Abstract
Background
Late outbreak identification is a common risk factor mentioned in case reports of large respiratory infection outbreaks in long-term care (LTC) homes.
Aim
To systematically measure the association between late SARS-CoV-2 outbreak identification and secondary SARS-CoV-2 infection and mortality in residents of LTC homes.
Methods
We studied SARS-CoV-2 outbreaks across LTC homes in Ontario, Canada from March to November 2020, before the COVID-19 vaccine rollout. Our exposure (late outbreak identification) was based on cumulative infection pressure (the number of infectious resident-days) on the outbreak identification date (early: ≤ 2 infectious resident-days, late: ≥ 3 infectious resident-days), where the infectious window was −2 to +8 days around onset. Our outcome consisted of 30-day incidence of secondary infection and mortality, based on the proportion of at-risk residents with a laboratory-confirmed SARS-CoV-2 infection with onset within 30 days of the outbreak identification date.
Results
We identified 632 SARS-CoV-2 outbreaks across 623 LTC homes. Of these, 36.4% (230/632) outbreaks were identified late. Outbreaks identified late had more secondary infections (10.3%; 4,437/42,953) and higher mortality (3.2%; 1,374/42,953) compared with outbreaks identified early (infections: 3.3%; 2,015/61,714; p < 0.001, mortality: 0.9%; 579/61,714; p < 0.001). After adjustment for 12 LTC home covariates, the incidence of secondary infections in outbreaks identified late was 2.90-fold larger than that of outbreaks identified early (OR: 2.90; 95% CI: 2.04–4.13).
Conclusions
The timeliness of outbreak identification could be used to predict the trajectory of an outbreak, plan outbreak measures and retrospectively provide feedback for quality improvement, with the objective of reducing the impacts of respiratory infections in LTC home residents.
Keywords: long term care facility, LTCF, nursing homes, aged care facilities, outbreak, timeliness, SARS-CoV-2, infection prevention and control
Key public health message.
What did you want to address in this study and why?
We aimed to measure the association between how late a SARS-CoV-2 outbreak is identified and the final size of the outbreak in long-term care homes. Such an indicator of the outbreak identification delay could help gauge the resources needed to control a SARS-CoV-2 outbreak and make comparisons across long-term care homes for quality improvement purposes.
What have we learnt from this study?
We classified outbreaks based on the cumulative number of infectious resident-days elapsed on the date the outbreak was identified, i.e. late ≥ 3 resident-days vs early ≤ 2 days. Across 632 outbreaks recorded in Ontario, Canada from March to November 2020, those identified late had a substantially higher incidence of secondary infections (10%) compared to outbreaks identified early (3%). Mortality was also higher in outbreaks identified late (3%) vs early (1%).
What are the implications of your findings for public health?
Outbreaks identified late evolved to be larger and more severe. Measurement of outbreak identification delays, leveraging the concept of resident infection pressure, could be used to plan outbreak responses and guide quality improvement initiatives.
Introduction
Following the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in early 2020, many countries faced frequent and large SARS-CoV-2 outbreaks in long-term care (LTC) homes; when LTC home residents were infected, advanced age and comorbidity burden drove high case fatality rates [1,2]. The result was a disproportionate burden of mortality among LTC home residents, which early in the pandemic represented 30–80% of deaths across European and North American countries, although LTC home residents represented less than 1% of these countries’ respective populations [3-5].
Several factors have been identified that affect SARS-CoV-2 introductions and transmission within LTC homes. Community transmission is known to be associated with the probability of introduction of SARS-CoV-2 into LTC homes by staff [6], and factors including for-profit status [7], LTC home quality rating [8] and shared multi-bedded rooms [9] have been shown to drive the size of outbreaks after introduction. Case identification delays are also known to be an important driver of SARS-CoV-2 transmission within LTC homes [10]. Research from 2020, early in the COVID-19 pandemic, identified a high incidence of infection among residents and staff already at the time of the first identified case (outbreak identification) [11]. Early outbreak identification can enable prompt implementation of measures to contain transmission, particularly in more crowded LTC homes where transmission can occur more rapidly [12]. In Ontario, Canada, access to timely testing in the general population and in LTC homes improved substantially over the course of 2020 with the addition of staff SARS-CoV-2 testing policies; data reflected this with higher testing rates and lower test positivity in fall of 2020 compared to spring of 2020 [13].
To our knowledge, the association between delays in SARS-CoV-2 outbreak identification and the eventual size of LTC home outbreaks has not been examined empirically to date. Using granular resident and staff information on SARS-CoV-2 infection outbreaks during the pandemic period before the rollout of SARS-CoV-2 vaccines in Ontario, Canada [14], we examined how delays in LTC home SARS-CoV-2 outbreak identification were associated with the subsequent incidence of infection and mortality.
Methods
Study population
We conducted a retrospective cohort study of SARS-CoV-2 outbreaks across 623 LTC homes in Ontario, Canada from 1 March 2020 to 14 November 2020, a period ending 1 month before the COVID-19 vaccine rollout in Ontario LTC homes. Homes could be included more than once if they experienced multiple outbreaks. Laboratory-confirmed LTC home resident and staff (including family caregivers) SARS-CoV-2 cases were included. SARS-CoV-2 testing in Ontario during this period was primarily based on nasopharyngeal swab specimens that were analysed using nucleic acid amplification.
Data sources
Data for all detected SARS-CoV-2-infected residents and staff related to LTC home outbreaks were obtained from Ontario’s Case and Contact Management (CCM) database. For each case, CCM included information on date of symptom onset (when symptomatic), specimen collection date and positive test result date. Monthly facility-level information on resident characteristics were obtained from the Continuing Care Reporting System, which is based on quarterly Resident Assessment Instrument Minimum Dataset (RAI-MDS) resident assessments [15]; information on occupancy, facility ownership and bed types were obtained from the Ontario Ministry of Long-Term Care inspections branch [9]. Datasets were cleaned and merged based on the facility name or identifier, using R version 4.0.4.
Outbreak definition
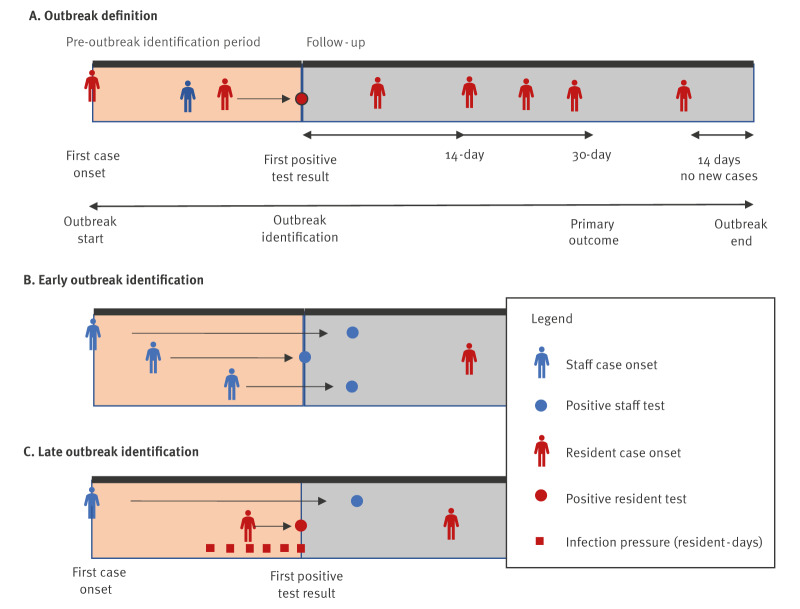
A distinct LTC home outbreak (Figure 1) was defined as an occurrence of one resident or staff case with onset at least 14 days since the last case (based on Ontario SARS-CoV-2 outbreak surveillance definitions [16]). Onset date was defined as the first of symptom onset date or specimen collection date, in order to capture the first evidence of SARS-CoV-2 infection for each case. All subsequent resident and staff cases were included in the outbreak until 14 days passed with no new cases with an onset. Outbreaks could include a single case, as per provincial outbreak definitions. For each outbreak, we defined the outbreak identification date as the first positive test result date, and based on this, we defined a pre-outbreak identification period and a follow-up period (Figure 1). The pre-outbreak identification period was the time between the first resident or staff onset date up to and including the outbreak identification date. The full follow-up period for each outbreak was the period from the day after the outbreak identification date until 14 days after the last onset date of the outbreak.
Figure 1.
Definitions used to define timeliness of SARS-CoV-2 outbreak identification in long-term care homes, Ontario, Canada, 1 March–14 November 2020
ID: identification.
Panel A shows an overview of outbreak definitions, including the pre-outbreak identification period and the follow-up period. The pre-outbreak identification period starts at the time of onset of the first identified case (resident or staff) and ends on the outbreak identification date (first positive test result date, resident or staff). The follow-up period starts on the day after the pre-outbreak identification period ends and continues for 30 days (primary outcome) or for 14 days (secondary outcome) or until the end of the outbreak, 14 days after the last case (resident or staff; secondary outcome). Only resident cases in the follow-up period are counted as part of the outcome.
Panel B shows an example of an outbreak identified early (≤ 2 days of infection pressure). This outbreak has been identified before there are any resident cases; as such there are 0 days of resident infection pressure at the time of outbreak identification.
Panel C shows an example of an outbreak identified late (≥ 3 days of infection pressure). In this example, the single resident case had symptoms that started 3 days before the outbreak was identified (based on their own positive test); as such, there were already 6 days of resident infection pressure at the time of outbreak identification.
Exposures – outbreak infection pressure and late outbreak identification
Outbreak infection pressure was defined as the number of infectious resident-days occurring in the window from 14 days prior (upper limit on one incubation period) to the outbreak identification date (Figure 1). We considered residents to be infectious for 10 days (from 2 days before onset to 8 days after), and stopped being infectious after that time, or earlier if they were hospitalised or died [12]. This definition measures the number of infectious resident-days of exposure occurring before the outbreak is identified, and relates to fundamental exposure measures in hospital-acquired infection epidemiology (colonisation pressure [17]) and invasion ecology (propagule pressure [18]). We truncated infection pressure at 25 infectious resident-days (the 98th percentile). Note that this measure excludes staff infection pressure, because of the known role of staff in propagating LTC outbreaks [2]; outbreaks without staff infection pressure are likely to represent outbreaks with poor staff case identification.
We categorised outbreaks based on infection pressure as being identified early (≤ 2 infectious resident-days) or late (≥ 3 infectious resident-days), as this distinguished an outbreak identified before vs on or after, respectively, the onset date of a single resident case. Because, by definition, staff person-days of infection pressure did not contribute to the measure; an outbreak could have 0 resident-days of infection pressure if the outbreak was first identified among staff more than 2 days prior to any resident cases (Figure 1B).
Outcomes
The primary outcome of this analysis was the 30-day secondary infection incidence, defined as the proportion of at-risk LTC home residents with onset within the first 30 days of the follow-up period. The number of at-risk residents was calculated as the occupancy of the LTC home in the month before the outbreak identification date, minus the number of LTC home residents infected in the pre-outbreak identification period. In addition, we examined two secondary follow-up windows: the infection incidence over the entire duration of the follow-up period and the 14-day infection incidence. For each of these three follow-up windows, we also examined the incidence of secondary mortality, which were SARS-CoV-2 deaths among those infected, for a total of six outcome measures.
Covariates
Resident characteristics included: the percentage of residents aged ≥ 85 years, the percentage of female residents, the percentage of residents with university education, the percentage of residents with dementia, as well as the mean number of comorbidities including congestive heart failure, chronic obstructive pulmonary disease (COPD), cancer, diabetes and renal failure, and average activities of daily living (ADL) impairment scale. Long-term care home structural characteristics included: size (< 100 or ≥ 100 beds), profit status (municipal, non-profit or for-profit) and the home crowding index (< 2 or ≥ 2 residents per room) [9]. The crowding index was derived based on an algorithm using LTC home bed license types, and captures the average number of LTC home residents per bedroom and bathroom [9]. Other characteristics included the number of outbreaks before the outbreak identification date (0, 1, ≥ 2), the outbreak identification date (Wave 1: 1 Mar–31 Aug 2020; Wave 2: 1 Sep–14 Nov 2020) and the community incidence of SARS-CoV-2 excluding LTC home and congregate setting outbreaks in the public health region (n = 32) in the month of the outbreak per 10,000 population, based on CCM data.
Statistical methods
To determine whether late outbreak identification and infection pressure were associated with secondary incidence of infection and mortality, logistic regression models were fitted to measure odds ratios (OR). All models were fitted using generalised additive modelling framework (mgcv package in R [19]). The outcome was specified as a quasibinomial count [20], with random intercepts accounting for facility-level clustering. Unadjusted models included only the exposure variable. Adjusted models included an additional 12 covariates: LTC home proportion of residents aged ≥ 85 years, proportion with female sex, proportion with university education, proportion with dementia, average number of comorbidities, average ADL impairment scale, logarithm of the size of the LTC home, profit status, crowding index > 2, number of prior outbreaks, public health unit community SARS-CoV-2 incidence and outbreak identification date. Outbreak identification date was included as a penalised spline with a knot for each 4-week period (n = 9 knots in main analysis). All unadjusted and adjusted models were fit once for the primary exposure, late outbreak identification, and once for the secondary exposure, outbreak infection pressure.
Additional analyses
Regression models were repeated to further evaluate the impact of late SARS-CoV-2 outbreak identification on secondary cases and deaths, when we restricted the analysis to: (i) outbreaks in the first SARS-CoV-2 pandemic wave only (1 Mar–31 Aug 2020), (ii) outbreaks in the second SARS-CoV-2 pandemic wave only (1 Sep–14 Nov 2020) and (iii) second or subsequent outbreaks. We also conducted population simulations to estimate the number of cases and deaths that could have been averted if all outbreaks identified late were instead identified early [21]. To ensure comprehensiveness, this analysis was based on the secondary cases and secondary deaths outcome that covered the entire outbreak follow-up period. This analysis was repeated for the first and second waves separately.
Results
Between 1 March 2020 and 14 November 2020, we identified 632 LTC home SARS-CoV-2 outbreaks, of which 412 (65.2%) occurred in Wave 1 and 220 (34.8%) occurred in Wave 2. These outbreaks occurred in 349 (56.0%) of Ontario’s 623 LTC homes. Across the 632 outbreaks (Table 1), there were 104,667 residents at risk of infection at the time of outbreak identification. The incidence of infection in residents at 30-days follow-up was 6.2% (n = 6,452), while the incidence of SARS-CoV-2 mortality was 1.9% (n = 1,953). The incidence of infection at 14 days was 3.5% (n = 3,643) while the incidence up to the end of the outbreak period was 7.6% (n = 7,904).
Table 1. Characteristics of long-term care home outbreaks identified early versus late, Ontario, Canada, 1 March–14 November 2020 (n = 632 outbreaks).
| Variables | Overall outbreaks n = 632 |
Outbreak identification | p valuee | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early (≤ 2 days of infection pressurea) n = 402 |
Late (≥ 3 days of infection pressurea) n = 230 |
|||||||||
| n | % | n | % | n | % | |||||
| Resident characteristics, mean (IDR)b | ||||||||||
| Per cent residents aged ≥ 85 years | 51.2 (35.8–64.3) | 50.9 (35.3–64.3) | 51.9 (37.5–65.1) | 0.29 | ||||||
| Per cent female | 68.5 (58.1–77.1) | 68.3 (58.2–77.0) | 69.0 (58.0–77.3) | 0.27 | ||||||
| Per cent with university education | 7.2 (1.3–14.8) | 7.2 (1.3–14.9) | 7.2 (1.4–14.5) | 0.96 | ||||||
| Per cent with dementia | 59.8 (45.8–72.6) | 60.3 (46.5–73.3) | 58.9 (45.0–71.4) | 0.12 | ||||||
| Number of comorbidities | 0.41 (0.31–0.52) | 0.41 (0.31–0.51) | 0.41 (0.30–0.53) | 0.78 | ||||||
| ADL impairment scale | 3.93 (3.50–4.33) | 3.92 (3.51–4.33) | 3.95 (3.50–4.36) | 0.28 | ||||||
| LTC home structural characteristics | ||||||||||
| Number of beds, mean (IDR) | 166 (68–254) | 163 (71–252) | 172 (66–262) | 0.76 | ||||||
| Profit status | ||||||||||
| Municipal | 361 | 57.1 | 235 | 58.5 | 126 | 54.8 | 0.09 |
|||
| Private for-profit | 170 | 26.9 | 100 | 24.9 | 70 | 30.4 | ||||
| Private non-profit | 101 | 16.0 | 67 | 16.7 | 34 | 14.8 | ||||
| Crowding index > 2 | 263 | 41.6 | 161 | 40.0 | 102 | 44.3 | 0.29 | |||
| Prior outbreaks | ||||||||||
| 0 | 349 | 55.2 | 204 | 50.7 | 145 | 63.0 | 0.09 |
|||
| 1 | 183 | 29.0 | 127 | 31.6 | 56 | 24.3 | ||||
| ≥ 2 | 100 | 15.8 | 71 | 17.7 | 29 | 12.6 | ||||
| Outbreak identification date | ||||||||||
| Wave 1 (1 Mar–31 Aug 2020) | 412 | 65.2 | 241 | 60.0 | 171 | 74.3 | 0.001 | |||
| Wave 2 (1 Sep–14 Nov 2020) | 220 | 34.8 | 161 | 40.0 | 59 | 25.7 | ||||
| SARS-CoV-2 incidence on week of outbreak identification (cases per 100,000), mean (IDR)c | 97.8 (16.6–232.9) | 103.2 (18.2–232.9) | 88.4 (15.3–236.7) | 0.16 | ||||||
| Outcomesd | ||||||||||
| Secondary SARS-CoV-2 infections | ||||||||||
| 30-day follow-up | 6,452 | 6.2 | 2,015 | 3.3 | 4,437 | 10.3 | < 0.001 | |||
| 14-day follow-up | 3,643 | 3.5 | 840 | 1.4 | 2,803 | 6.5 | < 0.001 | |||
| Full follow-up | 7,904 | 7.6 | 2,893 | 4.7 | 5,011 | 11.7 | < 0.001 | |||
| Secondary SARS-CoV-2 mortality | ||||||||||
| 30-day follow-up | 1,953 | 1.9 | 579 | 0.9 | 1,374 | 3.2 | < 0.001 | |||
| 14-day follow-up | 1,155 | 1.1 | 214 | 0.3 | 941 | 2.2 | < 0.001 | |||
| Full follow-up | 2,293 | 2.2 | 793 | 1.3 | 1,500 | 3.5 | < 0.001 | |||
ADL: activities of daily living; IDR: interdecile range (10–90th percentile); LTC: long-term care.
a Infection pressure is equal to the number of infectious resident-days at the time of outbreak identification, where residents were considered infectious from 2 days before onset to 8 days after onset.
b Count of congestive heart failure, chronic obstructive pulmonary disease, cancer, diabetes and renal failure.
c Health region community incidence excludes long-term care home and congregate care settings.
d Denominator for overall (n = 104,667), early (n = 61,714), late (n = 42,953). Full follow-up defined as time from outbreak identification date until 14 days after the last detected case.
e P values were based on Wald tests. For all variables except the outcomes, Wald tests were measured within a logistic regression model comparing outbreaks identified early versus late as the binary outcome, and using the variable as the only continuous or categorical predictor variable. For outcomes, these were measured within logistic count regression models with the count of cases and non-cases as the outcome and outbreak identification as the binary predictor variable.
Late outbreak identification
Among the 632 outbreaks, 36.4% (n = 230) were identified late, after 3 or more infectious resident-days. On average, outbreaks identified late had 11.0 infectious resident-days at the time of identification (interdecile range (IDR): 3.5–20.0). There was a substantial improvement in outbreak identification between the first and second wave. Compared with outbreaks identified early, outbreaks identified late were more likely to have occurred in the first COVID-19 wave in Ontario (late: 171/230, 74.3% vs early: 241/402, 60.0%; p < 0.001).
Association between late outbreak identification and outbreak incidence
At 30-days follow-up, outbreaks identified late had an incidence of 10.3% (4,437/42,953), compared with 3.3% (2,015/61,714) among outbreaks identified early (p < 0.001). Patterns were consistent when examining shorter (14-day follow-up: late 6.5% vs early 1.4%; p < 0.001) and longer follow-up windows (full follow-up: late 11.7% vs early 4.7%; p < 0.001) and when examining SARS-CoV-2-associated deaths (30-day follow-up: late 3.2% vs early 0.9%; p < 0.001).
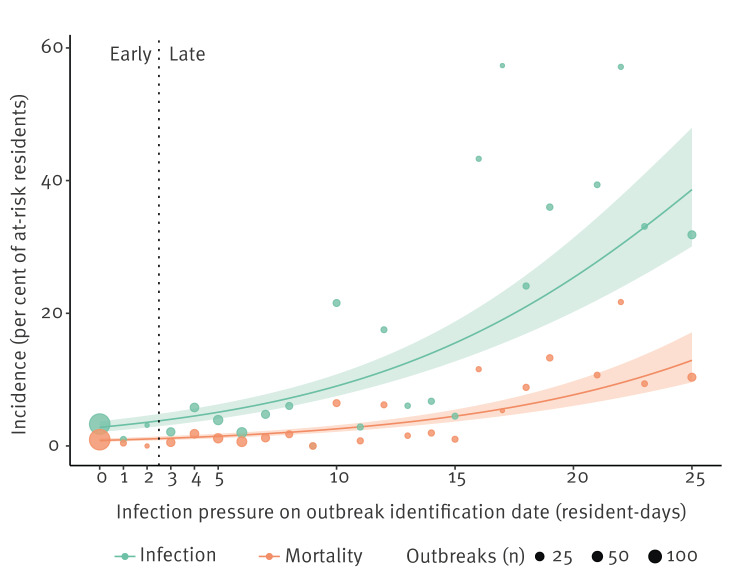
At 30-days follow-up, unadjusted incidence was 3.41 times higher for outbreaks with late identification (Table 2; unadjusted OR: 3.41; 95% confidence interval (CI): 2.24–5.19). Each additional resident-day of outbreak infection pressure was associated with a 1.13-fold (unadjusted OR: 1.13; 95% CI: 1.11–1.16) increase in the odds of infection among at-risk residents in the LTC home (Figure 2). Adjustment for 12 covariates led to moderately attenuated estimates of the impact of late identification (adjusted OR: 2.90; 95% CI: 2.04–4.13) and the impact of each additional day of delay (adjusted OR: 1.10; 95% CI: 1.08–1.12). Associations were similar for the 30-day SARS-CoV-2 mortality outcome (adjusted OR: 2.47; 95% CI: 1.77–3.46). Associations were stronger when examining incidence in the shorter 14-day follow-up window (adjusted OR: 4.47; 95% CI: 2.98–6.70), compared with the entire follow-up window (adjusted OR: 2.21; 95% CI: 1.55–3.16). Coefficient estimates for the adjustment covariates for incidence of infection and mortality at 30 days are provided in the Supplementary Materials.
Table 2. Association between late vs early outbreak identification and SARS-CoV-2 secondary infections and mortality in long-term care home residents, Ontario, Canada, 1 March–14 November 2020 (n = 632 outbreaks).
| Variables | Secondary infection incidence | Secondary mortality incidence | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 30-day follow-up | ||||||||
| Late (vs early) | 3.41 | 2.24–5.19 | 2.90 | 2.04–4.13 | 3.49 | 2.26–5.39 | 2.47 | 1.77–3.46 |
| Infection pressureb | 1.13 | 1.11–1.16 | 1.10 | 1.08–1.12 | 1.12 | 1.10–1.14 | 1.07 | 1.06–1.09 |
| 14-day follow-up | ||||||||
| Late (vs early) | 5.06 | 3.04–8.42 | 4.47 | 2.98–6.70 | 6.44 | 3.92–10.57 | 4.81 | 3.12–7.43 |
| Infection pressureb | 1.14 | 1.12–1.17 | 1.11 | 1.09–1.13 | 1.14 | 1.12–1.17 | 1.10 | 1.08–1.12 |
| Full follow-up c | ||||||||
| Late (vs early) | 2.68 | 1.79–4.02 | 2.21 | 1.55–3.16 | 2.78 | 1.84–4.20 | 1.96 | 1.42–2.72 |
| Infection pressureb | 1.12 | 1.10–1.15 | 1.09 | 1.07–1.11 | 1.11 | 1.09–1.13 | 1.06 | 1.05–1.08 |
CI: confidence interval; LTC: long-term care; OR: odds ratio.
a Adjusted models included the following 12 covariates: beds in the LTC home, LTC home proportion of residents aged ≥ 85 years, proportion of females, proportion with university education, proportion with dementia, average number of comorbidities, average activities of daily living (ADL) impairment scale, crowding index > 2, profit status, prior outbreaks, outbreak identification date (spline with one knot for each month) and public health unit community SARS-CoV-2 incidence.
b Infection pressure is the number of infectious resident-days occurring in the window from 14 days prior (upper limit on one incubation period) to the outbreak identification date. The numeric value represents the odds ratio associated with a 1-unit increase in infection pressure.
c Full follow-up defined as time from outbreak identification date until 14 days after the last detected case.
Figure 2.
Association between infection pressure on outbreak identification date and incidence of secondary SARS-CoV-2 infection and mortality, Ontario, Canada, 1 March–14 November 2020 (n = 632 outbreaks)
The 30-day SARS-CoV-2 incidence of infection (green) and mortality (orange) are shown as a function of the resident infection pressure on outbreak identification date (n = 632 outbreaks). Circle size is proportional to the number of outbreaks (n). Infection pressure is equal to the number of infectious resident-days at the time of outbreak identification, where residents were considered infectious from 2 days before onset to 8 days after onset. Outbreaks with infection pressure ≥ 3 were considered to be identified late, while outbreaks with infection pressure ≤ 2 were considered to be identified early.
Additional analyses
The adjusted association between late outbreak identification and the incidence of infection at 30-days was similar in the first and second waves (adjusted ORWave 1: 2.73; 95% CI: 1.88–3.97; adjusted ORWave 2: 3.64; 95% CI: 2.11–6.29). When we limited the analysis to the second or subsequent outbreaks, impacts were similar to the overall effect (adjusted OR: 3.97; 95% CI: 2.90–5.43). When we limited the analysis to late-identified outbreaks, the continuous measure of infection pressure at the time of outbreak identification remained associated with increased risk (adjusted OR: 1.10; 95% CI: 1.08–1.13).
Preventable cases through early outbreak identification
We conducted simulation analyses to determine the number of preventable cases and deaths if outbreak identification had occurred more rapidly. Starting from the observed outbreak identification delays, which yielded 7,698 cases and 2,219 deaths, if all outbreaks were identified early, rather than late, the simulation estimated that cases would have decreased to 5,455 (a reduction of 2,247 cases; 29.2%) and deaths would have decreased to 1,522 (a reduction of 697 deaths; 31.3%). When the same analysis was applied to Wave 1 and 2 separately, the simulation estimated that Wave 1 cases and deaths would have been reduced by 31.6% and 33.4%, respectively, while Wave 2 cases and deaths would have decreased by 23.2% and 24.3%, respectively.
Discussion
This study examined the timeliness of outbreak identification and the subsequent secondary incidence of SARS-CoV-2 infections and deaths in LTC homes. We found that outbreaks identified late, when at least one resident was already symptomatic, evolved to be much larger outbreaks. Further, we identified a strong linear association between resident infection pressure at the time of outbreak identification and the overall size of outbreaks, with a 14% increased odds of infection among at-risk residents per additional infectious resident-day. We observed substantial improvements in the timeliness of outbreak identification between the first and second SARS-CoV-2 waves in Ontario, and estimated further reductions in the overall burden of SARS-CoV-2 that would have been possible with improvements to outbreak identification.
Prior studies have demonstrated initial underdetection of SARS-CoV-2 cases as a common feature during severe LTC home outbreaks. A case study of one of the first severe SARS-CoV-2 outbreaks from Ontario, Canada, reported that, at the time of outbreak declaration, 12 staff, two visitors and nine residents had symptoms [22]. One study from Fulton County, Georgia, during March–May 2020, conducted facility-wide testing within 1–5 days of an index resident SARS-CoV-2 case, showing that, on average 28% of residents and 7% of staff were already test-positive [23]. Our study developed a measure to determine the extent of an outbreak at the time an outbreak is identified, which can be used for future quality improvement initiatives. A paradoxical feature of this indicator is that we exclude staff infection pressure. Because of the important role of staff in propagating outbreaks, outbreaks without staff infection pressure are likely to represent outbreaks with lapses in staff case identification, and as such, inclusion could have weakened the predictiveness of our outbreak severity indicator.
Test turnaround times, frequency and sensitivity all have substantial impacts on outbreak detection [24]. Systematic delays in test turnaround times are apparent in LTC homes, particularly in for-profit homes and those with lower quality scores [25]. Actions to improve the timeliness of outbreak identification could reduce the severity of outbreaks. These could include, among others: (i) measures to promote earlier and more complete identification and testing of staff with symptoms of respiratory virus infection, such as through active screening and improved paid sick leave policies [26,27], (ii) testing of residents in proportion to population prevalence of infection [28] and (iii) strategies to reduce test turnaround times [29], such as point-of-care testing. At a health system level, systematic facility-level reporting of staff and resident respiratory virus symptoms and testing rates, and delays in outbreak identification, would facilitate setting of timeliness benchmarks (e.g. the 7-1-7 target [30]) and quality improvement through audit and feedback [31].
We observed substantial improvements in the quality and timeliness of outbreak identification between the first and second wave and concomitant reductions in outbreak size. These improvements were likely attributable to recognition of the importance of asymptomatic/pre-symptomatic transmission and atypical presentation in frail older adults, alongside increases in testing capacity and policy changes that occurred in Ontario [13]. Our results alongside a recent study [28], suggest increased test frequency could lead to earlier outbreak identification, and possibly to lower attack rates during outbreaks.
This study is subject to several limitations. Firstly, it was conducted before vaccine availability and before the emergence of new SARS-CoV-2 variants such as Alpha, Delta and the currently predominant Omicron variant, which may limit generalisability. Nevertheless, the insights from this study are still relevant for SARS-CoV-2 and other respiratory viruses since vaccine effectiveness for Omicron variants is low and wanes over time, many other seasonal respiratory infections lack effective vaccines, and because emergent infections are likely to lack effective vaccines as well. Thus, improving the timeliness of outbreak detection remains an important avenue for minimising the impacts of SARS-CoV-2 and other viral respiratory infections. Secondly, we lacked information on measures (other than transfer to the hospital) taken after cases were identified. However we were able to control for factors at the LTC home level including home crowding, which likely impacted the ability to isolate patients in rooms separately [9], and profit status, which is associated with staffing levels and quality [32]. Finally, our estimates of potential cases and deaths prevented through early outbreak identification may not have been achievable, since it may have been challenging to identify all outbreaks early in the first wave given the limited testing capacity.
Conclusions
SARS-CoV-2 outbreaks in LTC homes, when identified late, evolved to be much larger than outbreaks identified early. The timeliness of outbreak identification can be used to predict the trajectory of an outbreak and to plan for increased staffing demands, infection control measures, and antiviral administration, with the goal of improving resident outcomes.
Ethical statement
This study was reviewed and approved by the research ethics board of Public Health Ontario.
Funding statement
This research did not receive external funding.
Use of artificial intelligence tools
None declared.
Data availability
Patient and nursing home-level data contain personal health information and cannot be shared publicly due to provincial data protection and confidentiality requirements.
Supplementary Data
Conflict of interest: Dr Allison McGeer reported research funds from Pfizer, Merck and Sanofi, personal honoraria from lectures/webinars from AstraZeneca, Merck, Moderna, and personal fees for advisory boards and DSMBs from AstraZeneca, GSK, Janssen, Moderna, Novavax, Pfizer and Sanofi. All other authors reported no conflicts.
Authors’ contributions: Conception and design (KAB, SAB and JMJ), acquisition (KAB and SAB), analysis (KAB and JMJ), interpretation of data (all authors), drafting of the manuscript (KB), critical review for important intellectual content (all authors), final approval of the version to be published (KAB, SAB, AKC, AC, ND, GG, MH, AJ, JMJ, DK, KM, RGM, AM, KLS, NMS, JJ).
References
- 1.Panagiotou OA, Kosar CM, White EM, Bantis LE, Yang X, Santostefano CM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-48. 10.1001/jamainternmed.2020.7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashan MR, Smoll N, King C, Ockenden-Muldoon H, Walker J, Wattiaux A, et al. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: A systematic review and meta-analysis. EClinicalMedicine. 2021;33:100771. 10.1016/j.eclinm.2021.100771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canadian Institute for Health Information (CIHI). Pandemic experience in the long-term care sector: how does Canada compare with other countries? Ottawa: CIHI; 2020. Available from: https://www.cihi.ca/sites/default/files/document/covid-19-rapid-response-long-term-care-snapshot-en.pdf
- 4.Fisman DN, Bogoch I, Lapointe-Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw Open. 2020;3(7):e2015957. 10.1001/jamanetworkopen.2020.15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danis K, Fonteneau L, Georges S, Daniau C, Bernard-Stoecklin S, Domegan L, et al. ECDC Public Health Emergency Team . High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Euro Surveill. 2020;25(22):2000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malikov K, Huang Q, Shi S, Stall NM, Tuite AR, Hillmer MP. Temporal associations between community incidence of COVID-19 and nursing home outbreaks in Ontario, Canada. J Am Med Dir Assoc. 2021;22(2):260-2. 10.1016/j.jamda.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stall NM, Jones A, Brown KA, Rochon PA, Costa AP. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ. 2020;192(33):E946-55. 10.1503/cmaj.201197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui DP, See I, Hesse EM, Varela K, Harvey RR, August EM, et al. Association between CMS quality ratings and COVID-19 outbreaks in nursing homes - West Virginia, March 17-June 11, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1300-4. 10.15585/mmwr.mm6937a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown KA, Jones A, Daneman N, Chan AK, Schwartz KL, Garber GE, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern Med. 2021;181(2):229-36. 10.1001/jamainternmed.2020.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul LA, Daneman N, Brown KA, Johnson J, van Ingen T, Joh E, et al. Characteristics associated with household transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Ontario, Canada: A Cohort Study. Clin Infect Dis. 2021;73(10):1840-8. 10.1093/cid/ciab186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang S, Sanchez Perez M, Saavedra-Campos M, Paranthaman K, Myers R, Fok J, et al. Mass testing after a single suspected or confirmed case of COVID-19 in London care homes, April-May 2020: implications for policy and practice. Age Ageing. 2021;50(3):649-56. 10.1093/ageing/afab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kain D, Stall N, Brown K, McCreight L, Rea E, Kamal M, et al. A longitudinal, clinical, and spatial epidemiologic analysis of a large COVID-19 long-term care home outbreak. J Am Med Dir Assoc. 2021;22(10):2003-2008.e2. 10.1016/j.jamda.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kain D, Stall NM, Allen V, Evans GA, Hopkins J, Kouyoumdjian FG, et al. Routine asymptomatic SARS-CoV-2 screen testing of Ontario long-term care staff after COVID-19 Vaccination. Ontario COVID-19 Science Advisory Table; 2021. Available from: https://covid19-sciencetable.ca/sciencebrief/routine-asymptomatic-sars-cov-2-screen-testing-of-ontario-long-term-care-staff-after-covid-19-vaccination
- 14.Brown KA, Stall NM, Vanniyasingam T, Buchan SA, Daneman N, Hillmer MP, et al. Early impact of Ontario’s COVID-19 vaccine rollout on long-term care home residents and health care workers. Ontario COVID-19 Science Advisory Table; 2021. Available from: 10.47326/ocsat.2021.02.13.1.0 [DOI]
- 15.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4) Suppl;III50-9. 10.1097/01.mlr.0000120104.01232.5e [DOI] [PubMed] [Google Scholar]
- 16.Ottawa Public Health. COVID-19 outbreak definitions in Ottawa over time. Ottawa: Ottawa Public Health; 2022. Available from: https://www.ottawapublichealth.ca/en/reports-research-and-statistics/resources/Documents/covid-19/outbreak_definitions_2022-02_EN.pdf
- 17.Ajao AO, Harris AD, Roghmann M-C, Johnson JK, Zhan M, McGregor JC, et al. Systematic review of measurement and adjustment for colonization pressure in studies of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and clostridium difficile acquisition. Infect Control Hosp Epidemiol. 2011;32(5):481-9. 10.1086/659403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20(5):223-8. 10.1016/j.tree.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Wood SN. Generalized Additive Models: An Introduction with R. 2nd ed. Chapman and Hall/CRC, 2017. Available from: 10.1201/9781315370279 10.1201/9781315370279 [DOI]
- 20.Douma JC, Weedon JT. Analysing continuous proportions in ecology and evolution: A practical introduction to beta and Dirichlet regression. Methods Ecol Evol. 2019;10(9):1412-30. 10.1111/2041-210X.13234 [DOI] [Google Scholar]
- 21.Manuel DG, Rosella LC, Hennessy D, Sanmartin C, Wilson K. Predictive risk algorithms in a population setting: an overview. J Epidemiol Community Health. 2012;66(10):859-65. 10.1136/jech-2012-200971 [DOI] [PubMed] [Google Scholar]
- 22.Murti M, Goetz M, Saunders A, Sunil V, Guthrie JL, Eshaghi A, et al. Investigation of a severe SARS-CoV-2 outbreak in a long-term care home early in the pandemic. CMAJ. 2021;193(19):E681-8. 10.1503/cmaj.202485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telford CT, Onwubiko U, Holland DP, Turner K, Prieto J, Smith S, et al. Preventing COVID-19 outbreaks in long-term care facilities through preemptive testing of residents and staff members - Fulton County, Georgia, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1296-9. 10.15585/mmwr.mm6937a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmdahl I, Kahn R, Hay JA, Buckee CO, Mina MJ. Estimation of transmission of COVID-19 in simulated nursing homes with frequent testing and immunity-based staffing. JAMA Netw Open. 2021;4(5):e2110071. 10.1001/jamanetworkopen.2021.10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarry BE, SteelFisher GK, Grabowski DC, Barnett ML. COVID-19 test result turnaround time for residents and staff in US nursing homes. JAMA Intern Med. 2021;181(4):556-9. 10.1001/jamainternmed.2020.7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson A, Stall NM, Born KB, Gibson JL, Allen U, Hopkins J, et al. Benefits of paid sick leave during the COVID-19 pandemic. Ontario COVID-19 Science Advisory Table. 2021. Available from: https://covid19-sciencetable.ca/sciencebrief/benefits-of-paid-sick-leave-during-the-covid-19-pandemic
- 27.Gussin GM, Singh RD, Tjoa TT, Saavedra R, Kaplan SH, Huang SS. Evaluating barriers and potential solutions to speaking up about coronavirus disease 2019 (COVID-19) symptoms: A survey among nursing home workers. Infect Control Hosp Epidemiol. 2023;44(11):1834-9. 10.1017/ice.2023.51 [DOI] [PubMed] [Google Scholar]
- 28.McGarry BE, Gandhi AD, Barnett ML. Covid-19 surveillance testing and resident outcomes in nursing homes. N Engl J Med. 2023;388(12):1101-10. 10.1056/NEJMoa2210063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. 10.1126/sciadv.abd5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bochner AF, Makumbi I, Aderinola O, Abayneh A, Jetoh R, Yemanaberhan RL, et al. Implementation of the 7-1-7 target for detection, notification, and response to public health threats in five countries: a retrospective, observational study. Lancet Glob Health. 2023;11(6):e871-9. 10.1016/S2214-109X(23)00133-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No.: CD000259. Available from: https://doi.wiley.com/10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed]
- 32.Comondore VR, Devereaux PJ, Zhou Q, Stone SB, Busse JW, Ravindran NC, et al. Quality of care in for-profit and not-for-profit nursing homes: systematic review and meta-analysis. BMJ. 2009;339(aug04 2):b2732-2732. 10.1136/bmj.b2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.