Abstract
Introduction
Epistaxis is the most common otorhinolaryngological emergency and historically there have been an important debate whether there is a cause-effect relationship with high blood pressure.
Aim
This retrospective study explored whether hypertension is a significant risk factor for epistaxis in Emergency Department (ED) patients and examined associations between blood pressure levels and epistaxis episodes.
Materials and Methods
Two groups were studied: Group A (patients with epistaxis) and Group B (control). Patient characteristics, comorbidities, and medication use were recorded. Blood pressure measurements were taken upon ED arrival and after specialist evaluation. Statistical analyses included descriptive statistics, T-test, χ2 test, and logistic regression.
Results
Group A, enrolled from April 2014 to February 2015, included 102 patients, mean age 67, male-female ratio 2:1. Blood pressure on arrival was over 140/90 mmHg in 73%, decreasing to 26% after 30 minutes. Group B, enrolled from May 2023 to August 2023, included 126 patients, mean age 59, male-female ratio 2:1. Blood pressure on arrival was over 140/90 mmHg in 60%, decreasing to 23% after 30 minutes. Both groups showed reduced blood pressure post-evaluation. Logistic regression identified anticoagulant and/or antiplatelet therapy as the main independent risk factor for epistaxis. Age, sex, blood pressure levels, and hypertension did not significantly influence epistaxis occurrence.
Conclusion
No significant correlation between hypertension and epistaxis was found. Anticoagulant and/or antiplatelet therapy was the primary independent risk factor, highlighting the importance of considering medication history in evaluating epistaxis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40292-024-00669-7.
Keywords: Hypertension, Epistaxis, Emergencies, Blood pressure
Introduction
Epistaxis is the most common otorhinolaryngological emergency with a lifetime prevalence of 60% in the general population [1]. It can occur at any age, with a bimodal distribution of children up to age 10 and adults older than age 50. Children with nosebleeds are rare and need to be examined for trauma, nasal foreign body and systemic hematological and/or medical condition [2]. Individuals older than age 50 represent 40% of those requiring medical attention and tend to have more serious bleedings [3]. Trauma from nose picking, inflammatory and neoplastic disease of the nasal cavity represent the most frequent causes of epistaxis in adult population. Moreover, the risk of nosebleeds is increased by treatment with anticoagulant and/or antiplatelet drugs, by the therapy with nasal vasoconstrictors or by chronic intranasal-drug use (e.g., cocaine), and by topical steroids [4, 5]. The same is true in patients affected by coagulopathies such as thrombocytopenia, von Willebrand disease or hemophilia. If nosebleeds are recurrent and difficult to control, the diagnosis of Osler-Weber-Rendu syndrome and carotid artery aneurysm should be taken into consideration [1]. For a long time, a relationship between arterial hypertension and epistaxis was hypothesized, but the etiologic role of hypertension in provoking nasal bleeding still remains controversial [6–10]. In the literature, some authors reported that high blood pressure values could increase the risk of epistaxis, as result of acute organ damage on the sinonasal vessels [11–14]. According to Guidelines of the French Society of Otorhinolaryngology, it is recommended to measure the blood pressure of patients in acute-phase epistaxis, to control high blood pressure in the acute phase of bleeding in order to reduce its duration, and to control high blood pressure medically in the waning phase to reduce the risk of recurrence [15]. On the contrary, other authors showed that hypertension could be the consequence of the stressful alarm reaction induced by the nosebleed itself [8] and some others suggested the possible role in determining the severity of bleeding [16]. Kikidis et al. [17] underscored the controversy in the literature examining the available studies, reporting on arterial hypertension and epistaxis. They conclude that the presence of high blood pressure values during the actual episode of nasal bleeding cannot establish a causative relationship with epistaxis, because of confounding stress and the possible white coat phenomenon. Min et al. [18] confirm that hypertension is significantly associated with the risk of epistaxis, but this association does not fully support a causal relationship. Moreover, in their meta-analysis that includes 10 studies, they show that there is a significant heterogeneity in the definition of hypertension and in the methods used to measure blood pressure in different studies. In addition, seasonal variation of epistaxis was not taken into account in the studies included in the metanalyses, although it is well known that weather conditions, such as low temperature or low air humidity may increase the risk of primary epistaxis. We investigated the relationship between epistaxis and changes in blood pressure, testing if higher values of blood pressure were correlated to higher risk of bleeding. Another purpose was to analyze blood pressure variation from the hospital admission to discharge after otorhinolaryngological evaluation, comparing the blood pressure variation between the group with epistaxis and the control group.
Methods
A monocentric study was carried out, prospectively collecting patients sent for a specialist evaluation at the department of Otorhinolaryngology and divided in two groups: group A, consisting of patients who reported active or recent epistaxis, and group B, reported as the control group, consisting of patients who came for reasons other than bleeding. In group A, consecutive patients were included, presenting for acute primary epistaxis at the Emergency Department (ED) of Spedali Civili of Brescia (Italy), from April 2014 to February 2015 and sent for a specialist evaluation at the department of Otorhinolaryngology of the same institution. Exclusion criteria were: (1) diagnosis of Osler-Weber-Rendu syndrome, (2) recent nose surgery or trauma (< 5 days), (3) bleeding because of neoplastic lesions, and (4) coagulopathies. Datas regarding management and characteristics of bleeding were recorded for all patients. In group B, consecutive patients aged 18 years or older were included, presenting for other reasons than epistaxis (i.e. dizziness, cough, ear pain, dysphonia, etc.) at the ED of Spedali Civili of Brescia (Italy), from May 2023 to August 2023 and sent, after presentation at the ED, for a specialist evaluation at the department of Otorhinolaryngology. Patients with any head and neck bleeding were excluded.
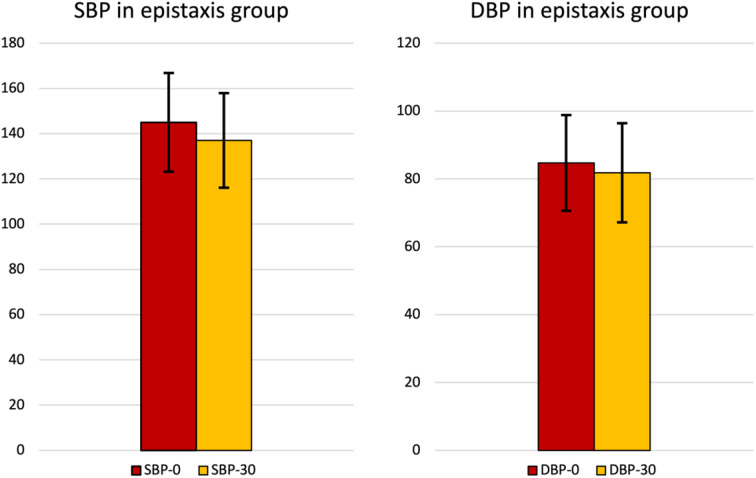
Fig. 2.
Blood pressure values in epistaxis group (A) (SBP-0 = systolic blood pressure at the arrival; SBP-30 = systolic blood pressure after 30 min; DBP-0 = diastolic blood pressure at the arrival; DBP-30 = diastolic blood pressure after 30 min)
For both groups, blood pressure and heart rate were measured in all patients, at arrival at the ED (t0) and 30 min after the otorhinolaryngology examination (t30). Blood pressure and heart rate measurements were performed according to 2013 ESC-ESH guidelines by the medical and nursing staff, using an automatic device (OMRON MT10-IT) [19]. A careful clinical history and a throughout clinical examination were performed. History of hypertension, chronic obstructive pulmonary disease (COPD), diabetes, dyslipidemia, and related treatment, with particular attention to the use of anticoagulant and/or antiplatelet drugs, were recorded. All clinical data were inserted in a dedicated database. The study was approved by the local ethical committee.
Descriptive statistics were used to present the demographic, anamnestic and clinical baseline characteristics. Student’s T test was used for quantitative variables and the χ2 test for categorical variables. Univariate correlations were analyzed by Pearson’s correlation coefficient, multivariate ones by linear regression analysis. Statistical analyses were performed by SPSS software version 20 (SPSS Inc, Chicago, Illinois).
Results
In Table 1, patients characteristics, use of anticoagulant (ACT) or antiplatelet (APT) therapy and mean values of systolic (SBP) and diastolic (DBP) blood pressure at arrival in the ED (t0) and 30 min after the otorhinolaryngology visit (t30) (with eventual hemostasis) are reported. From April 2014 to February 2015, 102 patients were enrolled into the study group (A) according to the predefined inclusion/exclusion criteria and to the protocol. Among all the patients, 60% of patients presented with active epistaxis, almost 40% with recent episode of nose bleeding occurred in the last 24 h. When required, patients underwent a hemostasis procedure during the otorhinolaryngology visit. The hemostasis was performed by bipolar cauterization of the nasal bleeding vessel under local anesthesia, or with nasal packing with resorbable nasal swab. Mean age was 67 years (range 15–93 years), and the male-female ratio was 2:1.
Table 1.
Patients characteristics, use of anticoagulant or antiplatelet therapy and mean values of systolic (SBP) and diastolic (DBP) blood pressure at arrival in the ED (t0) and 30 min after the otorhinolaryngology visit (t30). ACT = anticoagulant treatment: APT = antiplatelet treatment
| Group A | Group B | χ2 test | t-Student | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |||
| Age | 67,3 | 15,4 | 58,9 | 16,7 | p < 0,05 | |||
| Sex | 74 | 83 | p = 0,231 | |||||
| 27 | 43 | |||||||
|
HYPERTENSION (No/Yes) |
37 | 61 | p = 0,066 | |||||
| 65 | 65 | |||||||
| SBP-0 mmHg | 144,6 | 21,8 | 138,1 | 20,4 | p < 0,05 | |||
| DBP-0 mmHg | 84,7 | 14,1 | 83,3 | 11,9 | p > 0,05 | |||
| SBP-30 mmHg | 137,1 | 20,9 | 132,7 | 17,5 | p > 0,05 | |||
| DBP-30 mmHg | 81,8 | 14,6 | 81,6 | 11,6 | p > 0,05 | |||
| SBP variation mmHg | 7,5 | 11 | 5,4 | 4,6 | p > 0,05 | |||
| DBP variation mmHg | 2,9 | 9,2 | 1,8 | 1,4 | p > 0,05 | |||
| ACT and APT N | 8 | 3 | p < 0,05 | |||||
| ACT | 14 | 5 | p < 0,05 | |||||
| APT | 26 | 20 | p < 0,05 | |||||
Group B was identified from May 2023 to August 2023. It consists in 126 patients enrolled according to the predefined inclusion/exclusion criteria and to the protocol. The following diagnoses were recorded: vertigo was the most common diagnosis, affecting 31 patients (24.60%), rhinitis/sinusitis was diagnosed in 9 patients (7.14%), pharyngotonsillitis/tonsillar abscess/laryngitis affected 27 patients (21.43%), an ENT district neoplasm was found in 3 patients (2.38%), otitis was diagnosed in 24 patients (19.05%), sudden hearing loss was identified in 4 patients (3.17%), other ear conditions (foreign body, cerumen) were found in 9 patients (7.14%), traumas (ear, larynx) were diagnosed in 2 patients (1.59%), laryngeal dyspnea/airways obstruction was identified in 1 patient (0.79%), foreign body (ear, nose, pharynx) was diagnosed in 2 patients (1.59%), facial palsy was found in 3 patients (2.38%), acute infections of salivary glands were diagnosed in 3 patients (2.38%), neck abscess was identified in 1 patient (0.79%), examination negative or other cases were recorded in 7 patients (5.56%).
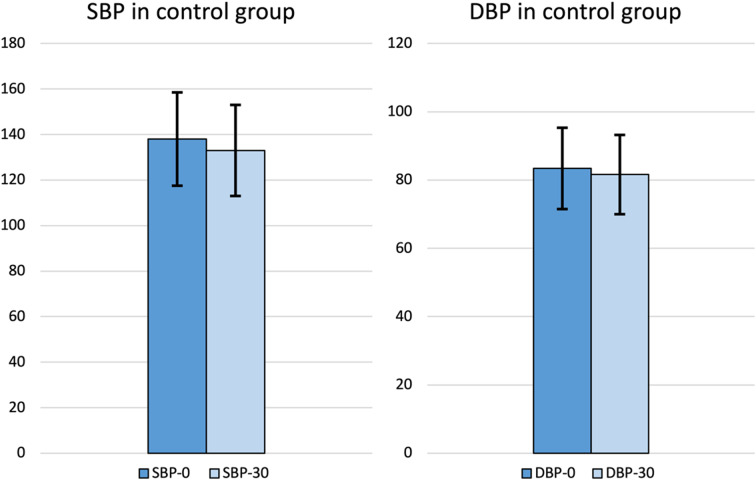
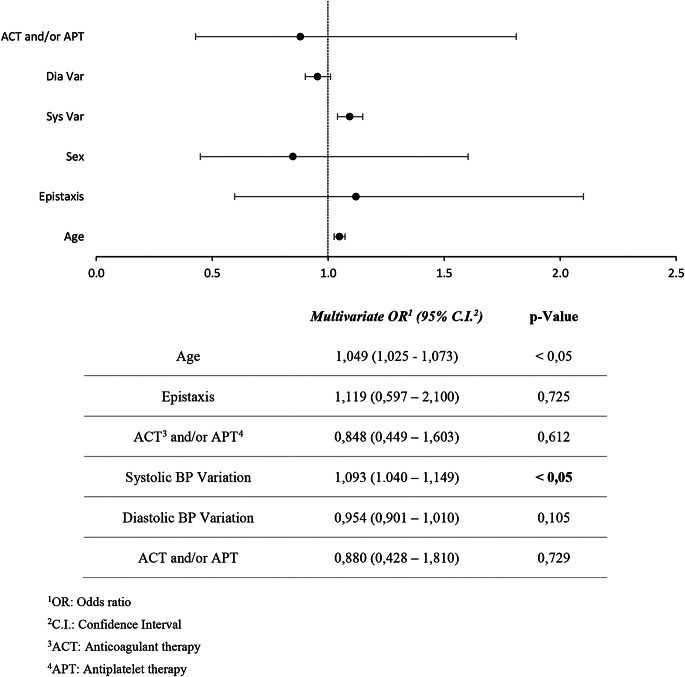
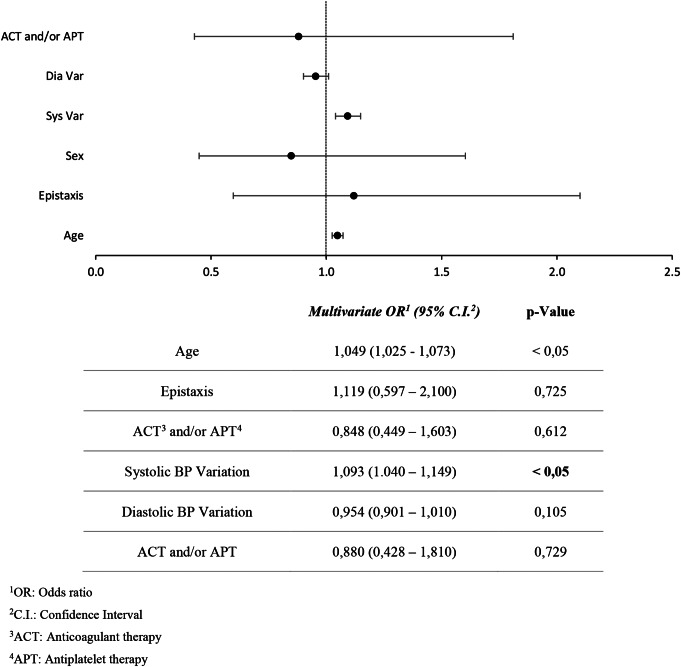
In both groups a progressive reduction of BP values from t0 to t30 was observed (p = 0.001). Taking into account group A, in 73% of cases (75 patients) BP values > 140/90 mmHg were measured at t0, and this percentage decreased to 26% (27 patients) at t30. The same was observed in group B, where BP values > 140/90 mmHg were recorded in 75 patients (60%) at t0, while at t30 only 29 patients (23%) had a high BP. The mean BP decreased from 145/85 mmHg to 137/82 mmHg in group A, and from 138/83 mmHg to 133/82 mmHg in group B (Fig. 1, 2). No anti-hypertensive treatment was given to the patients during the evaluation. SBP was higher in the group of patients with epistaxis (group A) compared to the control group (group B) while there were no differences regarding DBP and the prevalence of hypertension was not different between the two groups. However, the age of the patients belonging to group A was higher than that of group B (Table 1). For this reason, logistic regression was performed (Tables 2 and 3). Epistaxis was positively associated with ACT and/or APT, while it was not associated with systolic or diastolic blood pressure values. Multivariate logistic regression analysis screened out that the main independent risk factors for epistaxis (p-value = 0,016) is the use of ACT and/or APT, while sex, age, SBP, DBP, and high BP didn’t show a statistically significant result (p-value > 0,05) (Table 2). Considering hypertension as the dependent variable, SBP variations becomes statistically significant (p-value < 0,001), meaning that when BP is higher, there is a more important reduction in SBP than DBP (Table 3) ).
Fig. 1.
Blood pressure values in control group (B) (SBP-0 = systolic blood pressure at the arrival; SBP-30 = systolic blood pressure after 30 min; DBP-0 = diastolic blood pressure at the arrival; DBP-30 = diastolic blood pressure after 30 min)
Table 2.
Logistic regression model, dependent variable: Epistaxis
Table 3.
Logistic regression model, dependent variable: hypertension
Discussion
The main finding of our prospective study is the absence of a correlation between hypertension and epistaxis. We were able to show that a spontaneous reduction in blood pressure values occurred in all patients from admission to discharge. The demographic data of our population were consistent with the epidemiology described in the literature, indicating an increase in epistaxis with age, particularly after 50 years. Previous studies have shown that the majority of patients admitted to the ED for acute epistaxis had a documented history of hypertension and high blood pressure values upon admission. Conversely, the prevalence of hypertensive patients in our population cohort seems comparable to that observed in the general population, and no cause-effect relationship between the diagnosis of hypertension and nosebleeds was found, as the variable related to hypertension nullifies itself when considering age. A major strength of our study was the accuracy in measuring blood pressure values; measurements were performed in a standardized manner with the same semiautomatic device according to guidelines. To our knowledge, no other studies have used this methodology for blood pressure measurement, as many previous studies were based on a single value of blood pressure without specifying the timing and methods of acquisition. The results of our study indicate that a progressive reduction in blood pressure values may occur within about 30 min, without the administration of any drug treatment, suggesting that the higher values recorded at admission may reflect, to some extent, an alert reaction to bleeding, supported by concomitant heart rate changes during the same time interval. This reinforces the importance of repeated and accurate measurements of blood pressure values according to guidelines, as a single detection can be influenced by anxiety, stress, and error. High blood pressure values decreased in both the epistaxis and control groups, confirming that blood pressure rise could be considered a reaction to the stressful event. The use of 24-hour blood pressure ambulatory monitoring (ABPM) could have further helped in identifying patients with white coat or sustained hypertension. Another important finding concerns the strong relationship between the risk of epistaxis and the use of anticoagulant and antiplatelet therapy. Evidence from both clinical observations and experimental studies provides substantial support for the assertion that anticoagulant therapy (ACT) and antiplatelet therapy (APT) significantly impact the risk of epistaxis in hypertensive patients. Anticoagulant medications, such as warfarin or direct oral anticoagulants (DOACs), are known to interfere with the clotting cascade, thereby increasing the propensity for bleeding events. Similarly, antiplatelet agents like aspirin or clopidogrel inhibit platelet aggregation, potentially exacerbating bleeding tendencies in hypertensive individuals predisposed to vascular fragility. Moreover, the synergistic effect of hypertension-induced vascular remodeling and the antithrombotic properties of these medications further amplifies the likelihood of epistaxis occurrence. Consequently, meticulous consideration of the patient’s hypertensive status and concurrent pharmacotherapy is imperative in assessing and managing the risk of epistaxis, thereby optimizing therapeutic outcomes and patient safety.
Conclusions
According to our monocentric with prospective collection of data and retrospective analysis, epistaxis and hypertension do not show a clear association. On one hand the prevalence of the disease is superimposable to general population. On the other, measurement of blood pressure values, performed according to the guidelines recommendations, shows a spontaneous and statistically significant reduction, not attributable to any antihypertensive therapy. These data seem to suggest that epistaxis should not be considered secondary organ damage to an acute pressure rise but may rather be the cause of an alarm reaction in both normotensive and hypertensive patients.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Declarations
Competing Interests
None.
References
- 1.Morgan DJ, Kellerman R. Epistaxis: evaluation and treatment. Prim Care. 2014;41(1):63–73. 10.1016/j.pop.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Brown NJ, Berkowitz RG. Epistaxis in Healthy Children Requiring Hospital Admission. Int J Pediatr Otorhinolaryngol. 2004;68(9):1181–4. 10.1016/j.ijporl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Walker TW, Macfarlane TV, McGarry GW. 2007. The Epidemiology and Chronobiology of Epistaxis: An Investigation of Scottish Hospital Admissions 1995–2004. Clin Otolaryngol 32, no. 5 (Oct): 361-5. 10.1111/j.1749-4486.2007.01530.x [DOI] [PubMed]
- 4.Rosenblut A, Bardin PG, Muller B, Faris MA, Wu WW, Caldwell MF, Fokkens WJ. Long-term safety of Fluticasone Furoate Nasal Spray in adults and adolescents with perennial allergic Rhinitis. Allergy. 2007;62(9):1071–7. 10.1111/j.1398-9995.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Ando Y, Iimura J, Arai S, Arai C, Komori M, Tsuyumu M, Hama T, Shigeta Y, Hatano A, Moriyama H. Risk factors for recurrent Epistaxis: importance of initial treatment. Auris Nasus Larynx. 2014;41(1):41–5. 10.1016/j.anl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Petruson B, Rudin R, Svärdsudd K. Is high blood pressure an aetiological factor in Epistaxis? ORL J Otorhinolaryngol Relat Spec. 1977;39(3):155–60. 10.1159/000275350. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs FD, Moreira LB, Pires CP, Torres FS, Furtado MV, Moraes RS, Wiehe M, Fuchs SC. and J. F. Lubianca Neto. 2003. Absence of Association between Hypertension and Epistaxis: A Population-Based Study. Blood Press 12, no. 3: 145-8. 10.1080/08037050310001750 [DOI] [PubMed]
- 8.Herkner H, Havel C, Müllner M, Gamper G, Bur A, Temmel AF, Laggner AN, Hirschl MM. Active Epistaxis at Ed Presentation is Associated with arterial hypertension. Am J Emerg Med. 2002;20(2):92–5. 10.1053/ajem.2002.31577. [DOI] [PubMed] [Google Scholar]
- 9.Isezuo SA, Segun-Busari S, Ezunu E, Yakubu A, Iseh K, Legbo J, Alabi BS, Dunmade AE, Ologe FE. Relationship between Epistaxis and Hypertension: a study of patients seen in the Emergency Units of Two Tertiary Health Institutions in Nigeria. Niger J Clin Pract. 2008;11(4):379–82. [PubMed] [Google Scholar]
- 10.André N, Klopp-Dutote N, Biet-Hornstein A, Strunski V, Page C. Cardiovascular Risk and Severity factors in patients admitted to hospital for spontaneous Epistaxis. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(2):119–22. 10.1016/j.anorl.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Lubianca Neto JF, Fuchs FD, Facco SR, Gus M, Fasolo L, Mafessoni R, Gleissner AL. 1999. Is Epistaxis evidence of end-organ damage in patients with hypertension? Laryngoscope 109, 7 pt 1 (Jul): 1111–5. 10.1097/00005537-199907000-00019 [DOI] [PubMed]
- 12.Lubianca-Neto JF, Bredemeier M, Carvalhal EF, Arruda CA, Estrella E, Pletsch A, Gus M, Lu L, Fuchs FD. A study of the Association between Epistaxis and the severity of hypertension. Am J Rhinol. 1998;12(4):269–72. 10.2500/105065898781389985. [DOI] [PubMed] [Google Scholar]
- 13.Acar B, Yavuz B, Yıldız E, Ozkan S, Ayturk M, Sen O, Deveci OS. A possible cause of Epistaxis: increased masked hypertension prevalence in patients with Epistaxis. Braz J Otorhinolaryngol. 2017;83(1):45–9. 10.1016/j.bjorl.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page C, Biet A, Liabeuf S, Strunski V, Fournier A. Serious spontaneous Epistaxis and Hypertension in Hospitalized patients. Eur Arch Otorhinolaryngol. 2011;268(12):1749–53. 10.1007/s00405-011-1659-y. [DOI] [PubMed] [Google Scholar]
- 15.Michel J, Prulière Escabasse V, Bequignon E, Vérillaud B, Robard L, Crampette L, Malard O, Work-Group SFORL. Epistaxis and High Blood Pressure. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(1):33–5. 10.1016/j.anorl.2016.09.011. Guidelines of the French Society of Otorhinolaryngology (Sforl). [DOI] [PubMed]
- 16.Byun H, Chung JH, Lee SH, Ryu J, Kim C, Shin JH. Association of Hypertension with the risk and severity of Epistaxis. JAMA Otolaryngol Head Neck Surg. 2020;147(1):1–7. 10.1001/jamaoto.2020.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikidis D, Tsioufis K, Papanikolaou V, Zerva K, Hantzakos A. Is Epistaxis Associated with arterial hypertension? A systematic review of the literature. Eur Arch Otorhinolaryngol. 2014;271(2):237–43. 10.1007/s00405-013-2450-z. [DOI] [PubMed] [Google Scholar]
- 18.Min HJ, Kang H, Choi GJ, Kim KS. Association between Hypertension and Epistaxis: systematic review and Meta-analysis. Otolaryngol Head Neck Surg. 2017;157(6):921–7. 10.1177/0194599817721445. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force Members. 2013 Esh/Esc guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (Esh) and of the European Society of Cardiology (Esc). J Hypertens. 2013;31(7):1281–357. 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Michel J, Prulière Escabasse V, Bequignon E, Vérillaud B, Robard L, Crampette L, Malard O, Work-Group SFORL. Epistaxis and High Blood Pressure. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(1):33–5. 10.1016/j.anorl.2016.09.011. Guidelines of the French Society of Otorhinolaryngology (Sforl). [DOI] [PubMed]