger spleens, severalfold-increased IgG2a values, completely or partially inhibited LPS mitogen responses, and totally inhibited ConA responses. Thus, taken together with our anti-CD154 MAb blocking studies, these results confirmed that functional CD154 expression was required for LP-BM5-induced MAIDS pathogenesis.
FIG. 1.
B6 CD154 k.o. mice do not develop MAIDS after LP-BM5 infection. B6 w.t. or CD154 k.o. mice infected with LP-BM5 virus were sacrificed 8 weeks later. LP-BM5-infected w.t. B6, but not CD154 k.o., mice exhibited all of the positive readouts denoting LP-BM5-induced MAIDS which were statistically different from those responses seen for uninfected mice; splenomegaly, hyper-IgG2a, and diminished or nonexistent spleen cell response to LPS or ConA stimulation. Each bar represents a value for an individual mouse. P values were derived by the Student t test and are represented as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; NS (nonsignificant), P > 0.05. This experiment is representative of three other experiments.
To determine which cellular subset(s) that expresses CD154 is required for induction of MAIDS, various in vivo reconstitution experiments were undertaken. The first approach involved reconstituting B6 CD154 k.o. mice with purified CD4+ T cells (see Materials and Methods) from w.t. B6 donors, since CD154 expression has been found most prominently on the CD4+ T subpopulation. In three different experiments, approximately 70% of the CD4+ T-cell-reconstituted CD154 k.o. mice developed some clear signs of MAIDS after LP-BM5 challenge (data not shown).
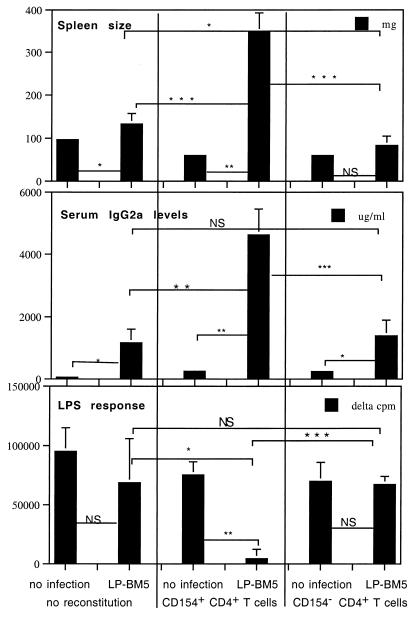
Because the results from this experimental approach were only suggestive that CD4+ T cells were the basis for the CD154 requirement in MAIDS pathogenesis, we alternatively reconstituted T-cell-deficient B6 nude mice (41), in which there was no possibility of dilution of w.t. donor, with resident k.o. recipient, CD4+ T cells. Nude mice, in particular B6 nude mice, have previously been shown to be resistant to MAIDS induction (37, 47). CD4+ T cells positively selected from B6 w.t. or CD154 k.o. spleen cell suspensions were transferred 2 days before inoculation with LP-BM5 virus. In four different experiments, only the CD154+ CD4+ T-cell-reconstituted, LP-BM5-infected nude mice exhibited MAIDS, and this was consistent across all disease readouts examined. This result was in sharp contrast to that for the LP-BM5-infected mice which had received CD154− CD4+ T cells. For example, as shown by the representative experiment in Fig. 2, comparision of the mean responses for the uninfected and infected CD154+ CD4+ T-cell-reconstituted nude mice showed that LP-BM5 infection led to spleen weights that were approximately 7-fold higher and serum IgG2a levels around 5,000-fold higher, and the LPS mitogenic response was almost completely inhibited. These MAIDS-related changes in the LP-BM5-infected, CD154+ CD4+ T-cell-reconstituted mice were also significantly different from results for both the infected nude mice that were not reconstituted and those that received CD154− CD4+ T cells prior to infection (Fig. 2). In addition, the ConA-dependent mitogenic response, another index of LP-BM5-induced immunodeficiency, also demonstrated the critical expression of CD154 by the CD4+ T-cell population for MAIDS pathogenesis. Thus, after infection, the ConA response for the CD154+ CD4+ T-cell-reconstituted nude mice was statistically significantly lower (P = 0.0146) than the response for the CD154− CD4+ T-cell-reconstituted mice (data not shown). Of note, we have reported in Fig. 2 and throughout the subsequent experiments all readout responses that we have routinely used to define MAIDS pathology (Fig. 1) except those that directly measure the responsiveness of the specifically transferred cell subset. These responses, although showing the same trend as the other disease parameters, were somewhat more variable, possibly because of slightly different effective cellular reconstitutions on a mouse-to-mouse basis.
FIG. 2.
LP-BM5-infected B6 nude mice, which are normally MAIDS resistant, become MAIDS susceptible after reconstitution with CD154+ CD4+ T cells but not CD154− CD4+ T cells. B6 nude mice were intravenously reconstituted with 2 × 107 purified B6 w.t. CD154+ or CD154− CD4+ T cells and 2 days later infected with LP-BM5 virus. At 8 weeks after LP-BM5 infection, only the CD154+ CD4+ T-cell-reconstituted mice had developed all of three positive disease readouts indicating MAIDS susceptibility (splenomegaly, hyper-IgG2a, and diminished spleen cell response to LPS), which were all statistically different from those obtained for control, reconstituted, uninfected mice and CD154− CD4+ T-cell-reconstituted, LP-BM5-infected nude mice. Each bar represents a mean value ± standard deviation. P values were derived as detailed in the legend to Fig. 1. There were three mice for the reconstituted, uninfected group and four mice for the reconstituted, LP-BM5-infected group. This experiment is representative of three other experiments.
In contrast to CD154+ CD4+ T-cell-reconstituted nude mice, in uninfected and infected CD154− CD4+ T-cell-reconstituted mice, the mean spleen sizes and LPS responses (Fig. 2) and ConA responses (data not shown) were essentially the same, indicating that MAIDS was not induced. The 10-fold elevation of the serum IgG2a level detected for the infected mice was minimal compared to the 5,000-fold elevation seen in the CD154+ CD4+ T-cell-reconstituted and LP-BM5-infected nude mice, and approximately 10-fold increases in IgG2a levels were also found for the nonreconstituted, infected nude mice. This pattern of slight IgG2a elevation, compared to the level for the CD154+ CD4+ T-cell transfer and infected group, was also observed in the three repeat experiments conducted and may represent the reported LP-BM5 virus-dependent but MAIDS-independent increases in multiple Ig isotypes that have been observed by two other laboratories in different k.o. mouse models (35, 54; see below). Similarly, there was a slight (statistically nonsignificant) increase in spleen weight for infected CD154− CD4+ T-cell-reconstituted nude mice, but a similar (and somewhat greater) increase was observed for nontransferred infected nude mice. FITC anti-CD4 labeling of spleen cells obtained on the day of sacrifice from CD154+ and CD 154− donor CD4+ T-cell-reconstituted nude mice, followed by flow cytometric analysis (see Materials and Methods), ruled out the possibility that the MAIDS-insusceptible CD154− CD4+ T-cell-transferred mice were not successfully reconstituted with CD4+ T cells (data not shown). From these data, we can conclude that CD4+ T cells are required and must express CD154 for LP-BM5-induced MAIDS pathogenesis.
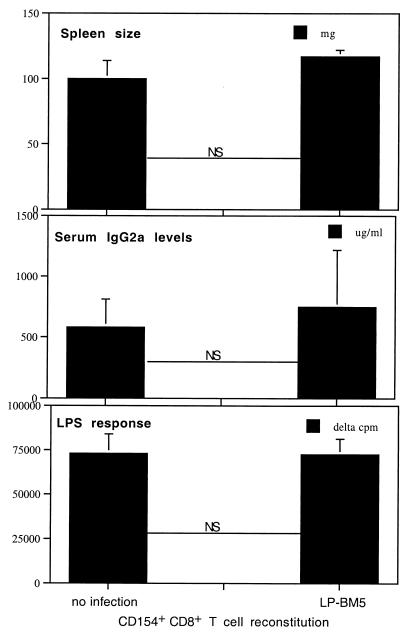
To test the possibility that cell subsets other than CD4+ T cells might also provide the CD154 expression requisite for MAIDS pathogenesis, we examined CD8+ T cells, a portion of which have been reported to express CD154 in response to antigenic stimulation (9, 19). We reconstituted B6 nude mice with w.t. CD8+ T cells (see Materials and Methods). In two experiments, no CD8+ T-cell-reconstituted, LP-BM5-infected nude mice presented with any positive readouts indicating LP-BM5-induced MAIDS. As shown by the representative experiment in Fig. 3, LP-BM5-infected, w.t. CD8+ T-cell-reconstituted nude mice exhibited mean responses for spleen size, serum IgG2a levels, and LPS stimulation that were not significantly different from the mean responses for control, uninfected, CD8+ T-cell-reconstituted nude mice. Spleen cells from the CD8+ T-cell-reconstituted, LP-BM5-infected nude mice were also able to mount an allo-CTL response (see Materials and Methods) that was not significantly different from that for uninfected CD8+ T-cell-transferred nudes (P = 0.1237 [data not shown]). Compared to the LP-BM5-infected, w.t. CD8+ T-cell-reconstituted mice represented in Fig. 3, LP-BM5-infected, CD154+ CD4+ but not CD154− CD4+ T-cell-reconstituted nude mice, which were also included in the experiment, exhibited the clear signs of MAIDS as shown in Fig. 2 (data not shown). Indeed, the responses of the CD154− CD4+ and w.t. CD154+ CD8+ T-cell-reconstituted and LP-BM5-infected mice were similar. These data strongly suggest that w.t. CD8+ T cells are not capable of providing the CD154 expression required for LP-BM5-induced MAIDS pathogenesis.
FIG. 3.
CD154+ CD8+ T-cell-reconstituted B6 nude mice remain MAIDS insusceptible after LP-BM5 infection. B6 nude mice were reconstituted with 107 purified CD154+ CD8+ T cells and 2 days later were infected with LP-BM5 virus. At 8 weeks postinfection, all recipient mice were sacrificed and the indicated disease parameters indicative of MAIDS were determined. Each bar represents a mean value ± standard deviation. This is representative of one other experiment. P values shown were derived by the Student t test (NS [not significant], P > 0.05).
Thus, CD4+ T cells appear to be uniquely able to provide the CD154 expression required for induction and progression of MAIDS. This finding is consistent with a variety of studies indicating defects in priming and/or expansion of CD4+ T cells in CD154 k.o. mice (17, 30, 33, 49, 52). Whether this requirement for CD154 in these previous studies and the present study reflects a signaling defect normally mediated by CD154 to directly activate the CD4+ T cell per se, as was originally suggested in some early studies (17, 52), or a failure to activate and mature CD40+ B cells or professional antigen-presenting cells to function and/or traffic appropriately (33) was unclear. However, because the bulk of the literature now generally favors an effect mediated by the lack of signaling through CD40 (30), we focused next on identification of the possible CD40+ cell subsets required for MAIDS.
Determination of which cellular subset(s) must express CD40 for MAIDS pathogenesis.
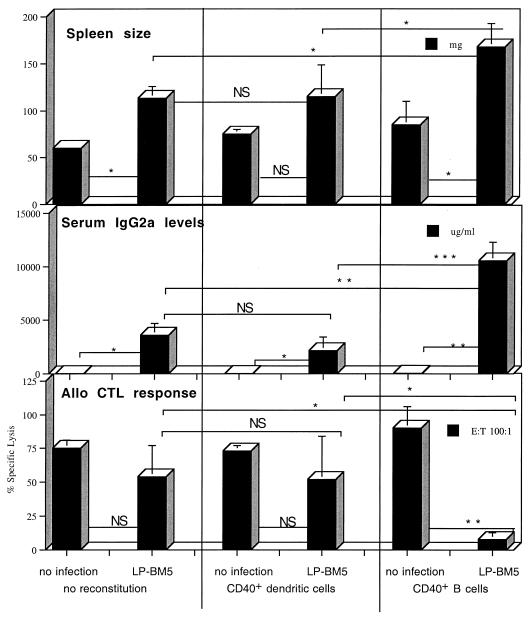
We and others (54) have found that, like CD154 k.o. mice, B6 CD40 k.o. mice are resistant to LP-BM5-induced MAIDS, although the degree of resistance is somewhat less dramatic than for CD154 k.o. mice. These findings provided confirmation of our conclusion that CD154-CD40 interactions are required, based on the results from our studies showing an interference with MAIDS initiation and progression by treatment with blocking anti-CD154 MAb (15, 16). CD40, originally identified as a B-cell molecule, has also been reported to be expressed prominently by DC and macrophages (48), as well as by other cell types. To determine which cellular subsets must express CD40 for LP-BM5-induced MAIDS pathogenesis, we performed parallel reconstitutions of normally MAIDS-resistant B6 CD40 k.o. mice with three different B6 background-derived sources of CD40+ cells: (i) highly purified CD40+ B cells; (ii) purified CD40+ DC; and (iii) B6 SCID spleen cells, representing a combined population of CD40+ DC and macrophages. We found that only CD40+ B-cell-reconstituted CD40 k.o. mice became MAIDS susceptible after LP-BM5 infection. Thus, compared to the minimal to moderate effects seen for LP-BM5 infection of nonreconstituted CD40 k.o. mice, CD40+ B-cell-reconstituted, LP-BM5-infected CD40 k.o. mice exhibited MAIDS by statistically significant differences for mean spleen size, serum IgG2a levels, allo-CTL responsiveness (Fig. 4), and LPS responsiveness (P = 0.0272, data not shown). Of note, the observed serum hyper-Ig levels were 2 fold higher than that seen in LP-BM5-infected B6 control mice (data not shown). These responses for CD40+ B-cell-reconstituted, LP-BM5-infected mice were also statistically different from those obtained for CD40+ DC-reconstituted, LP-BM5-infected CD40 k.o. mice (see also below). A similar pattern of results was found in another experiment in which CD40 k.o. mice were again reconstituted with 7 × 107 CD40+ B cells, representing approximately 1.1 splenic equivalents, before they were infected with LP-BM5. In two earlier experiments in which CD40 k.o. mice were reconstituted with either 2 × 107 or 5 × 107 CD40+ B cells, LPBM5-induced MAIDS also occurred, but there was more mouse-to-mouse variability.
FIG. 4.
MAIDS-resistant LP-BM5-infected B6 CD40 k.o. mice become MAIDS susceptible after reconstitution with CD40+ B cells but not after reconstitution with CD40+ DC. CD40 k.o. mice were intravenously reconstituted with 7 × 107 highly purified B6 CD40+ B cells or 5 × 106 CD40+ purified DC obtained from Flt3-treated B6 mice. Two days later they were infected with LP-BM5 virus, and at 11 weeks postinfection, the recipients were sacrificed for the indicated disease parameters indicating MAIDS susceptibility: splenomegaly, hyper-IgG2a, and diminished allo-CTL responses. Serum IgG2a levels for LP-BM5-infected nonreconstituted CD40 k.o. mice are higher than those for noninfected k.o. mice, apparently in accordance with the published observation that LP-BM5 causes hyper-IgG2a due to a virus-dependent but non-MAIDS-related isotype class switching in CD40 k.o. mice (54). Each bar represents the mean response ± standard deviation. P values were derived as detailed in the legend to Fig. 1. This experiment is representative of one other. A less consistent but similar trend of responses was seen in two other experiments in which 2 × 107 and 5 × 107 purified CD40+ B cells were transferred (see text). E:T, effector:target.
To address the possible ability of alternative CD40-expressing cellular subsets to provide the CD40 expression required for MAIDS pathogenesis in the absence of CD40-expressing B cells, we reconstituted CD40 k.o. mice in parallel with 5 × 106 (> 10 splenic equivalents) purified CD40+ DC. No MAIDS induction was observed in such CD40+ DC-reconstituted CD40 k.o. mice 11 weeks after LP-BM5 infection compared to nontransferred, LP-BM5-infected CD40 k.o. controls (Fig. 4). In addition to the allo-CTL data, there was no evidence for immunosuppression based on LPS responsiveness (P = 0.1066 [data not shown]). A repeat experiment in which more (107) DC were used for reconstitution produced the same result: all LP-BM5-infected, DC-reconstituted CD40 k.o. mice had spleen weights, IgG2a levels, and LPS and allo-CTL responses similar to those seen in LP-BM5-infected, nonreconstituted CD40 k.o. mice. Of note, CD40+ CD11c+ donor DC were detected in about half of the mice by flow cytometric analysis on the day of sacrifice in the spleens of reconstituted mice (mean delta percent positive of 1 to 2%). These results were confirmed by the presence of the expected 900-bp band indicative of w.t. CD40 following PCR amplification of DNA also extracted at sacrifice from the reconstituted recipients (see Materials and Methods). While it seems likely that more dramatic evidence controlling for the presence of the donor DC populations would have been obtained if recipients could have been analyzed before their sacrifice at 11 weeks after transfer and infection, and considering that because no disease occurred, there was no expansion of the transferred cells, we take these results to be reasonable evidence of the success of the reconstitution (see also Materials and Methods regarding the transfers).
As an alternative approach to assessing non-B-cell sources of CD40+ cells for their possible role in MAIDS, CD40 k.o. mice were alternatively reconstituted with 5 × 106 B6 SCID (4) spleen cells as a source of both CD40+ DC and macrophages. Of note, SCID mice have previously been shown by Simard et al. to be resistant to induction of MAIDS, albeit in experiments using the alternative Du5H (Du5H/Mo-LTR) rescued chimeric defective virus (47). Such transferred SCID mouse spleen cell subsets, which have been reported as competent antigen-presenting cells (10), did not render CD40 k.o. recipients MAIDS susceptible. Thus, there were no positive readouts indicating LP-BM5-induced MAIDS at 11 weeks after LP-BM5 infection (Fig. 5).
FIG. 5.
B6 CD40 k.o. mice do not develop LP-BM5-induced MAIDS after reconstitution with a cell preparation enriched for CD40+ DC and macrophages. CD40 k.o. mice were intravenously reconstituted with 5 × 106 spleen cells obtained form B6 SCID mouse donors; they were infected with LP-BM5 2 days later and assessed for the indicated MAIDS disease parameters at 11 weeks postinfection. Each bar represents the mean ± standard deviation. Serum IgG2a levels for LP-BM5-infected nonreconstituted CD40 k.o. mice are higher than those for uninfected k.o. mice, apparently in accordance with the published observation that LP-BM5 causes hyper-IgG1 and -IgE, (54) due to a virus-dependent but non-MAIDS-related isotype class switching in CD40 k.o. mice. P values were derived by the Student t test (NS [nonsignificant], P > (0.05). E:T, effector:target.
In conclusion, collectively these data strongly suggest that with respect strictly to the CD154+ and CD40+ cellular requirements for disease, CD4+ T cells expressing CD154 and B-cells expressing CD40 are necessary and sufficient for LP-BM5 MuLV-induced MAIDS pathogenesis. Further, in the absence of CD40+ B cells, macrophages, and/or DC, despite their CD40 positivity, are unable to provide the functional CD40 expression for LP-BM5-induced MAIDS. However, we cannot exclude a role for macrophages or DC in MAIDS pathogenesis in a manner that does not involve their display of CD40. Of note, flow cytometric analyses indicated that the highly purified B-cell preparations, which allowed for LP-BM5 induction of MAIDS in reconstituted CD40 k.o. mice, were negative not only for CD11c- or CD11b-expressing DC or macrophages but also for CD5+ CD19+ double-positive B cells. Thus, it would appear from our data that this latter B-la subset of B cells is not essential as the cellular basis of the CD40 requirement in MAIDS. This finding is relevant to the ongoing debate as to the importance of B-la B cells in MAIDS pathogenesis (20, 50). Our data clearly imply that if B-la B cells have any role in MAIDS pathogenesis, then at least this putative function does not depend on the expression of CD40.
The results from our previous studies and herein, in which we have defined CD154+ CD4+ T cells and CD40+ B cells as the cellular subsets which must express these two paired interacting molecules for LP-BM5-induced MAIDS initiation and progression, thus provide the basis for further experimentation in which we will explore the molecular signaling cascades emanating from CD40 (and possibly CD154) that appear central to MAIDS pathogenesis. However, other receptor-ligand pairs may also be critical to disease induction. Along these lines, De Leval et al. recently reported that MAIDS pathogenesis in LP-BM5-infected B6 mice could be partially blocked by in vivo treatment with soluble CTLA4Ig (12) or more completely by the transgenic overexpression of soluble CTLA4 (11). Whether the critical manifestation of CD40 signaling in MAIDS is indeed the upregulation on B cells of CD80 and/or CD86 and a subsequent interaction with T cells expressing CD28 and CTLA4 will await future studies. Similarly, evidence suggesting that the receptor ligands CD11a/CD18 (LFA-1) and CD54 (ICAM-1) are required for MAIDS development has been presented (31). Whether these molecules aid in the interaction of CD4+ T cells with B cells prior to delivery of signals from CD154 to CD40 or there is a role for LFA-1–ICAM-1 interactions subsequent to CD40 signaling is unclear.
Our results on the requirement for interactions between CD40+ B cells and CD154+ CD4+ T cells in the initiation and progression of MAIDS are interesting in the context of other retrovirus-induced immunodeficiencies, specifically human AIDS. Particularly relevant to the present study, several recent reports have defined CD154-CD40 interactions that may be important to HIV infectivity and/or pathogenesis. In a study by Poulin et al. (42), human B lymphocytes activated in vitro with murine cell surface-presented CD154 in the presence of interleukin-4 became infectable by HIV type 1 (HIV-1). When such infected B cells were cocultured with an HIV-1-susceptible CD4+ T-cell line, B-T cell fusion and syncytium formation led to direct infection of the T cells (42). In a follow-up report, it was further found that culturing freshly isolated B lymphocytes with soluble oligomeric CD154 plus interleukin-4 upregulated B-cell CD4 and CXCR4 receptor expression, with subsequent greatly increased susceptibility to infection by T-cell-tropic and dualtropic strains of HIV but not by macrophagetropic strains. The authors speculated that CD40 activation-dependent conversion of B cells to HIV-1 susceptibility may make the B lymphocyte a potential viral reservoir in AIDS patients (32). Similarly, Gras et al. (14) have provided data suggesting that activation through CD40 augments HIV replication in B cells. Furthermore, Muller et al. (38) have reported that the polyclonal hyper-Ig characteristic of HIV-infected patients may relate to the observed increases in the percentage of CD4+ T cells expressing CD154 and in the density of cell surface CD40 expressed by B cells.
Because evidence has been provided that in MAIDS B cells are the primary cell type for expression of the disease-causing defective retrovirus (21, 25), further study of the effects of CD154-CD40 activation and downstream molecular signaling interactions in LPBM5-infected B cells may provide additional insights into the role of B cells in MAIDS and potentially the pathogenesis of other retrovirus-induced immunodeficiencies, including AIDS. Such new information, particularly if B cells do constitute a retroviral reservoir, may be useful in improving existing, or developing new, strategies involving the use of antiviral drug therapies or other approaches to eliminate residual retrovirus.
ACKNOWLEDGMENTS
We thank Herbert C. Morse III, Jim Gorham, Hillary White, Bob Rich, On Ho, Sue Eszterhas, Loren Erickson, and Burkhard Becher for helpful discussions, and we thank Barbara Peterson-Cremer and Darshan Sappal for technical assistance and discussions in helping to complete the experiments described in this report.
This work was supported by Public Health Service grant CA50157. The DMS irradiation facilities and the flow cytometers were the generous gift of the Fannie Rippel Foundation and are partially supported by National Institutes of Health Core grant CA23108 for the Norris Cotton Cancer Center.
REFERENCES
- 1.Allen R C, Armitage R J, Conley M E, Rosenblatt H, Jenkins N A, Copeland N G, Bedell M A, Edelhoff S, Disteche J, Simoneaux D K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–995. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 2.Armitage R J, Fanslow W C, Strockbine L, Sato T A, Clifford K N, Madcuff B M, Anderson D M, Gimpel S D, Davis-Smith T, Maliszewski C R, Clark E A, Smith C A, Grabstein K H, Cosman D, Spriggs M K. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 3.Aziz D C, Hanna Z, Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukemia virus. Nature. 1989;338:505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- 4.Bosma M J, Carroll A M. The SCID mouse mutant: definition characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 5.Buller R M L, Yetter R A, Fredrickson T N, Morse H C., III Abrogation of resistance to severe mousepox in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. J Virol. 1987;61:383–387. doi: 10.1128/jvi.61.2.383-387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerny A A, Hugin W, Hardy R R, Hayakawa K, Zinkernagel R M, Makino M, Morse H C., III B cells are required for induction of T cell abnormalities in a murine retrovirus-induced immunodeficiency syndrome. J Exp Med. 1990;171:315–320. doi: 10.1084/jem.171.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattopadhyay S K, Morse III H C, Makino M, Ruscetti S K, Hartley J W. A defective virus is associated with induction of a murine retrovirus-induced immunodeficiency syndrome, MAIDS. Proc Natl Acad Sci USA. 1989;86:3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caligam J, et al., editors. Current protocols in immunology, section 3.7. New York, N.Y: John Wiley & Sons; 1998. p. 2. [Google Scholar]
- 9.Cronin D C, Stack R, Fitch F W. IL-4 producing CD8+ T cell clones can provide B cell help. J Immunol. 1995;154:3118–3127. [PubMed] [Google Scholar]
- 10.Czitrom A A, Edwards S, Phillips R A, Bosma M J, Marrack P, Kappler J W. The function of antigen-presenting cells in mice with severe combined immunodeficiency. J Immunol. 1985;134:2276–2280. [PubMed] [Google Scholar]
- 11.De Leval L, Debrus S, Lane P, Boniver J, Moutschen M. Mice transgenic for a soluble form of murine cytotoxic T lymphocyte antigen 4 are refractory to murine acquired immune deficiency syndrome development. Immunology. 1999;98:630–638. doi: 10.1046/j.1365-2567.1999.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Leval L, Colombi S, Debrus S, Demoitie M, Greimers R, Linsley P, Moutschen M, Boniver J. CD28–B7 costimulatory blockade by CTLA4Ig delays the development of retrovirus-induced murine AIDS. J Virol. 1998;72:5285–5290. doi: 10.1128/jvi.72.6.5285-5290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giese N A, Giese T, Morse H C., III Murine AIDS is an antigen-driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J Virol. 1994;68:5819–5824. doi: 10.1128/jvi.68.9.5819-5824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gras G, Legendre C, Krzysiek R, Dormont D, Galanaud P, Richard Y. CD40/CD40L interactions and cytokines regulate HIV replication in B cells in vitro. Virology. 1996;220:309–319. doi: 10.1006/viro.1996.0319. [DOI] [PubMed] [Google Scholar]
- 15.Green K A, Crassi K M, Laman J D, Schoneveld A, Strawbridge R R, Foy T M, Noelle R J, Green W R. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J Virol. 1996;70:2569–2575. doi: 10.1128/jvi.70.4.2569-2575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green K A, Noelle R J, Green W R. Evidence for a continued requirement for CD40/CD40 ligand (CD154) interactions in the progression of LP-BM5 retrovirus-induced murine AIDS. Virology. 1998;241:260–268. doi: 10.1006/viro.1997.8970. [DOI] [PubMed] [Google Scholar]
- 17.Grewal I S, Xu J, Flavell R A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–623. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 18.Hartley J W, Fredrickson T N, Yetter R A, Makino M, Morse H C., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1230. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann P, Van Kooten C, Gaillard C, Bancherreau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25:2972–2977. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 20.Hitoshi Y, Okada Y, Sonoda E, Tominaga A, Makino M, Suzuki K, Kinoshita J, Komuro K, Mizuoci T, Takatsu K. Delayed progression of a murine retrovirus-induced acquired immunodeficiency syndrome in X-linked immunodeficient mice. J Exp Med. 1993;177:621–626. doi: 10.1084/jem.177.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M, Simard C, Kay D G, Jolicoeur P. The majority of cells infected with the defective murine AIDS virus belong to the B-cell lineage. J Virol. 1991;65:6562–6571. doi: 10.1128/jvi.65.12.6562-6571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M, Simard C, Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989;246:1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- 23.Hugo P, Kappler J W, Marrack P C. Positive selection of TcR alpha beta thymocytes: is cortical epithelium an obligatory participant in the presentation of major histocompatibility complex protein? Immunol Rev. 1993;135:134–155. doi: 10.1111/j.1600-065x.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;3:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim W K, Tang Y, Kenny J J, Longo D L, Morse H C., III In murine AIDS, B cells are early targets of defective virus and are required for efficient infection and expression of defective virus in T cells and macrophages. J Virol. 1994;68:6767–6776. doi: 10.1128/jvi.68.10.6767-6769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinken S P, Fredrickson T N, Hartley J W, Yetter R A, Morse H C., III Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988;140:1123–1131. [PubMed] [Google Scholar]
- 27.Klinman D M, Morse H C., III Characteristics of B cell proliferation and activation in murine AIDS. J Immunol. 1989;142:1144–1149. [PubMed] [Google Scholar]
- 28.Laurence H M, Miga A J, Vanderlugt C L, Dal Canto M C, Laman J D, Noelle R J, Miller S D. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Investig. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legrand E, Daculsi R, Duplan J F. Characteristics of the cell populations involved in extrathymic lymphosarcoma induced in C57BL/6 mice by RadLV-RS. Leuk Res. 1981;5:223–233. doi: 10.1016/0145-2126(81)90107-7. [DOI] [PubMed] [Google Scholar]
- 30.Mackey M F, Barth R J, Noelle R J. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 31.Makino M, Kazuhiko Y, Azuma M, Okada Y, Hitoshi Y, Yagita H, Takatsu K, Komuro K. Rapid development of murine AIDS is dependent on signals provided by CD54 and CD11a. J Immunol. 1995;155:974–981. [PubMed] [Google Scholar]
- 32.Moir S, Lapointe R, Malaspina A, Ostrowski M, Cole C E, Chun T, Adelsberger J, Baeseler M, Hwu P, Fauci A S. CD40 mediated induction of CD4 and CXCR4 on B lymphocytes correlates with restricted susceptibility to human immunodeficiency virus type 1 infection: potential role of B lymphocytes as a viral reservoir. J Virol. 1999;73:7972–7980. doi: 10.1128/jvi.73.10.7972-7980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moodycliffe A M, Shreedhar V, Ullrich S E, Walterscheld J, Bucana C, Kripke M L, Flores-Romo L. CD40-CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J Exp Med. 2000;191:2011–2020. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse H C, III, Yetter R A, Via C S, Hardy R R, Cerny A, Hayakawa K, Hugin A W, Miller M W, Homes K L, Shearer G M. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J Immunol. 1989;143:844–850. [PubMed] [Google Scholar]
- 35.Morse H C, III, McCarthy T, Giese N A, Taddesse-Heath L, Grusby M J. Stat6-deficient mice exhibit normal induction of murine AIDS and expression of immunoglobulin E following infection with LP-BM5 murine leukemia viruses. J Virol. 1999;73:7093–7095. doi: 10.1128/jvi.73.8.7093-7095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosier D E, Yetter R A, Morse H C., III Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosier D E, Yetter R A, Morse H C., III Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS) J Exp Med. 1987;165:1732–1742. doi: 10.1084/jem.165.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller F, Aukrust P, Nordoy I, Froland S S. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood. 1998;92:3721–3729. [PubMed] [Google Scholar]
- 39.Noelle R J, Roy M, Shepherd D M, Stamenkovic I, Ledbetter J A, Aruffo A. A novel ligand on activated T helper cells binds CD40 and transduces the signal for the cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pattengale P K, Taylor C R, Twoney P, Hill S, Jonasson J, Beardsley T, Haas M. Immunopathology of B cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV) Am J Pathol. 1982;107:362–377. [PMC free article] [PubMed] [Google Scholar]
- 41.Pelletier M, Montplaisir S. The nude mouse: a model of deficient T-cell function. Methods Achiev Exp Pathol. 1975;7:149–166. [PubMed] [Google Scholar]
- 42.Poulin L, Paquette N, Moir S, Lapointe R, Darveau A. Productive infection of normal CD40-activated human B lymphocytes by HIV-1. AIDS. 1994;8:1539–1544. doi: 10.1097/00002030-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Pulendran B, Lingappa J, Kennedy M K, Smith J, Teepe M, Rudensky A, Maliszewski C R, Maraskovsky E. Developmental pathways of dendritic cells in vivo. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 44.Ranheim E A, Kipps T J. Activated T cells induce expression of B7/BB 1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renshaw B R, Fanslow III W C, Armitage R J, Campbell K A, Liggitt D, Wright B, Davidson B L, Maliszewski C R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 47.Simard C, Klein S J, Mak T, Jolicoeur P. Studies of the susceptibility of nude, CD4 knockout, and SCID mutant mice to the disease induced by the murine AIDS defective virus. J Virol. 1997;71:3013–3022. doi: 10.1128/jvi.71.4.3013-3022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stout R D, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 49.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–697. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Kim W, Holmes K L, Hugin A W, Kenny J J, Chattopadhyay S K, Hartley J W, Morse H C., III Contribution of B cell subsets to delayed development of MAIDS in xid mice. Cell Immunol. 1995;165:1–6. doi: 10.1006/cimm.1995.1179. [DOI] [PubMed] [Google Scholar]
- 51.Uckun F M, Schieven G L, Dibirdik I, Chandan L M, Tuel A L, Ledbetter J A. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the pan-B-cell receptor CD40/Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991;266:17478–17485. [PubMed] [Google Scholar]
- 52.Van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;38:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 53.Yetter R A, Buller R M L, Lee J S, Elkins K L, Mosier D E, Fredrickson T N, Morse H C., III CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS) J Exp Med. 1988;168:623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu P, Morawetz R A, Chattopadhyay S, Makino M, Kishimoto T, Kikutani H. CD40-deficient mice infected with the defective murine leukemia virus LP-BM5def do not develop murine AIDS but produce IgE and IgGl in vivo. Eur J Immunol. 1999;29:615–625. doi: 10.1002/(SICI)1521-4141(199902)29:02<615::AID-IMMU615>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]