Abstract
The diversity of physiological roles of the endocannabinoid system has turned it into an attractive yet elusive therapeutic target. However, chemical probes with various functionalities could pave the way for a better understanding of the endocannabinoid system at the cellular level. Notably, inverse agonists of CB2R – a key receptor of the endocannabinoid system – lagged behind despite the evidence regarding the therapeutic potential of its antagonism. Herein, we report a matched fluorescent probe pair based on a common chemotype to address and visualize both the active and inactive states of CB2R, selectively. Alongside extensive cross-validation by flow cytometry, time-lapse confocal microscopy, and super-resolution microscopy, we successfully visualize the intracellular localization of CB2R pools in live cells. The synthetic simplicity, together with the high CB2R-selectivity and specificity of our probes, turns them into valuable tools in chemical biology and drug development that can benefit the clinical translatability of CB2R-based drugs.
Selective visualization of the active and inactive states of CB2R by a matched fluorescent agonist and inverse agonist probe pair.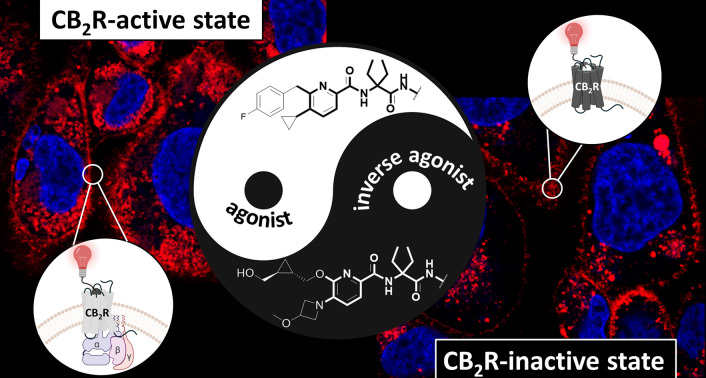
Introduction
The endocannabinoid system (ECS) is a complex lipid-based signalling network involved in a wide variety of physiological and cognitive processes such as pain regulation, immune response, appetite control, learning and memory formation, cardiovascular regulation, and addictive-like behaviour.1 The ECS consists of two cannabinoid receptor subtypes (CB1R and CB2R) that belong to the class A G protein-coupled receptor (GPCR) family. Arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) are the endogenous ligands of both CB1R and CB2R.2 Regardless of their high homology, the key difference between the two receptors is their distribution.3 CB1R is predominantly expressed in the central nervous system with the highest density in the cerebellum, hippocampus, and cerebral cortex,4–6 while CB2R is more abundant in peripheral organs, such as the spleen and tonsils, and is mainly expressed in cells associated with the immune system.3 It has been shown that expression of CB2R is strongly upregulated under pathological conditions such as cancer,7,8 immunological disorders,9 inflammation, neurodegenerative diseases,10,11 and drug abuse.12 Therefore, modulating CB2R activation will be a valuable therapeutic approach for several diseases including inflammation, autoimmune and metabolic disorders, chronic pain, multiple sclerosis and cancer. For example, agonist-mediated activation of CB2R was previously shown to be beneficial for neuroprotection in chronic neurodegenerative disorders such as Huntington's and Alzheimer's diseases.13 Conversely, inactivation of CB2R via an inverse agonist/antagonist was found to have therapeutic potential for treatment of various diseases associated with neuroinflammation and the immune system.14,15 Due to different expression patterns of CB2R and CB1R, as well as their distinct functions, the selective activation or deactivation of CB2R does not involve undesired psychotropic responses which is considered a major therapeutic advantage and makes it a more attractive target compared to CB1R. However, despite its great potential, no CB2R-selective drug has made its way to the market to date, as clinical translatability from preclinical models deduced from different species is currently challenging.14 This is largely attributed to the highly inducible nature and complexity of CB2R signalling pathways at the cellular level and the unclear understanding of its expression, localization and function.16 For example, a number of studies indicate that the cellular responses associated with CB2R activation are not only limited to plasmalemmal receptors but also to the intracellular pools.17–19 The absence of CB2R-specific monoclonal antibodies, which are important tools for obtaining expression data at a cellular or tissue level, is further aggravating this situation.
Recent CB2R-selective agonist fluorescent probes20–22 could partially fill these gaps by addressing the activated state and providing information on CB2R localization, expression, target engagement, pharmacokinetics and dynamics in real-time; the scarcity of labeled CB2R-selective inverse agonist probes has resulted in a lack of information on the distribution of intra- and extracellular CB2R pools in the inactivated state. There are only a limited number of reports about inverse agonist fluorescent probes labeling CB2R. For example, the chromenopyrazole-based inverse agonist probe was originally generated from an agonist but upon attachment of the Cy5 fluorescent dye, the functionality was altered.23 The surface receptors of CB2R expressing HEK-293 cells were labeled via the aforementioned probe.23 Another example is NIR-mbc94, an analogue of selective inverse agonist SR144528, which has been shown to be an imaging agent for the unbiased high-throughput screening of compounds interacting with CB2R as a therapeutic target.24 Despite the wide range of applications and a high demand for CB2R inverse agonist probes, no versatile probe platform with diverse fluorophores is available so far.
We have previously reported a high-affinity, cell-permeable fluorescent CB2R probe 3 based on a reverse-design approach using a preclinically validated drug-derived CB2R agonist 1 (Fig. 1 and 2).20 The probe successfully detected CB2R in several in vitro and in vivo settings across species. For example, 3 was also recently used to visualize the high expression levels of CB2R in primary neonatal microglia isolated from wild-type and Tg2576 mice, the latter being used as an Alzheimer's Disease (AD) model.25
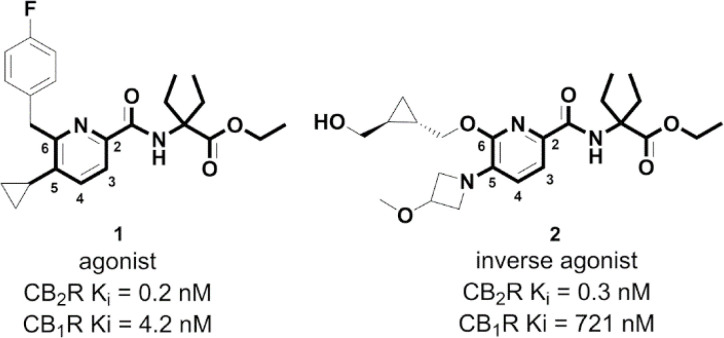
Fig. 1. Chemical structures of CB2R agonist 1 and inverse agonist 2 sharing a 5- and 6-substituted picolinamide core.
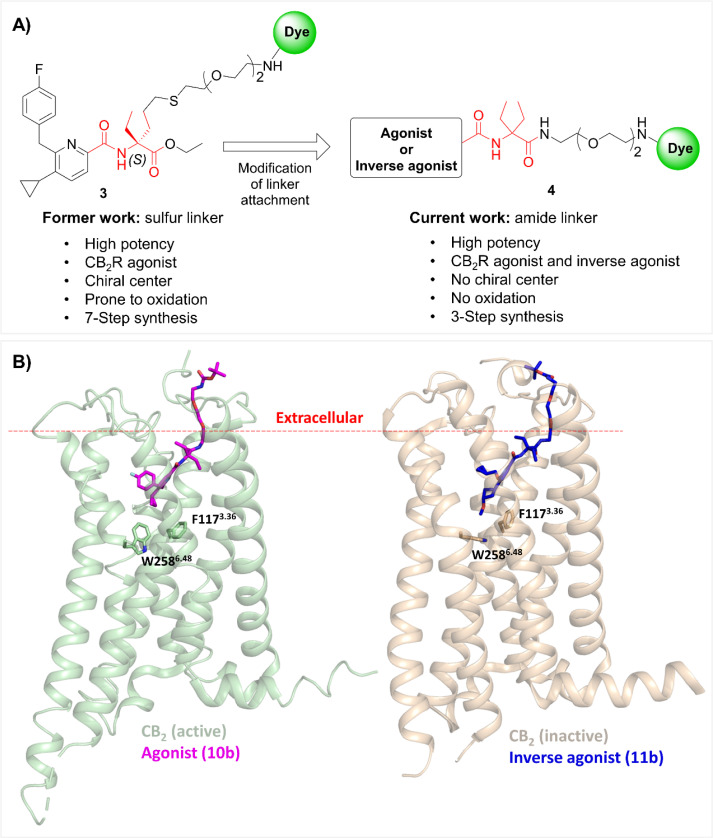
Fig. 2. (A) Modification of linker attachment from previous work (compound 3; sulfur linker) led to the discovery of a new versatile exit vector (compound 4; amide linker), and the diethylglycine moiety as the centerpiece hub in red. (B) Docking poses of compounds 10b (magenta sticks) and 11b (blue sticks) within active (green; PDB: 6KPF) and inactive (orange; PDB: 5ZTY) states of CB2R, respectively. The red dashed line indicates the approximate boundary of the lipid bilayers. For a detailed description of the docking studies see ESI, S3.†.
However, for any chemical probe approach, it is desirable to have access to a matched molecular pair of agonist and inverse agonist with high structural similarity which are correspondingly labeled. Such chemical probes are most suitable to address distinct mechanisms of action, e.g. by distinguishing the activated or resting state of the receptor or allowing differential analysis of agonist-stimulated internalization of the receptor, while excluding the cellular phenotype.
With the goal of expanding the scope of our probe platform and addressing both active and inactive states of the receptor, we designed a matched molecular pair of CB2R agonist and antagonist fluorescent probes derived from highly similar chemotypes of advanced preclinical CB2R agonist 1 (ref. 26) and inverse agonist 2 (ref. 27) drug candidates (Fig. 1). In addition, we were able to attach a variety of fluorescent dyes leading to CB2R probes that span a broad range of physicochemical properties. At last, varying combinations of agonist and antagonist with cell-permeable (e.g. TAMRA) or impermeable (e.g. Alexa488) fluorophores gave us access to a valuable toolbox suitable for detecting extra- and intracellular receptor pools. To explore the spatial–temporal dynamics of CB2R, we employed these novel probes to investigate the expression and subcellular localization of the active and inactive states of CB2R in living cells, utilizing super-resolution confocal imaging techniques.
Results and discussion
Probe design and molecular modelling
Our previous probes were derived from a drug-like CB2R agonist bearing a 5,6-substituted picolinamide 1 (Fig. 1). Interestingly, it was shown that different substitutions at the 5- and 6-positions of picolinamide could alter the functionality of the ligand while maintaining high CB2R affinity.27,28 For example, the replacement of the cyclopropyl moiety at position 5 of agonist 1 with 3-methoxy-azetidine alters the functionality from agonism to inverse agonism (2). This substitution causes a flip of the side chain of the toggle switch residue W2586.48 (Ballesteros–Weinstein numbering in superscript, Fig. 2B).29 Besides the 5,6-substituents, both ethyl side chains are involved in favorable van der Waals interactions with surrounding phenylalanine side chains F91, F94, and F106.
The drug-derived inverse agonist 2 possessing an exceptional selectivity profile (CB2R Ki = 0.3 nM; CB1R Ki = 721 nM; a selectivity factor of 1403 over CB1R) was an ideal starting point for generating a matched agonist and inverse agonist-based probe pair with a 5,6-substituted picolinamide core in common.28,30,31
The first and the most critical step in probe design is the identification of a suitable attachment point between the recognition element and the reporter unit, i.e. the ligand and fluorescent dye, respectively. In most cases, the recognition element and the fluorescent dye are distanced using a suitable linker, which allows the dye to access the extracellular space without compromising overall binding affinity.32 Previously, we introduced a hybrid of thio- and polyether chains to one of the ethyl groups of the diethylglycine moiety as the centerpiece hub (3, Fig. 2A). Even though our previous probes showed highly consistent interspecies affinity and potency for both human and mouse CB2R, the presence of the sulfur atom in the linker posed a possible experimental imponderability in some of the advanced settings, as sulfur might be prone to oxidation.20,33 Therefore, with the goal of improving the physicochemical properties and simplifying the synthetic strategy, SAR studies were performed to investigate alternative sites for linker attachment at the diethylglycine centerpiece hub. For this, we used the ester functionality which after substitution by an amide moiety served as an attachment point. This design approach has the advantage that no chiral center is present and the synthesis route is greatly simplified compared to our previous probes (4, Fig. 2A).
Docking experiments were conducted to estimate the required linker length to reach out into the extracellular space and support linkerology studies. In Fig. 2B the best docking poses for agonist (10b) and inverse agonist (11b) pharmacophores, respectively, are depicted. For both ligands, the proper range of polyethylene glycol PEG chains to access the extracellular space for insertion of the fluorescent dye was estimated to be n = 2.
Chemistry
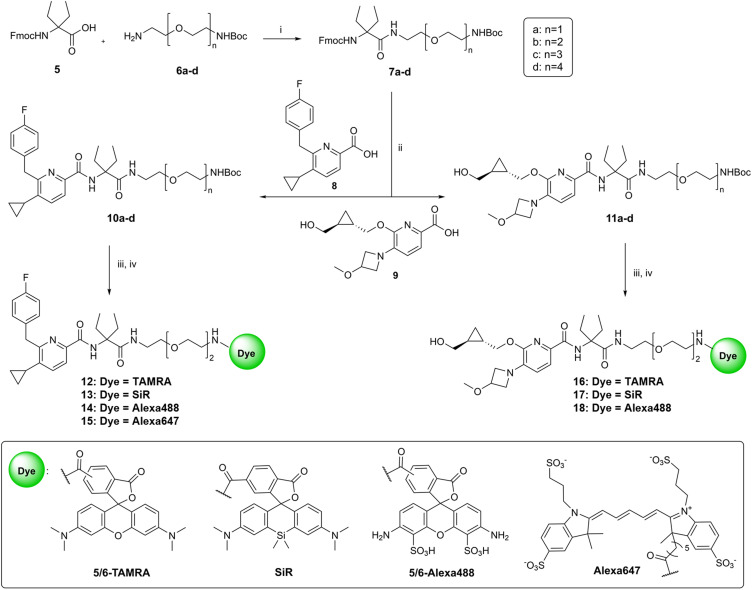
The synthetic pathway to access the Boc-protected intermediates and target fluorescent probes bearing various dyes is outlined in Scheme 1. Fmoc-protected diethylglycine 5 was used as the centerpiece unit to connect the 5,6-substituted picolinamide recognition elements to the desired linker. In order to elaborate the optimal linker length for dye attachment, compound 5 was functionalized with a series of linkers 6a–d with different lengths under HATU-mediated amide coupling conditions. Fmoc-protecting group removal of compounds 7a–d using DBU was followed by coupling to agonist 8 or inverse agonist 9 precursors in situ to afford Boc-protected congeners with matched chemotypes (10a–d and 11a–d). Compounds 8 (ref. 20) and 9 (ref. 31) were synthesized according to literature protocols. The final step was to conjugate a variety of broadly used fluorophores such as 5/6-TAMRA, SiR, 5/6-Alexa488 and Alexa647 to the selected intermediates (10b and 11b) with a linker length of n = 2, which turned out to be optimal in subsequent SAR studies. For this purpose, the Boc-protecting group of 10b or 11b was first cleaved using TFA. Subsequently, the resulting free amines were coupled with the desired fluorescent dyes either under suitable amide coupling conditions to furnish probes 12–15 and 17–18. Compound 16 was synthesized via a variation of the aforementioned synthetic route starting with coupling of 5/6-TAMRA-COOH to Boc-deprotected 7b followed by another amide coupling reaction to 9 using HATU as the coupling reagent (ESI, S32†).
Scheme 1. Synthesis of fluorescent probes. Reagents and conditions: (i) HATU, DIPEA, DMF, rt, 1 h; (ii) (1) DBU, HOAt, DMF, rt, 20 min; (2) 8 or 9, HATU, DIPEA, DMF, rt, 10 h; (iii) TFA (9 equiv.), CH2Cl2, 0 °C to rt, 3 h; (iv) for compounds 13, 15, 17 and 18: HATU, dye, DIPEA, DMF, rt, 10 h; for compound 12: EDC·HCl, HOAT, dye, DIPEA, DMF, rt, 10 h; for compound 14: dye, DIPEA, DMF, rt, 10 h; for compound 16: see the ESI.†.
Evaluation of the appropriate linker length
To identify the optimal linker length, binding affinities of unlabeled precursors 10a–d and 11a–d were measured via a competitive radioligand binding assay on CHO membranes stably expressing hCB1R or hCB2R (Table 1).
Binding affinities and potency of the Boc-protected intermediates.
| Cmpd | Linker length | K i a (nM) | Selectivityb | EC50 or IC50c (nM) | E max d (%) | Function | Parent cmpd | |
|---|---|---|---|---|---|---|---|---|
| hCB1R | hCB2R | |||||||
| 1 | n.a. | 4.2 | 0.2 | 21 | 6.5 | 102 | Agonist | n.a. |
| 2 | n.a. | 721 | 0.3 | 1403 | 4.4 | −130 | Inverse agonist | n.a. |
| 10a | n = 1 | 836 | 28 | 30 | n.d. | n.d. | n.d. | 1 |
| 10b | n = 2 | 466 | 6 | 78 | 49 | 83 | Agonist | 1 |
| 10c | n = 3 | 748 | 63 | 12 | n.d. | n.d. | n.d. | 1 |
| 10d | n = 4 | 1472 | 144 | 10 | n.d. | n.d. | n.d. | 1 |
| 11a | n = 1 | >10 000 | 2061 | >4 | n.d. | n.d. | n.d. | 2 |
| 11b | n = 2 | >10 000 | 106 | >94 | 88 | −63 | Inverse agonist | 2 |
| 11c | n = 3 | >10 000 | >10 000 | n.a. | n.d. | n.d. | n.d. | 2 |
K i (nM) values obtained from [3H]CP55940 displacement assays on CHO membranes stably expressing hCB1R or hCB2R. Values are means of at least three independent experiments performed in duplicate. For details, see the ESI.
Selectivity was determined by calculating the ratio of Ki (CB1R)/Ki (CB2R).
The potency (EC50 or IC50) of the selected compounds was measured using cells stably expressing hCB2R in homogeneous time-resolved fluorescence (HTRF) cAMP assay. The data are the means of four independent experiments performed in technical replicates.
Maximum effect (Emax in %) was normalized to reference full agonist APD371. n.a. is not applicable. n.d. is not determined.
For both agonist and inverse agonist chemotypes, the PEG chain with n = 2 (10b and 11b, respectively) showed the highest affinity and selectivity for CB2R and was therefore chosen as the optimal linker length. The observed trend confirmed the predictions based on our docking studies (Fig. 2B). To assess whether the attachment of the linker affects receptor function, the efficacy of selected precursors was determined in a cAMP assay (Table 1). Notably, 10b and 11b preserved partial agonist and inverse agonist activity, respectively, with high potency (CB2R cAMP EC50 = 49 nM for 10b and IC50 = 88 nM for 11b, respectively).
Pharmacological characterization of fluorescent probes 12–18
Compared to their unlabeled congeners, the binding affinity of the fluorescent probes 12–18 indicated fluorescent dye dependency which was not unexpected as the structural nature of the fluorophore alters the membrane interactions of the constructs. Overall, we observed a certain drop in affinity for all dye-bearing probes, consistent with previous results.20,21,34 This decrease is believed to mainly originate from additional configurational and steric penalties due to conjugation.35 However, most of the probes retained high affinity and selectivity for CB2R (ESI, Table S-2†).
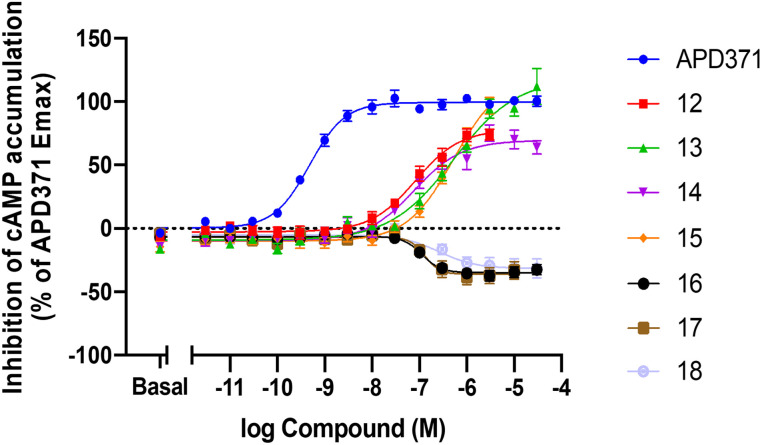
In functional studies (Table 2), probes 13 and 15 showed full agonism with potencies (EC50) of approximately 525 nM, while 12 and 14 showed partial agonism with higher potencies (EC50) of approximately 80 nM. As anticipated, the functional mode of action of probes 16–18 fully retained their inverse agonism with IC50 values in the range of 114–262 nM (Table 2 and Fig. 3).
hCB2R potency and predicted log D7.4 of fluorescent probes 12–18.
| Cmpd | EC50 or IC50a (nM) | E max b (%) | Function | Parent cmpds | Dye | c log D7.4c |
|---|---|---|---|---|---|---|
| 12 | 82 | 80 | Partial agonist | 1/10b | TAMRA | 3.7 |
| 13 | 528 | 127 | Full agonist | 1/10b | SiR | 3.9 |
| 14 | 77 | 79 | Partial agonist | 1/10b | Alexa488 | −0.5 |
| 15 | 523 | 129 | Full agonist | 1/10b | Alexa467 | −0.9 |
| 16 | 114 | −29 | Inverse agonist | 2/11b | TAMRA | 3.6 |
| 17 | 129 | −29 | Inverse agonist | 2/11b | SiR | 2.2 |
| 18 | 262 | −26 | Inverse agonist | 2/11b | Alexa488 | −0.9 |
The potency (EC50 or IC50) of fluorescent probes 12–18 was measured using cells stably expressing hCB2R in homogeneous time-resolved fluorescence (HTRF) cAMP assay. The data are the means of four or seven independent experiments performed in technical replicates.
Maximum effect (Emax in %) was normalized to reference full agonist APD371.
For computational calculation of c log D7.4 see ref. 36.
Fig. 3. Inhibition of cAMP accumulation on hCB2R was determined with a (HTRF) cAMP assay. The maximum effect (Emax in %) was normalized to reference full agonist APD371.
To assess the specificity of the probes and scout for putative off-targets, TAMRA-probe pairs 12 and 16 were screened against a customized panel of 50 representative receptors and enzymes.37 Both probes were devoid of any relevant off-target interactions thus confirming their suitability for specific CB2R detection studies (ESI, Table S-1†). Furthermore, kinetic binding characterization of agonist 12 and inverse agonist 16 using HEK293-hCB2R cell membranes revealed fast association and appropriate residence time (ESI, Fig. S-8†).
For high probe quality, a lower lipophilicity is crucial, as it significantly reduces non-specific binding.38 Therefore, it is noteworthy that all predicted c log D7.4 values of our drug-derived probes showed an overall significantly lower lipophilicity compared to phytocannabinoids21 and are in a favorable drug-like range (Table 2). Moreover, except for highly ionized sulfonated fluorescent dyes such as Alexa488 and Alexa647 (14, 15 and 18), attachment of TAMRA (12 and 16) and SiR (13 and 17) did not lead to a significant change in the lipophilicity of the final probes compared to their unlabeled counterparts (10bc log D7.4 = 3.8 and 11bc log D7.4 = 3.2).
Cellular imaging by flow cytometry and confocal microscopy in live cells
The probe's specificity and suitability for imaging were further validated by flow cytometry. All the fluorescent probes were incubated at various concentrations ranging from 0.014 to 10 μM – with live CHO cells overexpressing hCB1R or hCB2R and wild-type (wt) CHO cells as the control. Despite some differences observed in the mean fluorescence intensity (MFI) of probes bearing the same dyes, most of the tested probes indicated decent selectivity and specificity for hCB2R in flow cytometry, suggesting their suitability for imaging applications.
Probes carrying sulfonated dyes such as Alexa488 14 and Alexa647 18 showed high selectivity even at very high concentrations, which is in line with our previous data (ESI, Fig. S-1†).20 To exclude unspecific binding, we further examined the effect of preincubation of cells with high-affinity competitor ligands such as agonist JWH133 (ref. 39) and inverse agonist RO6851228 (ref. 40) on probe 15 binding (ESI, Fig. S-2†). Both ligands competed with 15 in a dose-dependent manner confirming a high target specificity of 15 for CB2R.
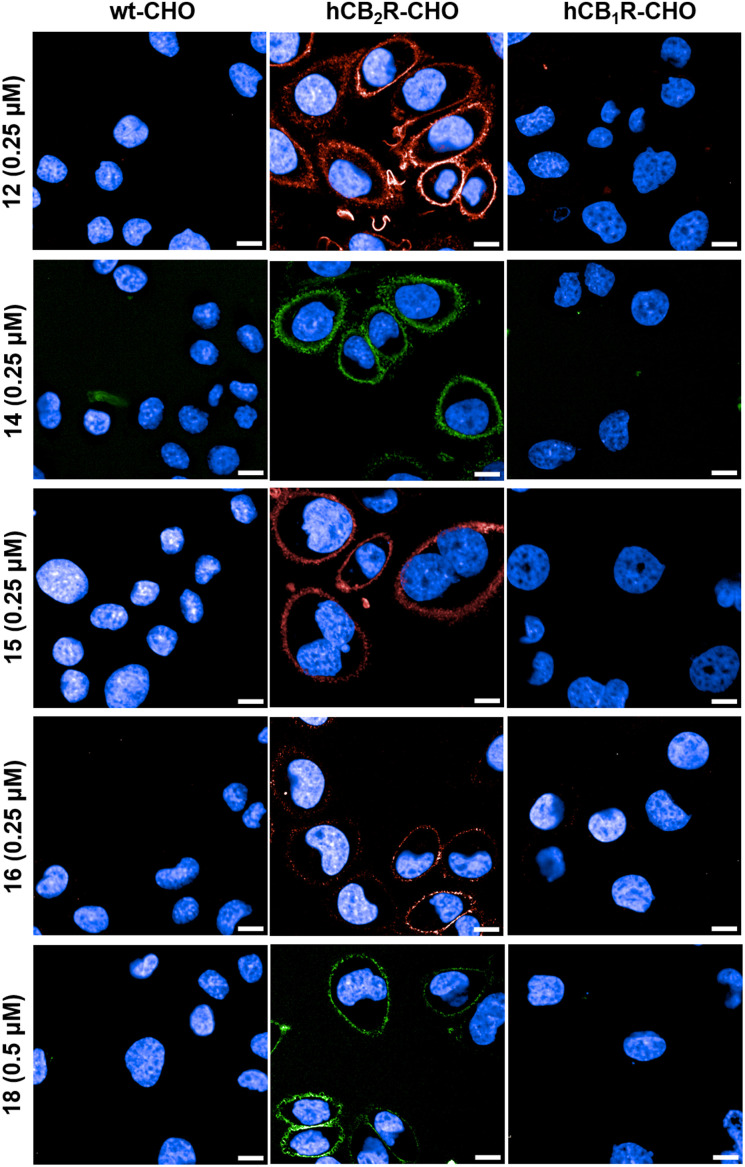
Based on the high specificity of the novel probes we continued our investigation by performing time-lapse confocal microscopy experiments to visualize hCB2R in live CHO cells. Fig. 4 displays the frames of CHO cells overexpressing hCB2R and hCB1R along with parental CHO cells 10 min after administration of probes 12, 14–16 and 18. All probes selectively stained CB2R on the cell membrane, but also intracellular staining was observed to some extent, especially for probe 12. We monitored the staining of 12 in a time-lapse setting and could follow the permeation kinetics through the outer cellular membrane over time, clearly evidencing internalization (ESI, Fig. S-4†). For selected probes, blocking experiments with competitive non-labeled ligands RO6851228 (ref. 40) (CB2R-inverse agonist) and/or RO6871304 (ref. 41) (CB2R-agonist) were carried out as well (ESI, Fig. S-3†). Both unlabelled ligands completely blocked the staining of all tested fluorescent probes in hCB2R expressing CHO cells, confirming the high binding specificity and being fully consistent with the results obtained for WT-CHO and hCB1R expressing CHO cells.
Fig. 4. Time-lapse confocal microscopy in live cells. The wild type (wt) and overexpressing hCB2R- and hCB1R-CHO cells were co-stained with probes 12, 14–16 and 18 and Hoechst 33342 (blue, nucleus counterstain). The images were recorded 10 min after probe incubation. Images are representative of two independent experiments. Scale bars, 10 μm.
To further validate the results obtained for CHO cells we continued with imaging human-derived live HEK293 cells expressing hCB2R. We followed the probe 12 distribution in the same manner over 10 min (ESI, Fig. S-5†). Probe 12 exhibited a similar distribution pattern in the human-derived HEK293 cells as in the CHO system, predominantly staining cellular membranes but also the intracellular compartments.
We then set out to visualize endogenous CB2R expression aiming to ascertain different patterns of activation and localization of CB2R pools induced by agonist and inverse agonist binding by using non-transfected primary cultures of human macrophages derived from healthy donors. Upon staining of human macrophages with probes 12 and 16 a markedly different distribution pattern was observed (ESI, Fig. S-6†). While agonist probe 12 was widely distributed within the cell, inverse agonist probe 16 was mainly localized on cellular membranes and partially in perinuclear dots, suggesting a distinct localization of inactive and activated CB2R pools.
Super-resolution confocal imaging of hCB2R-CHO cells
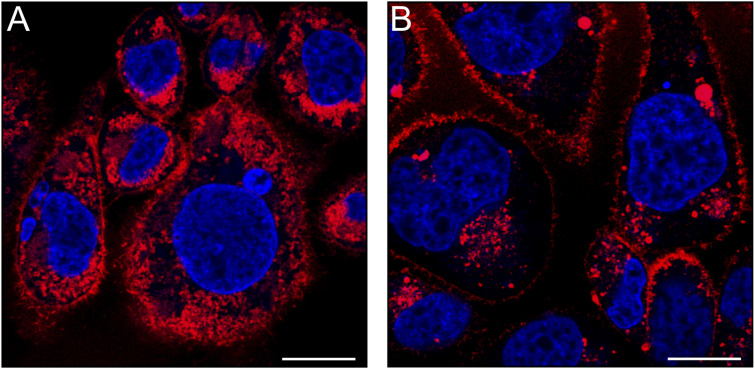
To examine in more depth the localization and activation states of the CB2R pools, we resorted to super-resolution confocal imaging of hCB2R overexpressing CHO cells. First, we assessed the intensity and subcellular distribution of fluorescence staining produced by the matched TAMRA-probe pair 12 and 16. Image acquisition at higher magnification and resolution was performed after 15 minutes of incubation with 12 and 16 and revealed that both probes yielded a similar localization pattern, yet with distinct labeling intensity. Indeed, for both probes the CB2R staining was largely observed intracellularly, especially in the perinuclear membranes that are suggestive of the Golgi apparatus and the endoplasmic reticulum; however, appreciable fluorescence was also present on the cell membrane (Fig. 5A and B). Notably, these results corroborate and extend the findings previously reported by den Boon et al.18
Fig. 5. Super-resolution confocal imaging of overexpressing hCB2R CHO cells. The cells were stained for 15 min with (A) 12 (0.8 μM, red) or (B) 16 (0.8 μM, red) and Hoechst 33342 (blue, nucleus counter stain). Cells were optically sectioned using confocal laser-scanning microscopy equipped with an Airyscan detector. For quantifying the relative levels of labeling using the two TAMRA probes, identical imaging settings (i.e., objective, light path, laser power, gain, offset, frame size, zoom and scan speed) were maintained throughout the acquisition process. Images are representative of three independent experiments. Scale bars, 10 μm.
It is noteworthy that the analysis of MFI of 12-labeled CB2R showed a plasma membrane labelling intensity of CB2R approximately six-fold higher than that of 16-labeled CB2R (12-labeled CB2R = 2090 ± 175 MFI; 16-labeled CB2R = 350 ± 4 MFI; unpaired t-test, t = 19.94, df = 4, and p-value < 0.0001). Similarly, when examining the intracellular compartments, a marked difference in MFI values was observed (12-labeled CB2R = 2575 ± 100 MFI; 16-labeled CB2R = 340 ± 13 MFI; unpaired t-test, t = 37.57, df = 4, and p-value < 0.0001). This marked difference in labelling intensity still persisted even when differences in binding affinities were compensated for by increasing the concentration of 16versus12 (ESI, Fig. S-7†). Moreover, comparable quantum yields of probes 12 and 16 in more lipophilic media (ESI, Table S-3†) ruled out potential interference with the observed differences in MFI.
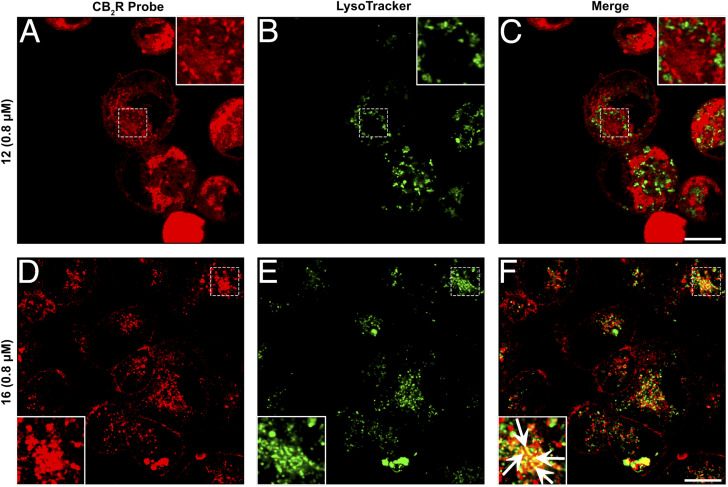
Given that probes 12 and 16 are conjugated with the identical fluorophore and exhibit comparable physicochemical features, including their binding affinity for CB2R, respectively (ESI, Table S-2†), the more pronounced signal detected with probe 12 relative to probe 16 indicates that under steady-state conditions in living cells, CB2R is present predominantly in its active conformation. It is also plausible that the inactive form of the receptor is less accessible for interaction with probe 16 resulting in weaker labeling when compared to probe 12.42,43 We further examined the different distribution patterns of the two probes by co-staining with AlexaFluor488 LysoTracker. Fig. 6 indicates that probe 12 had partial overlap but no clear co-localization with the LysoTracker staining. In contrast, co-staining with probe 16 exhibited a clear co-localization pattern. These results are in line with the observations made on human macrophages (vide supra), indicating a distinct intracellular distribution of CB2R pools, which is influenced by the activation state and may have further implications on cellular signalling pathways. This is consistent with previous extensive studies showing that localization of G protein-coupled receptors (GPCRs) is not restricted to the plasma membrane, but rather is in dynamic exchange with localization in various intracellular compartments, including endosomes, the Golgi apparatus, the endoplasmic reticulum, the nucleus and the mitochondria.44 This dynamic localization is often influenced by the receptor's activation state, with both active and inactive forms potentially residing within intracellular organelles. CB2R, similar to CB1R, undergoes constitutive endocytosis, resulting in significant intracellular accumulation.18,45–47 This distribution pattern has been confirmed in leukocytes using anti-CB2R antibodies.48 Functionally, CB2R can be either active or inactive within these subcellular compartments.17,47 Our findings reveal that inactive CB2R is presumably partially sequestered in lysosomes and in an as-yet uncharacterized vesicular compartment. We propose that this sequestration may serve as a regulatory mechanism, preventing CB2R activation and subsequent signalling until the receptor is recycled to the plasma membrane or degraded in the lysosome.
Fig. 6. Co-staining studies utilizing super-resolution confocal microscopy images of hCB2R-CHO cells. (A–F) Double-staining of hCB2R-overexpressing CHO cells exposed for 15 min with 12 (A) or 16 (D) together with LysoTracker, a lysosome marker (B and E, green). Merged images are shown in the panels (C) and (F). The superimposition of the two stainings revealed that 12 and LysoTracker did not colocalize, while 16 and LysoTracker partially co-stained in several dot structures (white arrows in the magnified box). The images are representative of three independent experiments, and in each case, five fields were examined. Scale bars, 10 μm.
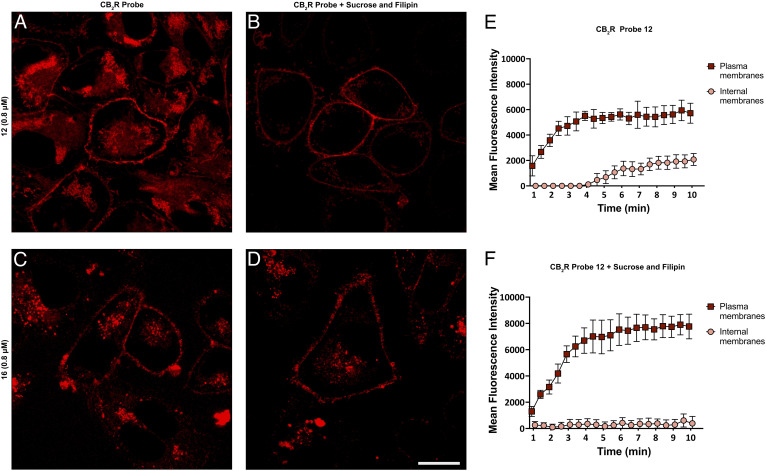
To determine the degree of endocytosis-mediated receptor internalization influenced by the receptor functional state, we performed blocking experiments. Although the experimental setting did not allow for an entire block of endocytosis, a significant reduction was achieved by treating the cells with 0.4 M sucrose and 5 μg mL−1 filipin,21 which can indirectly abrogate multiple pathways of endocytosis.49 This treatment reduced the intracellular staining of 12 by 70% without affecting that of 16 (see Fig. 7). We recorded the fluorescence intensity of probe 12 by time-lapse imaging and observed signal saturation in both native and blocker-pre-treated cells, both for cytoplasm and internal membranes. In cells pretreated with the endocytotic blockers sucrose and filipin the plasma membrane signal reached the same intensity plateau as in untreated cells, but was negligible for the internal membranes during the entire course of the experiment. The observation that suppression of endocytosis affects the internalization of 12 but does not reduce that of 16 aligns with the behavior of agonists and inverse agonists. Unlike agonists, inverse agonists stabilize the inactive form of a GPCR, which does not promote endocytosis from the membrane.46,50,51 However, this finding also clearly demonstrates that both probes 12 and 16 are able to reach a significant intracellular concentration by passive permeation in almost the same concentration range. Further investigation into the molecular basis of the differences in localization and activity states might provide valuable insights into the interaction dynamics between CB2R and its ligands, potentially informing the design of more effective probes or therapeutic agents targeting this receptor.
Fig. 7. Endocytosis blocking studies using super-resolution confocal microscopy in hCB2R-CHO cells. Cells were stained for 10 min with 12 (A) or 16 (C). In order to minimize endocytosis, hCB2R-CHO cells were pretreated for 30 min with 400 μM sucrose and 5 μg mL−1 filipin and then incubated for 10 min either with 12 (B) or 16 (D). Association curve of 12 on the plasma membrane and internal membranes of hCB2R-CHO cells without sucrose and filipin (E) and in the presence of both (F). Images are representative of three independent experiments. Scale bars, 10 μm. (E) and (F) demonstrate internal and external fluorescence over time.
Conclusions
In this study, we have developed the first general CB2R selective matched agonist or inverse agonist platform using validated drug-derived CB2R ligands 1 and 2, respectively, as starting points. We designed fluorescent probes sharing highly similar drug-derived chemotypes but addressing either the active or inactive state of CB2R. Due to our reverse-design approach using highly drug-like precursors, all labeled probes exhibited favorable physicochemical properties. The key for the receptor recognition element and fluorophore attachment was a common centrepiece that allowed straightforward linker attachment by amide coupling. The probes retained good binding affinity towards CB2R and high selectivity against CB1R upon conjugation of fluorescent dyes. When investigating the functional responses in a cellular cAMP accumulation assay, the labeled probes were able to evoke a similar functional response as their unlabeled congeners. In particular, the labeled inverse agonist enabled us to address the resting state of CB2R. This is of great benefit and importance for various control experiments in live cells which are now possible. With matching TAMRA probes in hand we performed time-lapse confocal microscopy imaging studies in various biological models, including hCB2R-CHO cells, hCB2R-HEK293 cells and human primary macrophages. In all settings, it was clearly demonstrated that hCB2R has different localization and internalization patterns, depending on the functional state. Most notably, our probes allowed the assessment of localization and distribution of the active and inactive conformations of CB2R through super-resolution confocal microscopy analysis. These studies indicate that within living cells, a considerable number of CB2Rs are located intracellularly and are in an active state. The differential content of the active and inactive states of CB2R suggests a complex regulatory mechanism governing its activity and interactions with intracellular signalling pathways. These observations, enabled by our matched fluorescent agonist and inverse agonist probe pair, suggest significant implications for understanding the receptor's role in physiological and pathological processes. Furthermore, our approach demonstrates the potential for super-resolution imaging of our CB2R probes in studying membrane receptors representing a powerful tool for future research in cellular biology and pharmacology.
Data availability
Primary data for probe synthesis, characterization, photophysical measurements, and fluorescence imaging are provided in the ESI.†
Author contributions
M. N. and U. G. conceived the research and acquired funding for this project. M. W.-K., A. O., L. M., Y. M., T. G., B. B., W. G., D. V., L. H. H., S. O., M. Ma., U. G., and M. N. designed the research approach. M. W.-K., A. O., L. M., L. S., J. B., X. L., S. R., Y. M., M. S., T. G., C. v. d. H., B. B., A. H., Y. K., W. G., D. S., J. P. v. K., J. Br., T. H., D. V., and S. O. performed experiments, analyzed the raw data, and worked on probe validation. M. W.-K., A. O., L. M., Y. M., S. O., M. Ma., U. G., and M. N. analyzed all data generated and wrote the manuscript. J. B., Y. M., W. G., J. Br., T. H., D. V., and L. H. H. provided useful comments and feedback for the manuscript. All authors have given approval to the final version of the manuscript.
Conflicts of interest
The authors declare no competing financial interest.
Supplementary Material
Acknowledgments
The authors are grateful to Marta Diceglie, Peter Lindemann, Edgar Specker, Katy Franke, Ramona Birke, Andreas Oder, Astrid Mühl, Peter Schmieder (all Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)), Isabelle Kaufmann, Christian Bartelmus, Oliver Scheidegger, and Claudia Korn (all Roche) for their excellent support in sample handling, analytical compound characterization and helpful discussions. S. O. and M. Ma. thank Dr Franca Abbruzzese (Università Campus Bio-Medico di Roma) for her technical assistance in live cell imaging. This research was partly funded by the European Union – Next Generation EU – NRRP M6C2 – Investment 2.1 Enhancement and strengthening of biomedical research in the NHS (project no. PNRR-MAD-2022-12376730) to S. O. and M. Ma., and EU-OPENSCREEN-DRIVE (Ensuring long-term sustainability of excellence in chemical biology within Europe and beyond) grant no. 823893 to M. N.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc00402g
References
- Friedman D. French J. A. Maccarrone M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019;18:504. doi: 10.1016/S1474-4422(19)30032-8. [DOI] [PubMed] [Google Scholar]
- Basavarajappa B. S. Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr. Neuropharmacol. 2007;5:81. doi: 10.2174/157015907780866910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M. Marzo V. D. Gertsch J. Grether U. Howlett A. C. Hua T. Makriyannis A. Piomelli D. Ueda N. Stelt M. v. d. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023;75:885. doi: 10.1124/pharmrev.122.000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C. and Abood M. E., in Adv. Pharmacol., ed. D. Kendall and S. P. H. Alexander, Academic Press, 2017, vol. 80, p. 169 [Google Scholar]
- Lu H.-C. Mackie K. Review of the Endocannabinoid System. Biol. Psychiatry. 2021;6:607. doi: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Moreno E. Cavic M. Krivokuca A. Casadó V. Canela E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019;10:339. doi: 10.3389/fphar.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi A. Mattei V. Martellucci S. Manganelli V. Saccomanni G. Garofalo T. Sorice M. Manera C. Misasi R. Anti-Proliferative Properties and Proapoptotic Function of New CB2 Selective Cannabinoid Receptor Agonist in Jurkat Leukemia Cells. Int. J. Mol. Sci. 2018;19:1958. doi: 10.3390/ijms19071958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez M. S. Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2010;25:111. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- McGeer P. L. McGeer E. Conquering Alzheimer's Disease by Self Treatment. J. Alzheimer's Dis. 2018;64:S361. doi: 10.3233/JAD-179913. [DOI] [PubMed] [Google Scholar]
- Cassano T. Calcagnini S. Pace L. De Marco F. Romano A. Gaetani S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z. X. Peng X. Q. Li X. Song R. Zhang H. Y. Liu Q. R. Yang H. J. Bi G. H. Li J. Gardner E. L. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat. Neurosci. 2011;14:1160. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J. Romero J. Velasco G. Tolón R. M. Ramos J. A. Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol. Sci. 2007;28:39. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Morales P. Hernandez-Folgado L. Goya P. Jagerovic N. Cannabinoid receptor 2 (CB2) agonists and antagonists: a patent update. Expert Opin. Ther. Pat. 2016;26:843. doi: 10.1080/13543776.2016.1193157. [DOI] [PubMed] [Google Scholar]
- Bu W. Ren H. Deng Y. Del Mar N. Guley N. M. Moore B. M. Honig M. G. Reiner A. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front. Neurosci. 2016;10:1. doi: 10.3389/fnins.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B. Wu J. Foss J. F. Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018;31:407. doi: 10.1097/ACO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu G. C. Deliu E. Marcu J. Hoffman N. E. Console-Bram L. Zhao P. Madesh M. Abood M. E. Brailoiu E. Differential Activation of Intracellular versus Plasmalemmal CB2 Cannabinoid Receptors. Biochemistry. 2014;53:4990. doi: 10.1021/bi500632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon F. S. Chameau P. Schaafsma-Zhao Q. van Aken W. Bari M. Oddi S. Kruse C. G. Maccarrone M. Wadman W. J. Werkman T. R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3534. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda J. T. Harui A. Roth M. D. Regulation of Cell Surface CB(2) Receptor during Human B Cell Activation and Differentiation. J. Neuroimmune Pharmacol. 2017;12:544. doi: 10.1007/s11481-017-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzi T. Brennecke B. Atz K. Korn C. Sykes D. Forn-Cuni G. Pfaff P. Sarott R. C. Westphal M. V. Mostinski Y. Mach L. Wasinska-Kalwa M. Weise M. Hoare B. L. Miljuš T. Mexi M. Roth N. Koers E. J. Guba W. Alker A. Rufer A. C. Kusznir E. A. Huber S. Raposo C. Zirwes E. A. Osterwald A. Pavlovic A. Moes S. Beck J. Nettekoven M. Benito-Cuesta I. Grande T. Drawnel F. Widmer G. Holzer D. van der Wel T. Mandhair H. Honer M. Fingerle J. Scheffel J. Broichhagen J. Gawrisch K. Romero J. Hillard C. J. Varga Z. V. van der Stelt M. Pacher P. Gertsch J. Ullmer C. McCormick P. J. Oddi S. Spaink H. P. Maccarrone M. Veprintsev D. B. Carreira E. M. Grether U. Nazaré M. Detection of cannabinoid receptor type 2 in native cells and zebrafish with a highly potent, cell-permeable fluorescent probe. Chem. Sci. 2022;13:5539. doi: 10.1039/D1SC06659E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarott R. C. Westphal M. V. Pfaff P. Korn C. Sykes D. A. Gazzi T. Brennecke B. Atz K. Weise M. Mostinski Y. Hompluem P. Koers E. Miljuš T. Roth N. J. Asmelash H. Vong M. C. Piovesan J. Guba W. Rufer A. C. Kusznir E. A. Huber S. Raposo C. Zirwes E. A. Osterwald A. Pavlovic A. Moes S. Beck J. Benito-Cuesta I. Grande T. Ruiz de Martín Esteban S. Yeliseev A. Drawnel F. Widmer G. Holzer D. van der Wel T. Mandhair H. Yuan C.-Y. Drobyski W. R. Saroz Y. Grimsey N. Honer M. Fingerle J. Gawrisch K. Romero J. Hillard C. J. Varga Z. V. van der Stelt M. Pacher P. Gertsch J. McCormick P. J. Ullmer C. Oddi S. Maccarrone M. Veprintsev D. B. Nazaré M. Grether U. Carreira E. M. Development of High-Specificity Fluorescent Probes to Enable Cannabinoid Type 2 Receptor Studies in Living Cells. J. Am. Chem. Soc. 2020;142:16953. doi: 10.1021/jacs.0c05587. [DOI] [PubMed] [Google Scholar]
- Spinelli F. Giampietro R. Stefanachi A. Riganti C. Kopecka J. Abatematteo F. S. Leonetti F. Colabufo N. A. Mangiatordi G. F. Nicolotti O. Perrone M. G. Brea J. Loza M. I. Infantino V. Abate C. Contino M. Design and synthesis of fluorescent ligands for the detection of cannabinoid type 2 receptor (CB2R) Eur. J. Med. Chem. 2020;188:112037. doi: 10.1016/j.ejmech.2020.112037. [DOI] [PubMed] [Google Scholar]
- Singh S. Oyagawa C. R. M. Macdonald C. Grimsey N. L. Glass M. Vernall A. J. Chromenopyrazole-based High Affinity, Selective Fluorescent Ligands for Cannabinoid Type 2 Receptor. ACS Med. Chem. Lett. 2019;10:209. doi: 10.1021/acsmedchemlett.8b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M. Woodruff G. Horne E. A. Lin Y. H. Muccioli G. G. Bai M. Stern E. Bornhop D. J. Stella N. NIR-mbc94, a fluorescent ligand that binds to endogenous CB(2) receptors and is amenable to high-throughput screening. Chem. Biol. 2011;18:563. doi: 10.1016/j.chembiol.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scipioni L. Tortolani D. Ciaramellano F. Fanti F. Gazzi T. Sergi M. Nazaré M. Oddi S. Maccarrone M. Aβ Chronic Exposure Promotes an Activation State of Microglia through Endocannabinoid Signalling Imbalance. Int. J. Mol. Sci. 2023;24:1. doi: 10.3390/ijms24076684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissantz C., Grether U., Hebeisen P., Kimbara A., Liu Q., Nettekoven M., Prunotto M., Roever S., Rogers-Evans M., Schulz-Gasch T., Ullmer C., Wang Z. and Yang W., US Pat., US20120316147A1, 2012
- Ametamey S. M., Atz K., Gobbi L., Grether U., Guba W. and Kretz J., WO2020002270A1, 2020
- Haider A. Kretz J. Gobbi L. Ahmed H. Atz K. Bürkler M. Bartelmus C. Fingerle J. Guba W. Ullmer C. Honer M. Knuesel I. Weber M. Brink A. Herde A. M. Keller C. Schibli R. Mu L. Grether U. Ametamey S. M. Structure–Activity Relationship Studies of Pyridine-Based Ligands and Identification of a Fluorinated Derivative for Positron Emission Tomography Imaging of Cannabinoid Type 2 Receptors. J. Med. Chem. 2019;62:11165. doi: 10.1021/acs.jmedchem.9b01280. [DOI] [PubMed] [Google Scholar]
- Li X. Chang H. Bouma J. de Paus L. V. Mukhopadhyay P. Paloczi J. Mustafa M. van der Horst C. Kumar S. S. Wu L. Yu Y. van den Berg R. Janssen A. P. A. Lichtman A. Liu Z. J. Pacher P. van der Stelt M. Heitman L. H. Hua T. Structural basis of selective cannabinoid CB(2) receptor activation. Nat. Commun. 2023;14:1447. doi: 10.1038/s41467-023-37112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik R. Grether U. Müller Herde A. Gobbi L. Fingerle J. Ullmer C. Krämer S. D. Schibli R. Mu L. Ametamey S. M. Discovery of a High Affinity and Selective Pyridine Analog as a Potential Positron Emission Tomography Imaging Agent for Cannabinoid Type 2 Receptor. J. Med. Chem. 2015;58:4266. doi: 10.1021/acs.jmedchem.5b00283. [DOI] [PubMed] [Google Scholar]
- Haider A. Gobbi L. Kretz J. Ullmer C. Brink A. Honer M. Woltering T. J. Muri D. Iding H. Bürkler M. Binder M. Bartelmus C. Knuesel I. Pacher P. Herde A. M. Spinelli F. Ahmed H. Atz K. Keller C. Weber M. Schibli R. Mu L. Grether U. Ametamey S. M. Identification and Preclinical Development of a 2,5,6-Trisubstituted Fluorinated Pyridine Derivative as a Radioligand for the Positron Emission Tomography Imaging of Cannabinoid Type 2 Receptors. J. Med. Chem. 2020;63:10287. doi: 10.1021/acs.jmedchem.0c00778. [DOI] [PubMed] [Google Scholar]
- Guberman M. Kosar M. Omran A. Carreira E. M. Nazaré M. Grether U. Reverse-Design toward Optimized Labeled Chemical Probes – Examples from the Endocannabinoid System. Chimia. 2022;76:425. doi: 10.2533/chimia.2022.425. [DOI] [PubMed] [Google Scholar]
- Kim G. Weiss S. J. Levine R. L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta, Gen. Subj. 2014;1840:901. doi: 10.1016/j.bbagen.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach L. Omran A. Bouma J. Radetzki S. Sykes D. A. Guba W. Li X. Hoffelmeyer C. Hentsch A. Gazzi T. Mostinski Y. Wasinska-Kalwa M. de Molnier F. van der Horst C. von Kries J. P. Vendrell M. Hua T. Veprintsev D. B. Heitman L. H. Grether U. Nazare M. Highly Selective Drug-Derived Fluorescent Probes for the Cannabinoid Receptor Type 1 (CB(1)R) J. Med. Chem. 2024;67:11841. doi: 10.1021/acs.jmedchem.4c00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. G. MacDonald C. Glass M. Hook S. Tyndall J. D. A. Vernall A. J. Alkyl indole-based cannabinoid type 2 receptor tools: Exploration of linker and fluorophore attachment. Eur. J. Med. Chem. 2018;145:770. doi: 10.1016/j.ejmech.2017.11.076. [DOI] [PubMed] [Google Scholar]
- Broccatelli F. Trager R. Reutlinger M. Karypis G. Li M. Benchmarking Accuracy and Generalizability of Four Graph Neural Networks Using Large In Vitro ADME Datasets from Different Chemical Spaces. Mol. Inf. 2022;41:2100321. doi: 10.1002/minf.202100321. [DOI] [PubMed] [Google Scholar]
- Bendels S. Bissantz C. Fasching B. Gerebtzoff G. Guba W. Kansy M. Migeon J. Mohr S. Peters J.-U. Tillier F. Wyler R. Lerner C. Kramer C. Richter H. Roberts S. Safety screening in early drug discovery: an optimized assay panel. J. Pharmacol. Toxicol. Methods. 2019;99:106609. doi: 10.1016/j.vascn.2019.106609. [DOI] [PubMed] [Google Scholar]
- Honer M. Gobbi L. Martarello L. Comley R. A. Radioligand development for molecular imaging of the central nervous system with positron emission tomography. Drug Discovery Today. 2014;19:1936. doi: 10.1016/j.drudis.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Huffman J. W. Liddle J. Yu S. Aung M. M. Abood M. E. Wiley J. L. Martin B. R. 3-(1′,1′-Dimethylbutyl)-1-deoxy-Δ8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 1999;7:2905. doi: 10.1016/S0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Ouali Alami N. Schurr C. Olde Heuvel F. Tang L. Li Q. Tasdogan A. Kimbara A. Nettekoven M. Ottaviani G. Raposo C. Röver S. Rogers-Evans M. Rothenhäusler B. Ullmer C. Fingerle J. Grether U. Knuesel I. Boeckers T. M. Ludolph A. Wirth T. Roselli F. Baumann B. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018;37:e98697. doi: 10.15252/embj.201798697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. F. Szczesniak A. M. Toguri J. T. Gebremeskel S. Johnston B. Lehmann C. Fingerle J. Rothenhäusler B. Perret C. Rogers-Evans M. Kimbara A. Nettekoven M. Guba W. Grether U. Ullmer C. Kelly M. E. M. Selective Cannabinoid 2 Receptor Agonists as Potential Therapeutic Drugs for the Treatment of Endotoxin-Induced Uveitis. Molecules. 2019;24:1. doi: 10.3390/molecules24183338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Hua T. Vemuri K. Ho J.-H. Wu Y. Wu L. Popov P. Benchama O. Zvonok N. Locke K. a. Qu L. Han G. W. Iyer M. R. Cinar R. Coffey N. J. Wang J. Wu M. Katritch V. Zhao S. Kunos G. Bohn L. M. Makriyannis A. Stevens R. C. Liu Z.-J. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell. 2019;176:459. doi: 10.1016/j.cell.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C. Zhuang Y. Xu T.-H. Feng Z. Zhou X. E. Chen M. Wang L. Meng X. Xue Y. Wang J. Liu H. McGuire T. F. Zhao G. Melcher K. Zhang C. Xu H. E. Xie X.-Q. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell. 2020;180:645. doi: 10.1016/j.cell.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasciani I. Carli M. Petragnano F. Colaianni F. Aloisi G. Maggio R. Scarselli M. Rossi M. GPCRs in Intracellular Compartments: New Targets for Drug Discovery. Biomolecules. 2022;12:1343. doi: 10.3390/biom12101343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C. Bonnard D. Carrel D. Rossier J. Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 2004;279:36013. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- Leterrier C. Laine J. Darmon M. Boudin H. Rossier J. Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006;26:3141. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salort G. Alvaro-Bartolome M. Garcia-Sevilla J. A. Regulation of cannabinoid CB(2) receptor constitutive activity in vivo: repeated treatments with inverse agonists reverse the acute activation of JNK and associated apoptotic signaling in mouse brain. Psychopharmacology. 2017;234:925. doi: 10.1007/s00213-017-4537-5. [DOI] [PubMed] [Google Scholar]
- Castaneda J. T. Harui A. Kiertscher S. M. Roth J. D. Roth M. D. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J. Neuroimmune Pharmacol. 2013;8:323. doi: 10.1007/s11481-012-9430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens K. M. Kolhatkar R. B. Swaan P. W. Ghandehari H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharm. 2008;5:364. doi: 10.1021/mp700089s. [DOI] [PubMed] [Google Scholar]
- Oliveira de Souza C. Sun X. Oh D. Metabolic Functions of G Protein-Coupled Receptors and beta-Arrestin-Mediated Signaling Pathways in the Pathophysiology of Type 2 Diabetes and Obesity. Front. Endocrinol. 2021;12:715877. doi: 10.3389/fendo.2021.715877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnot C. Miserey-Lenkei S. Bardin S. Corvol P. Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol. Metab. 2002;13:336. doi: 10.1016/S1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data for probe synthesis, characterization, photophysical measurements, and fluorescence imaging are provided in the ESI.†