Abstract
The aim of this study was to investigate whether a reduction in accelerometer-measured sedentary behavior (SB) improves blood lipids in inactive adults with metabolic syndrome (MetS). Sixty-four participants were randomly assigned into intervention (INT, n = 33) and control (CONT, n = 31) groups. The INT group was instructed to reduce SB by 1 h/day without increasing formal exercise, whereas the CONT group was advised to maintain usual SB habits. SB and physical activity (PA) were measured with accelerometers throughout the intervention. Plasma lipid concentrations and dietary intake by food diaries were assessed at baseline and at the end of the intervention. High-density lipoprotein percentage of total cholesterol decreased during the intervention similarly in both groups (p = 0.047). Other blood lipids did not change from baseline to six months in either group. The CONT group had a statistically significant reduction in the intake of saturated fatty acids compared to the INT group (p = 0.03). Intervention resulting in a 40-minute reduction in daily SB and 20-minute increase in habitual MVPA seems to not be effective in improving blood lipids in adults with MetS. Reducing SB together with a higher volume and/or intensity of PA and proper nutrition may be needed to reduce the risk of cardiometabolic diseases.
Trial registration. This study is registered at ClinicalTrials.gov (NCT03101228, 05/04/2017). https://www.clinicaltrials.gov/ct2/show/NCT03101228?term=NCT03101228&draw=2&rank=1.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75579-8.
Keywords: Metabolic syndrome, Blood lipids, Sedentary behavior, Dietary intake, Randomized controlled trial
Subject terms: Medical research, Risk factors
Introduction
Excess sedentary behavior (SB) increases the risk of premature mortality and the incidence of diabetes and cardiometabolic diseases1–4. Metabolic syndrome (MetS) refers to a set of various cardiometabolic risk factors including dyslipidemias (high triglycerides [TG], low high-density lipoprotein cholesterol [HDL]), high blood pressure, high fasting glucose and central obesity5,6. These elements are associated with a high amount of device-measured SB7, and an increased risk of cardiovascular diseases8,9 and mortality10.
Recent meta-analyses have reported heterogenous and inconsistent results when investigating the ability of SB reduction to modify blood lipid concentrations11–14. Most commonly, increased HDL levels have been observed after SB interventions11,15. Previous studies have investigated mainly the effect of structured physical activity (PA) on blood lipids16–19, whereas evidence on the effects of PA in free living setting is lacking. However, PA has been associated with favorable blood lipid concentrations in cross-sectional settings20,21 and replacing SB with PA may have acute favorable effects on blood lipids12,22. Nevertheless, it seems that approximately 30–40 min of moderate-to-vigorous PA (MVPA) per day might be needed to attenuate the detrimental effects of SB23. However, a major proportion of adults are physically inactive24, and thus it is important to investigate whether favorable health effects can be achieved by reducing SB without intentionally adding exercise. The possible benefits of reducing SB on blood lipids in free living setting remain unconfirmed.
In most previous 6–12-month SB-focused intervention studies, SB and PA of the study participants have been measured with accelerometers for relatively short periods (approximately seven days at the beginning and end of the intervention)25,26 instead of the entire intervention period. Consequently, there is a lack of long-term intervention studies that have used accelerometers throughout the whole study period and investigated the intervention effects on blood lipids, especially in people with cardiometabolic risk factors. Therefore, the purpose of this six-month randomized controlled trial (RCT) was to determine whether an intervention aiming at a one-hour reduction in daily SB improves blood lipids in sedentary and physically inactive adults with MetS. Additionally, due to the potential confounding role, the effects on dietary outcomes and associations between changes in lipids and diet were assessed.
Results
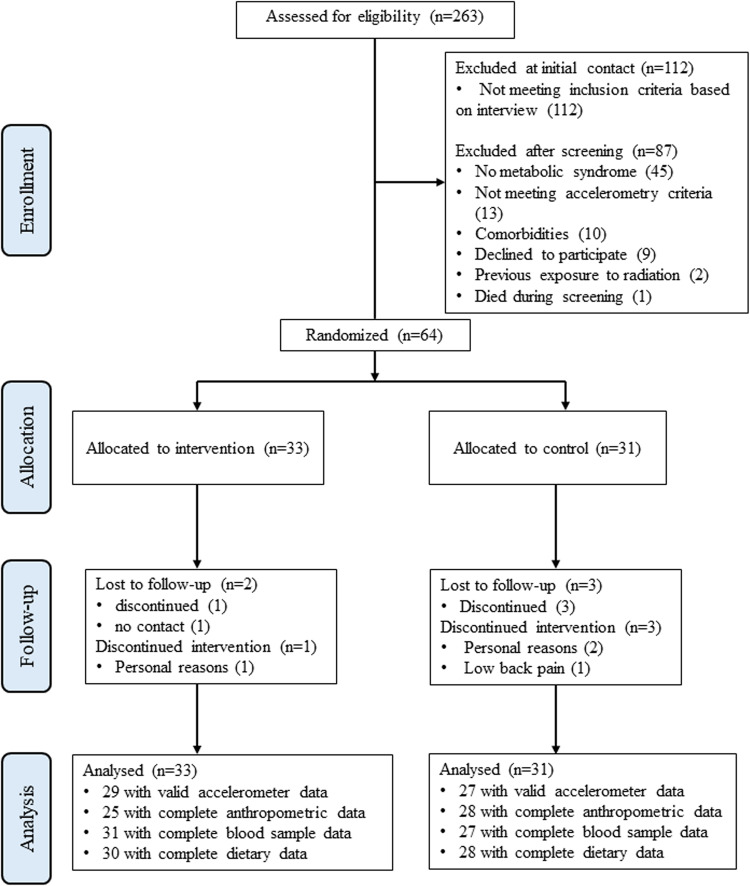
A total of 263 people volunteered, of which 151 were screened to fulfill the target of 64 eligible participants. Fourteen of the 64 participants (22%) were using cholesterol-lowering medication (statins). Two participants were lost to follow-up in the INT group: one discontinued the study for personal reasons, and one did not respond to contact attempts. Three participants discontinued in the CONT group, two for personal reasons and one due to back pain (see study flow diagram Fig. 1). Baseline characteristics of the INT and CONT groups are presented in Table 1.
Fig. 1.
CONSORT flow diagram of the study.
Table 1.
Baseline characteristics of the intervention group (INT) and the control group (CONT) presented as mean (SD) or n (%) .
| INT (n = 33) | CONT (n = 31) | |
|---|---|---|
| Age, years | 59 (6) | 57 (8) |
| Sex, n (%) females (n) | 20 (60) | 17 (53) |
| Body weight, kg | 92.4 (16.6) | 94.4 (15.8) |
| BMI, kg/m2 | 31.0 (4.0) | 31.8 (4.6) |
| Waist circumference, cm | 111.1 (11.6) | 110.8 (11.1) |
| Body fat, % | 43.1 (8.0) | 43.1 (8.0) |
| FFM, kg | 52.6 (11.9) | 53.5 (9.8) |
| Cholesterol medication, n (%) | 9 (27) | 5 (16) |
| Fasting plasma lipids | ||
| HDL mmol/l | 1.3 (0.3) | 1.4 (0.4) |
| LDL, mmol/l | 3.1 (0.9) | 3.0 (0.7) |
| Total cholesterol, mmol/l | 4.8 (1.0) | 4.6 (0.7) |
| HDL% | 28 (6.8) | 31 (8.7) |
| TG, mmol/l | 1.6 (0.7) | 1.1 (0.5) |
| FFA, mmol/l | 0.6 (0.2) | 0.59 (0.2) |
| Activity outcomes | ||
| SB, h/day | 10.0 (0.9) | 10.1 (1.1) |
| LPA, h/day | 1.7 (0.4) | 1.8 (0.5) |
| MVPA, h/day | 0.96 (0.31) | 0.97 (0.3) |
| Standing, h/day | 1.8 (0.6) | 1.8 (0.6) |
| Steps/day | 5204 (1910) | 5091 (1760) |
| Dietary intake | ||
| Energy intake, kJ/day | 7271 (1603) | 7789 (1725) |
| Energy intake/body mass, kJ/kg/day | 79.7 (16.1) | 84.8 (22.1) |
| Protein, g/day | 77.4 (20.7) | 79.5 (20.0) |
| CHO, g/day | 165.0 (46.6) | 180.9 (50.6) |
| MUFA, g/day | 26.5 (9.0) | 28.4 (9.6) |
| PUFA, g/day | 12.1 (5.0) | 13.0 (5.1) |
| SFA, g/day | 26.7 (8.8) | 30.4 (8.7) |
| Cholesterol, mg/day | 223.1 (104.4) | 287.2 (158.7) |
| Fiber, g/day | 18.5 (6.6) | 20.1 (7.6) |
BMI = body mass index; FFM = fat free mass; HDL = high density lipoprotein; LDL = low density lipoprotein; HDL% = total cholesterol divided by HDL; TG = triglycerides; FFA = free fatty acids; LPA = light physical activity; MVPA = moderate to vigorous activity; CHO = carbohydrate; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; SFA = saturated fatty acid.
SB and PA
The changes in accelerometer-measured SB and PA during the intervention have been reported previously27. In short, the changes during the intervention in SB, MVPA and steps were statistically different between groups: in the INT group SB decreased approximately by 40 (95% CI: 17, 65) min/dayand MVPA increased by 2011,28 min/day compared to the baseline, whereas there was no significant change in the CONT group. LPA increased in both groups by 104,15 min on average with no difference between groups. Steps/day increased in both groups: INT 3300 (2400, 4200) vs. CONT 1600 (600, 2600). Standing time did not significantly change during the intervention in either group. Participants in the INT group achieved the 1 h/day SB reduction on 39% o the measured days, and the CONT group reduced their SB by 1 h/day on 27% o the measured days, as reported earlier28.
Fasting lipids
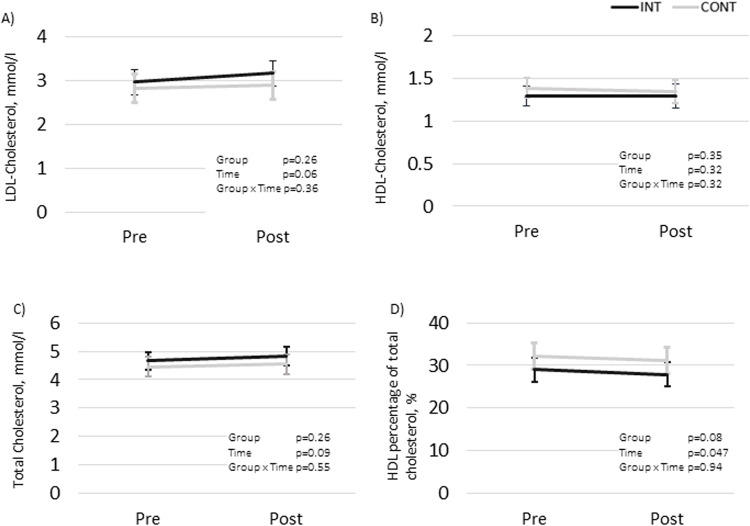
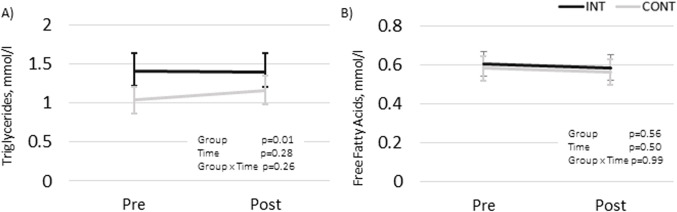
The mean changes over time did not differ statistically significantly between the groups in any of the blood lipid outcomes (LDL, HDL, TC, HDL%, TG, or FFA). The results remained non-significant when the participants using cholesterol-lowering medication were excluded from the analysis (data not shown). The changes in LDL, HDL, TC levels (mmol/L) and the HDL% are presented in Fig. 2 and TG and FFA in Fig. 3.
Fig. 2.
Fasting LDL- and HDL-cholesterol, total cholesterol, and proportion of HDL (%) in the intervention (INT) and the control (CONT) groups before and after the intervention presented as model-based means with 95% CI. Sex and possible usage of cholesterol-lowering medication were included as co-variables in all the analyses. (A) LDL, mmol/l; (B) HDL, mmol/l; (C) TC, mmol/l; (D) HDL%.
Fig. 3.
Fasting triglycerides and free fatty acids in intervention (INT) and control (CONT) groups before and after the intervention presented as model-based means with 95% CI. (A) triglycerides, mmol/l; (B) free fatty acids, mmol/l.
Confounding factors
The users of cholesterol-lowering medication had significantly lower LDL (p = 0.02) and higher HDL% values (p = 0.02) compared to the unmedicated participants. The medication did not have statistically significant effects in the TG and FFA analyses (Fig. 3). Therefore, medication was not included in the final models analyzing FFA and TG. Women had higher HDL (p = 0.001) and FFA (p = 0.002) compared to men.
Diet
As previously reported27, the total energy intake did not change statistically significantly during the intervention. The intake of SFA (g/day) decreased statistically significantly in the CONT group compared to the INT during the intervention (p = 0.03) (Table 2). The intake of CHO, protein, and total fat (g/day) did not change significantly during the intervention (Table 2). We also analyzed the changes in macronutrient (CHO, fats, and proteins) intake during the intervention as a percent of total daily energy intake (E%). The CONT group decreased their E% of SFA (p = 0.03), and E% of total fat (p = 0.049) compared to the INT. We did not find statistically significant changes or differences between groups in any other dietary variable (MUFA, PUFA, CHO and protein E%) (data not shown).
Table 2.
Macronutrient intakes (g/day) based on 4-day food diaries collected before and at the end of the intervention presented as model-based mean (95% CI).
| Baseline | 6 months | p | ||||||
|---|---|---|---|---|---|---|---|---|
| INT (n = 33) | CONT (n = 31) | INT (n = 30) | CONT (n = 28) | Group | Time | Sex | Group x Time | |
| SFA, g | 27.0 (24.0, 30.1) | 30.6 (27.5, 33.7) | 27.4 (24.2, 30.5) | 26.5 (23.3, 29.7) * | 0.50 | 0.06 | 0.12 | 0.03 |
| PUFA, g | 12.3 (10.6, 14.0) | 13.1 (11.3, 14.8) | 12.6 (10.9, 14.4) | 12.6 (10.8, 14.5) | 0.71 | 0.97 | 0.13 | 0.56 |
| MUFA, g | 26.9 (23.9, 29.9) | 28.5 (25.5, 31.6) | 26.2 (23.1, 29.4) | 27.0 (23.8, 30.2) | 0.52 | 0.33 | 0.07 | 0.68 |
| Fat, g | 76.2 (68.7, 83.7) | 83.9 (76.2, 91.5) | 77.4 (69.7, 85.2) | 76.7 (68.8, 84.6) | 0.47 | 0.26 | 0.10 | 0.11 |
| CHO, g | 166.1 (147.7, 184.6) | 181.5 (162.6, 200.3) | 167.2 (148.1, 186.3) | 184.9 (165.3, 204.4) | 0.17 | 0.73 | 0.37 | 0.86 |
| Prot, g | 78.4 (71.9, 85.0) | 80.0 (73.2, 86.7) | 75.5 (68.7, 86.7) | 75.7 (68.7. 82.6) | 0.84 | 0.13 | 0.03 | 0.77 |
| Fibers, g | 18.5 (16.1, 20.9) | 20.1 (17.7, 22.6) | 18.4 (15.9, 20.9) | 18.8 (16.3, 21.4) | 0.53 | 0.32 | 0.72 | 0.40 |
| Chol, mg | 205.5 (176.9, 239.0) | 256.7 (220.1, 299.6) | 225.9 (193.1, 264.3) | 227.6 (193.8, 267.2) | 0.22 | 0.83 | 0.22 | 0.08 |
SFA = saturated fatty acids; PUFA = polyunsaturated fatty acids; MUFA = monounsaturated fatty acids; CHO = carbohydrates; Prot = protein; Chol = cholesterol. * The within-group change in CONT p = 0.03.
Associations between blood lipids and PA
Changes in LDL correlated positively with changes in MVPA and LPA (r = 0.28, 0.35, p = 0.04, 0.01, respectively), and inversely with changes in SB (r= -0.31, p = 0.02) (Supplementary table). However, when adjusted for cholesterol-lowering medication, the association between changes in LDL and changes in MVPA, turned non-significant (p = 0.07). There were no significant correlations between changes in other lipids and changes in SB or PA, whereas changes in BMI, WC, and body mass correlated negatively with changes in PA (Supplementary table). Additionally, a positive correlation was found between changes in TG and changes in FFM (r = 0.31, p = 0.02). Changes in macronutrient intake did not correlate with changes in blood lipids (Supplementary table ).
Discussion
In this six-month RCT, we investigated the effects of a SB reduction on the blood lipids in adults with MetS. Despite differences in SB and PA, there were no statistically significant differences in the changes of fasting lipid levels between the INT and CONT groups during the intervention. Interestingly, the CONT group also increased steps/day, as well as reduced their SFA intake during the intervention. Additionally, the CONT group increased MVPA during the last quartile of the intervention period, as previously reported27, which may partly explain why the achieved reduction of SB in INT was not successful in improving the blood lipid levels in comparison to CONT. These changes in the CONT group may have narrowed the differences between the groups, even though the INT group increased the amount of MVPA 20 min/day27. However, our participants had less MVPA, and higher sedentary time compared to the PA and SB levels of a population-based sample of Finnish adults assessed with similar accelerometry methods29. It should be noted that the amount of measured MVPA depends on the analysis method30, and, for example, the epoch length has a significant impact on the estimates as recently shown31. The accelerometer data of this study was analyzed in six-second epochs to capture every meaningful movement of the participants, and thus includes even short MVPA bouts. Therefore the total amount of MVPA in this study may appear relatively high and should not be compared to studies using longer epoch lengths or self-reports.
The novelty of our study was the six-month accelerometer measurement of SB and PA, whereas in previous RCT studies the accelerometer data has been collected for shorter periods25,26, which may not have been able to detect the actual behavioral changes during the intervention. In our study, the INT group reduced their SB for 40 min per day instead of the targeted one-hour reduction per day27. Changing the passive behavior has also been challenging in previous studies: Aadahl et al.26 did not reach statistically significant changes in accelerometer-measured SB following a six-month community-based motivational counseling intervention aiming to reduce SB. Biddle and colleagues25 identified several reasons for the lack of commitment observed in their 12-month structured education intervention: e.g., the lack of perceived health risk in the current behavior and the preference to adopt more active PA behaviors rather than to sit less. Additionally, participants may experience social and physical barriers to sitting less, and this might be a critical hindrance to changing behavior. There might also be too few contacts with the researcher and difficulties with the self-monitoring tool25. All of these phenomena may have occurred in the current study, too.
Our present results are in line with prior studies that have been 6 to 12 months in duration25,26 and a meta-analysis by Chastin and colleagues14, which have not found statistically significant effects on blood lipid concentrations with SB reductions. However, a few studies have reported increased HDL-levels after SB interventions11,15,32. Interestingly, in our study the change in TG levels during the first three months of the study was different between groups, favoring INT33, but from baseline to six months the difference was no longer statistically significant. Furthermore, HDL% did decrease in both groups and LDL cholesterol tended to increase in both groups during the intervention. We previously reported similar results regarding TC and LDL changes at the 3-month timepoint as well33. It thus appears that blood lipids may fluctuate and worsen over time in this kind of a risk population as MetS often progresses towards cardiometabolic disease development. Therefore, it may be concluded that reducing SB alone is not effective for favorable and sustainable modification of blood lipids and focus also on physical exercise and nutrition is likely required. Despite no difference in the total energy intake between the groups27, the CONT group had a statistically significant reduction in SFA intake, which may have had a positive effect on plasma cholesterol and LDL levels8,34,35. This dietary change in the CONT group, along with increased MVPA in the last quartile of the intervention30, may partly explain why there was no difference in blood lipids between the groups at the six-month time point. Previous RCTs aiming to reduce SB25,26,32 have not assessed dietary habits, and thus this is a novel finding.
Surprisingly, increases in LPA and MVPA were associated with increases in LDL in the present study34,35. However, when the association between changes in LDL and changes in MVPA was controlled for cholesterol-lowering medication, the association turned non-significant (p = 0.07). Furthermore, changes in TG correlated positively with changes in FFM, indicating that an increase in FFM possibly also increased TG. Fat mass and FFM have both been associated with elevated TG levels in previous studies36,37.
Despite unchanged blood lipid levels, WC and body mass decreased slightly in both groups, and these decreases were associated with increases in PA, as previously reported27. Thus, the unintended behavioral changes in CONT may have resulted in similar changes in body composition in both groups and partly explain why there were no differences in the blood lipid levels between the INT and the CONT group after the intervention. In line with our results, previous interventions aiming to reduce SB have had favorable effects on body composition25,26. Indeed, weight loss is likely to have a positive effect on blood lipids in overweight and obese adults36,38 and it is a part of a recommended lifestyle modification when treating MetS or dyslipidemias5,8,38. Therefore, reducing SB may be used together with other strategies as a method to support weight loss in order to improve blood lipids, although this particular intervention was not effective. We have previously reported that this SB reduction did not improve maximal aerobic fitness either, but the more daily steps participants took during the intervention, the more their fitness improved28.
The major strengths of this study are the RCT study design and the accelerometer data collection throughout the whole six-month intervention, as in previous RCT studies accelerometers have been used only for shorter periods25,26. Additionally, validated algorithms were used to measure SB and PA during the entire six-month trial with six-second intervals39,40. The MAD-method has about 1.2 MET accuracy, when estimating energy expenditure during bipedal locomotion across a broad spectrum of speeds25, while the APE-method has an accuracy rate of approximately 90% in classifyng sitting and standing in free-living conditions26. Another strength is the consideration of the role of dietary intake on the blood lipids, yet the nutrition may have affected the lipid concentrations34,35. However, it may be considered a limitation that the four-day food diaries were assessed only at baseline and at six-month time points of the intervention. Another limitation is the relatively small sample size, which was calculated for the primary outcome of the study, while blood lipids are a secondary outcome. This may have resulted in inadequate study power. Also, blinding of the participants was not possible due to the nature of the intervention.
This RCT provides important information about the lacking effects of reduced SB on blood lipids. Therefore, these results may have clinically meaningful implications in terms of considering a cardiometabolic risk reduction: it seems that an intervention reducing daily SB by ~ 40 min together with a 20-minute increase in MVPA might not be sufficient to improve blood lipid levels. Therefore, future studies with more pronounced reductions in SB and/or increased volume or intensity of PA are warranted.
Conclusion
This six-month intervention reduced daily SB by 40 min and led to a concomitant 20-minute increase in MVPA. However, such an intervention does not seem to be effective in improving blood lipids in inactive adults with metabolic syndrome. Thus, reducing SB together with increased PA and proper nutrition may be needed to reduce the risk of cardiometabolic diseases. Future studies are warranted.
Methods
Study design
This is a secondary analysis of a RCT that was conducted at the Turku PET Centre, Turku, Finland, between April 2017 and March 2020. The trial consisted of a one-month screening period and a six-month intervention.
Participants
Participants (n = 64) were recruited by bulletin leaflets and newspaper advertisements from the local community. The inclusion criteria were BMI 25–40 kg/m2, age 40–65 years, blood pressure < 160/100 mmHg, fasting glucose < 7.0 mmol/L, sedentary time of at least 10 h or 60% of the accelerometer wear time per day during the screening measurement, fulfillment of the MetS criteria according to Alberti et al.6 and physical inactivity (not meeting the PA recommendations according to self-reports). The exclusion criteria were diagnosed diabetes, history of a cardiac event, diagnosed depression or bipolar disorder, any chronic disease that could create a safety hazard for the participant or study design, abundant use of alcohol, smoking, using snuff tobacco or narcotics, and inability to understand written Finnish.
Randomization
The participants were randomly allocated into the intervention (INT) and control (CONT) groups by a statistician. Randomization was performed with random permuted blocks with stratification for sex, a block size of 44, and 1:1 allocation ratio in SAS (version 9.4 for Windows).
Intervention and control groups
All participants were able to monitor their daily SB and PA using a mobile application (ExSed, UKK Terveyspalvelut Oy, Tampere, Finland) during the intervention period. The participants in the INT group received a 1-hour personal counselling session with a physiotherapist and were individually guided to reduce their daily SB by, for example, adding standing and PA whenever possible (short walks, choosing stairs, and breaking up sitting by moving). The daily SB goal was set at one hour less compared to the individual SB during screening. Correspondingly, one hour was added to standing, light-intensity PA (LPA), and/or MVPA goals on the mobile application. The intervention aimed at reducing SB by increasing PA in daily living activities, without increasing exercise training per se, and a maximum of 20 min/day was added to MVPA, according to individual preferences. The participants in the CONT group were guided to maintain their normal PA and SB habits (e.g. MVPA, LPA, standing time and steps), and the goals were set equal to the screening. A detailed description of the research design has been published previously21,27,33.
Measurements
Accelerometer data
During the four-week screening phase, the baseline SB and PA were determined using hip-worn tri-axial accelerometers during waking hours (except when exposed to water) (UKK AM30, UKK Terveyspalvelut Oy, Tampere, Finland). Through the whole six-month intervention, interactive hip-worn tri-axial accelerometers (Movesense, Suunto, Vantaa, Finland) with embedded measurement and analysis algorithms and a connected smartphone application (ExSed, UKK Terveyspalvelut Oy, Tampere, Finland) were used in both INT and CONT groups to measure SB and PA during waking hours, as previously reported27. Mean amplitude deviation (MAD)39 and angle for posture estimation (APE) methods40 were used to analyze the accelerometer data in six-second intervals40,41. PA was defined as energy expenditure higher than 1.5 metabolic equivalents (MET) with corresponding MAD cut-point value above 22.5 mg (milligravity). The MAD cut-point was 91 mg for 3.0 METs (separating moderate-intensity from light-intensity PA), and 414 mg for 6.0 METs (separating vigorous-intensity from moderate-intensity PA)25. Posture was analyzed for epochs having MAD value less than 22.5 mg, and the posture classification was based on the APE value. The orientation of the accelerometer during walking served as the reference vector, and APE was calculated as the angle between the concurrent accelerometer orientation vector and the reference vector. In validation studies, APE has been shown to be able to separate standing from sitting or lying with 100% accuracy and siting from lying with 95% accuracy in stanardized conditions. In real-world, free-living conditions, simultaneous data collected from thigh- and hip-worn accelerometers yielded an agreement rate of approximately 90%. Posture was clasified as standing if the APE value was less than 11.6°, and otherwise as SB26. Periods longer than 120 min without movement were classified as non-wear time.
Blood samples
Venous blood samples were drawn in Turku University Hospital Laboratory after at least 10 h of fasting. Fasting plasma lipids, including TG, total cholesterol (TC), low- and high-density lipoprotein cholesterol (LDL- and HDL), the proportion of HDL of TC (HDL%), and free fatty acids (FFA), were determined by enzymatic colorimetric tests (Cobas 8000 c702, Roche Diagnostics GmbH, Mannheim, Germany).
Anthropometric measures
Air displacement plethysmography (the Bod Pod system, COSMED, Inc., Concord, CA, USA) was used to measure body weight, fat mass, body fat-%, and fat free mass (FFM) after at least four hours of fasting. Waist circumference (WC) was measured during normal exhalation by a measuring tape (at the midpoint of the iliac crest and the lowest rib). To obtain an accurate measure, the measurement was repeated until the same measure was obtained twice. All WC measurements were performed by one assessor.
Dietary intake
The participants were instructed to not alter their diet during the intervention. The intake of total energy; carbohydrates (CHO); total fat; saturated (SFA), polyunsaturated (PUFA) and monounsaturated (MUFA) fatty acids; protein; cholesterol; and fiber were assessed by 4-dayfood diaries (including one weekend day) at baseline and at the end of the intervention. The food diaries were inspected for completeness and accuracy with the help of a portion picture booklet and the mean daily intakes of nutrients were calculated with a computerized software (AivoDiet 2.2.0.1, Aivo, Turku) using the Food and Nutrient Database of the National Institute for Health and Welfare (Fineli, https://fineli.fi/fineli/en/).
Statistical analyses
The power calculations were done for the primary outcome of the whole study project (whole-body insulin sensitivity), as previously reported27. The normal distribution of variables was evaluated by visual analysis, and the normal distribution of residuals was also evaluated by Cook’s distance and leverage values. Logarithmic transformation (log10) was performed for HDL and TG to achieve a normal distribution of the residuals. A dichotomous variable was created for self-reported use of cholesterol-lowering medication (yes/no). Linear mixed model for repeated measurements was used to determine the changes between and within groups over time, as well as their interaction. To adjust for confounding factors, sex was added to all the models and the possible presence of cholesterol-lowering medication was added to the models evaluating the changes in LDL, HDL, TC, and HDL%. Multiple comparisons were adjusted using the Tukey-Kramer method, and compound symmetry covariance structure was used for time. Associations between changes in measured outcomes (i.e. post value – pre value) were assessed with Pearson’s correlation coefficient. Linear analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and correlation analyses with IBM SPSS 26.0 (IBM Corp., Armonk, NY, USA). Model-based means are presented with 95% cnfidence interval (CI), and p < 0.05 (two-tailed) was considered as statistically significant.
Supplementary Information
Acknowledgements
This study was conducted within the Centre of Excellence in Cardiovascular and Metabolic Research, supported by the Academy of Finland, the University of Turku, Turku University Hospital, and Åbo Akademi University. We thank the staff in the Turku PET Centre, University of Turku, and the laboratory personnel in the Turku University Hospital Laboratory for their proficient assistance in conducting the study.
Abbreviations
- APE
angle for posture estimation
- BMI
Body Mass Index
- CHO
Carbohydrates
- CONT
Control group
- FFA
Free fatty acids
- FFM
Free fat mass
- HDL
High-density lipoprotein
- HDL%
Proportion of HDL
- INT
Intervention group
- LDL
Low-density lipoprotein
- LPA
Light-intensity physical activity
- MAD
Mean amplitude deviation
- MET
Metabolic Equivalent
- MetS
Metabolic Syndrome
- MUFA
Monounsaturated fatty acid
- MVPA
Moderate-to-vigorous physical activity
- PA
Physical activity
- PUFA
Polyunsaturated fatty acid
- RCT
Randomized controlled trial
- SB
Sedentary Behavior
- SFA
Saturated fatty acid
- TC
Total Cholesterol
- TG
Triglyceride
- WC
Waist circumference
Authors’ contributions
Author contributions are the following: I.H.A., J.K. T.V. and T.S. conception and design of research; T.S., M.K., S.L., T.G., O.E. and N.H. performed the experiments, V.Y., H.V-Y., T.G., T.S., N.H., J.N., and E.L. analysed the data; V.Y. drafted the manuscript; all authors edited and revised the manuscript; all authors approved the final version of the manuscript.
Funding
The study was financially supported by the Academy of Finland, the Finnish Cultural Foundation, the Juho Vainio Foundation, the Hospital District of Southwest Finland, the Yrjö Jahnsson Foundation, the Turku University Foundation, Diabetestutkimussäätiö (the Finnish Diabetes Research Foundation), and TYKS-foundation.
Data availability
Data and materials are available upon a reasonable request from authors.
Declarations
Ethics approval and consent to participate
The study was conducted at the Turku PET Centre, Turku, Finland between April 2017 and March 2020 according to good clinical practice and the Declaration of Helsinki. The study was approved by the Ethics Committee of the Hospital District of Southwestern Finland (16/1801/2017) and is registered at ClinicalTrials.gov (NCT03101228, 05/04/2017).
Consent for publication
The participants gave their informed consent before entering the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biswas, A. et al. Sedentary Time and Its Association With Risk for Disease Incidence, Mortality, and Hospitalization in Adults. Ann Intern Med162(2), 123–32. 10.7326/M14-1651 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Chau, J. Y. et al. Daily sitting time and all-cause mortality: a Meta-analysis. PLoS One. 8 (11), e80000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson, R. et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol33(9), 811–29. 10.1007/s10654-018-0380-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates, T. et al. Prospectively Reallocating Sedentary Time: Associations with Cardiometabolic Health. Med Sci Sports Exerc52(4), 844–50. https://pubmed.ncbi.nlm.nih.gov/31688653/ (2020) (Cited 2023 Nov 13). [DOI] [PubMed] [Google Scholar]
- 5.Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet365(9468), 1415–28 (2005) https://www.sciencedirect.com/science/article/pii/S0140673605663787. [DOI] [PubMed] [Google Scholar]
- 6.AlbertiKGMM et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120 (16), 1640–1645 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Powell, C., Herring, M. P., Dowd, K. P., Donnelly, A. E. & Carson, B. P. The cross-sectional associations between objectively measured sedentary time and cardiometabolic health markers in adults – a systematic review with meta-analysis component. Obes Rev19(3), 381–95. https://onlinelibrary.wiley.com/doi/full/10.1111/obr.12642 (2018) (Cited 2023 Nov 13). [DOI] [PubMed] [Google Scholar]
- 8.Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J.41(1), 111–88. 10.1093/eurheartj/ehz455 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard, B. G., Benn, M., Schnohr, P. & Tybjærg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA.298(3), 299–308. 10.1001/jama.298.3.299 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Jung, E. et al. Serum Cholesterol Levels and Risk of Cardiovascular Death: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public. Health.19(14). https://pubmed.ncbi.nlm.nih.gov/35886124/. (2022). Cited 2023 Apr 14. [DOI] [PMC free article] [PubMed]
- 11.Hadgraft, N. T. et al. Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta-analyses. Br J Sports Med.55(3), 144 (2021) http://bjsm.bmj.com/content/55/3/144.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh, R., Stamatakis, E., Folkerts, D., Allgrove, J. E. & Moir, H. J. Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sports Med.50(2), 295–330. 10.1007/s40279-019-01183-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buffey, A. J., Herring, M. P., Langley, C. K., Donnelly, A. E. & Carson, B. P. The Acute Effects of Interrupting Prolonged Sitting Time in Adults with Standing and Light-Intensity Walking on Biomarkers of Cardiometabolic Health in Adults: A Systematic Review and Meta-analysis. Sports Med. 52 (8), 1765–1787 (2022). [DOI] [PMC free article] [PubMed]
- 14.Chastin, S. F. M., Egerton, T., Leask, C. & Stamatakis, E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obes. (Silver Spring). 23 (9), 1800–1810 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Pinto, A. J. et al. Physiology of sedentary behavior. Physiol Rev.103(4), 2561–622. 10.1152/physrev.00022.2022 (2023) (Cited 2023 Nov 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henson, J. et al. Physical activity and lipidomics in a population at high risk of type 2 diabetes mellitus. J Sports Sci.38(10), 1150–60. 10.1080/02640414.2020.1744836 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Kujala, U. M., Mäkinen, V. P., Heinonen, I., Soininen. P., Kangas, A. J., Leskinen, T. H., Rahkila, P., Würtz. P., Kovanen, V., Cheng. S., Sipilä, S., Hirvensalo, M., Telama, R., Tammelin, T., Savolainen, M. J., Pouta, A., O'Reilly, P. F., Mäntyselkä, P., Viikari, J., Kähönen, M., Lehtimäki, T., Elliott, P., Vanhala, M.J., Raitakari, O. T., Järvelin, M. R., Kaprio, J., Kainulainen, H. & Ala-Korpela, M. Long-term leisure-time physical activity and serum metabolome. Circulation. 127 (3), 340–8. 10.1161/CIRCULATIONAHA.112.105551 (2013). [DOI] [PubMed]
- 18.Halbert, J. A., Silagy, C. A., Finucane, P., Withers, R. T. & Hamdorf, P. A. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: A meta-analysis of randomized, controlled trials. Eur J Clin Nutr.53(7), 514–22. 10.1038/sj.ejcn.1600784 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Wang, Y. & Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis.16(1), 132. 10.1186/s12944-017-0515-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon, A. S. & Sanchez, O. A. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc.33(6 Suppl), S502-15 (2001) (discussion S528-529). [DOI] [PubMed] [Google Scholar]
- 21.Sjöros, T. et al. Both sedentary time and physical activity are associated with cardiometabolic health in overweight adults in a 1-month accelerometer measurement. Sci Rep.10(1), 20578. 10.1038/s41598-020-77637-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler, M. J. et al. Combined effects of continuous exercise and intermittent active interruptions to prolonged sitting on postprandial glucose, insulin, and triglycerides in adults with obesity: a randomized crossover trial. Int J Behav Nutr Phys Act.17(1), 152. 10.1186/s12966-020-01057-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekelund, U. et al. Joint associations of accelerometer-measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med.54(24), 1499 (2020) http://bjsm.bmj.com/content/54/24/1499.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 6 (10), e1077–e1086 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Biddle, S. J. H. et al. A Randomised Controlled Trial to Reduce Sedentary Time in Young Adults at Risk of Type 2 Diabetes Mellitus: Project STAND (Sedentary Time ANd Diabetes). PLoS One.10(12), e0143398. 10.1371/journal.pone.0143398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aadahl, M. et al. Motivational Counseling to Reduce Sitting Time: A Community-Based Randomized Controlled Trial in Adults. Am J Prev Med.47(5), 576–86. 10.1016/j.amepre.2014.06.020 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Sjöros, T. et al. Reducing Sedentary Time and Whole-Body Insulin Sensitivity in Metabolic Syndrome: A 6-Month Randomized Controlled Trial. Med Sci Sports Exerc.55(3), 342 (2023) (http:///pmc/articles/PMC9924963/. Cited 2023 Apr 19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norha, J. et al. Effects of reducing sedentary behavior on cardiorespiratory fitness in adults with metabolic syndrome: A 6-month RCT. Scand J Med Sci Sports.00, 1–10. https://onlinelibrary.wiley.com/doi/full/10.1111/sms.14371 (2023) (Cited 2023 Apr 21). [DOI] [PubMed] [Google Scholar]
- 29.Husu, P. et al. Objectively measured sedentary behavior and physical activity in a sample of Finnish adults: A cross-sectional study. BMC Public Health.16(1), 1–11. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-3591-y (2016) (Cited 2023 Jan 14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vähä-ypyä, H., Sievänen, H., Husu, P., Tokola, K. & Vasankari, T. Intensity Paradox—Low-Fit People Are Physically Most Active in Terms of Their Fitness. Sensors (Basel).21(6), 1–12 (2021). Available from: /pmc/articles/PMC8002087/. Cited 2023 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vähä-Ypyä, H. et al. How adherence to the updated physical activity guidelines should be assessed with accelerometer?. Eur J Public Health.32(Suppl 1), (2022). /pmc/articles/PMC8002087/. (Cited 2024 Jun 11). [DOI] [PMC free article] [PubMed]
- 32.Pesola, A. J. et al. Accelerometer-assessed sedentary work, leisure time and cardio-metabolic biomarkers during one year: Effectiveness of a cluster randomized controlled trial in parents with a sedentary occupation and young children. PLoS One.12(8), e0183299. 10.1371/journal.pone.0183299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garthwaite, T. et al. Effects of reduced sedentary time on cardiometabolic health in adults with metabolic syndrome: A three-month randomized controlled trial. J Sci Med Sport.25(7), 579–85. 10.1016/j.jsams.2022.04.002 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Mente, A. et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol.5(10), 774–87. 10.1016/S2213-8587(17)30283-8 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Mensink, R. P., Zock, P. L., Kester, A. D. & Katan, M. B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr.77, 1146–55. https://academic.oup.com/ajcn/article/77/5/1146/4689813 (2003) (Cited 2023 Jan 12). [DOI] [PubMed] [Google Scholar]
- 36.Hasan, B. et al. Weight Loss and Serum Lipids in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab.105(12), 3695–703. 10.1210/clinem/dgaa673 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Mensink, R. P. & Organization, W. H. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva: World Health Organization; (2016). https://apps.who.int/iris/handle/10665/246104
- 38.Poobalan, A. et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes – a systematic review. Obesity Reviews.5(1), 43–50. 10.1111/j.1467-789X.2004.00127.x (2004). [DOI] [PubMed] [Google Scholar]
- 39.Vähä-Ypyä, H. et al. Validation of Cut-Points for Evaluating the Intensity of Physical Activity with Accelerometry-Based Mean Amplitude Deviation (MAD). PLoS One. 10(8). (2015). https://pubmed.ncbi.nlm.nih.gov/26292225/. Cited 2023 Apr 14. [DOI] [PMC free article] [PubMed]
- 40.Vähä-Ypyä, H., Husu, P., Suni, J., Vasankari, T. & Sievänen, H. Reliable recognition of lying, sitting, and standing with a hip-worn accelerometer. Scand J Med Sci Sports.28(3), 1092–102. https://pubmed.ncbi.nlm.nih.gov/29144567/ (2018) (Cited 2023 Apr 14). [DOI] [PubMed] [Google Scholar]
- 41.Vähä-Ypyä, H., Vasankari, T., Husu, P., Suni, J. & Sievänen, H. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin Physiol Funct Imaging.35(1), 64–70. https://pubmed.ncbi.nlm.nih.gov/24393233/ (2015) (Cited 2022 Dec 7). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are available upon a reasonable request from authors.