Abstract
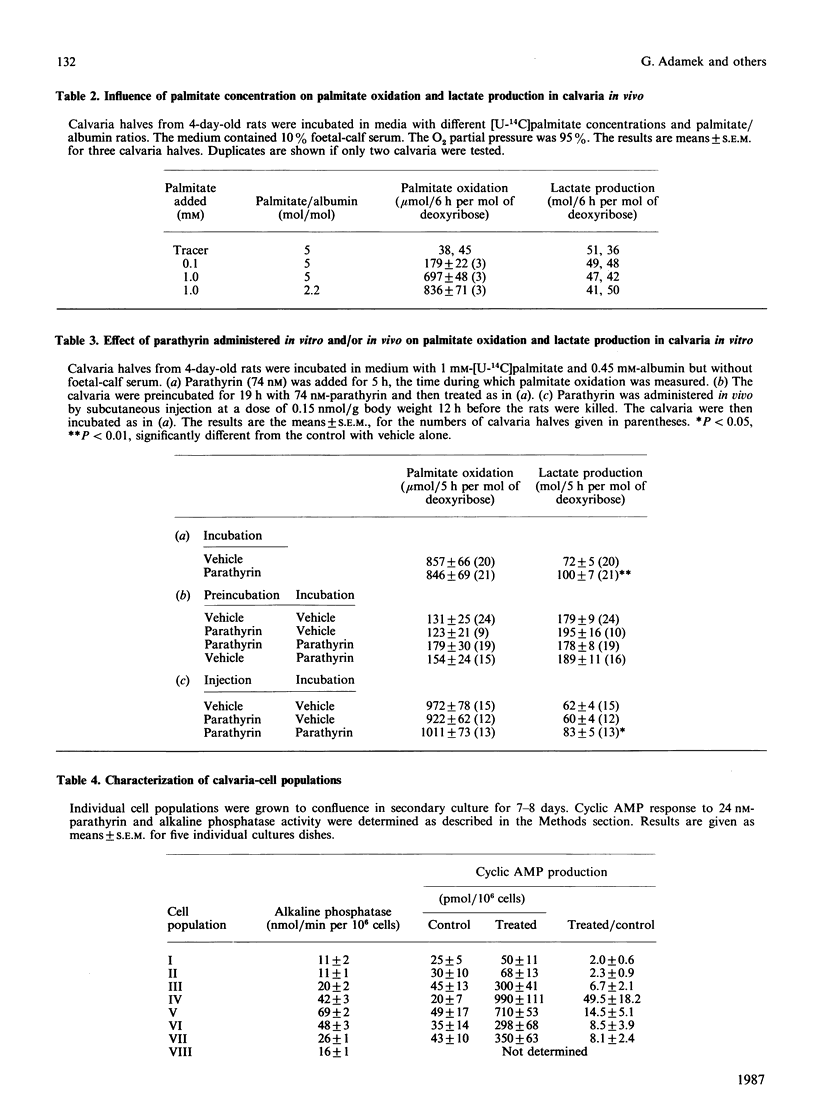
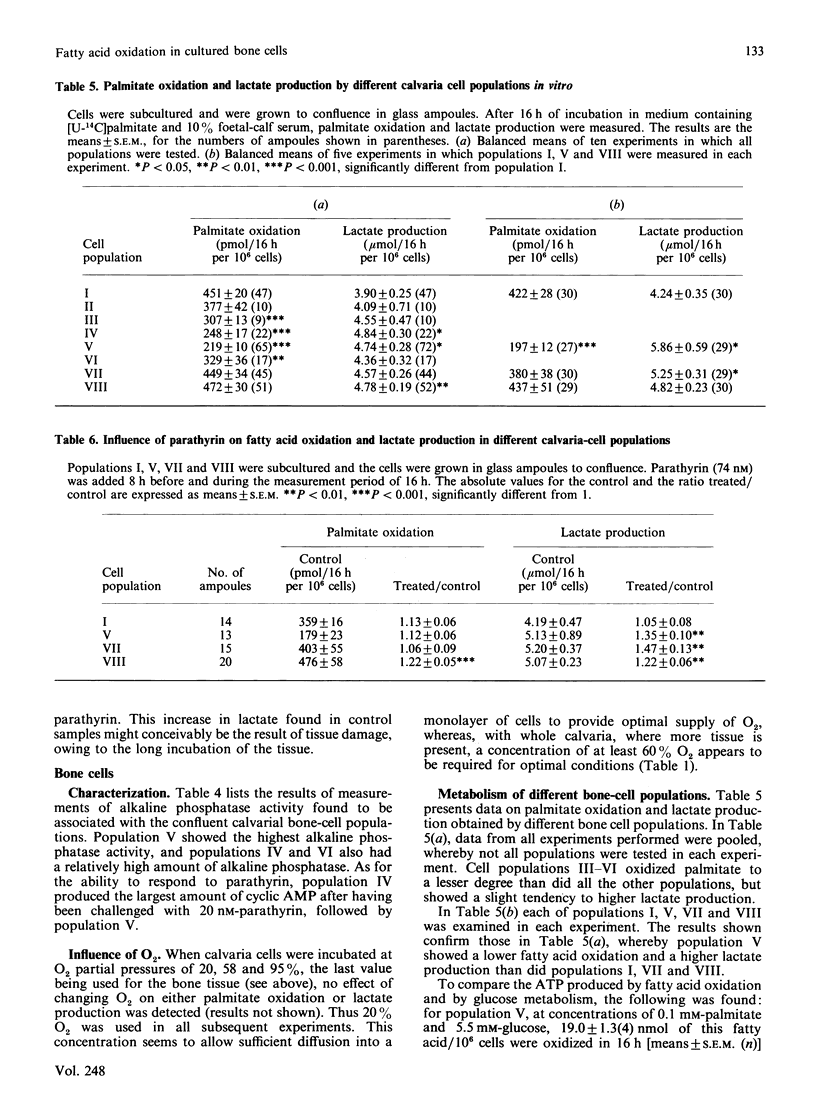
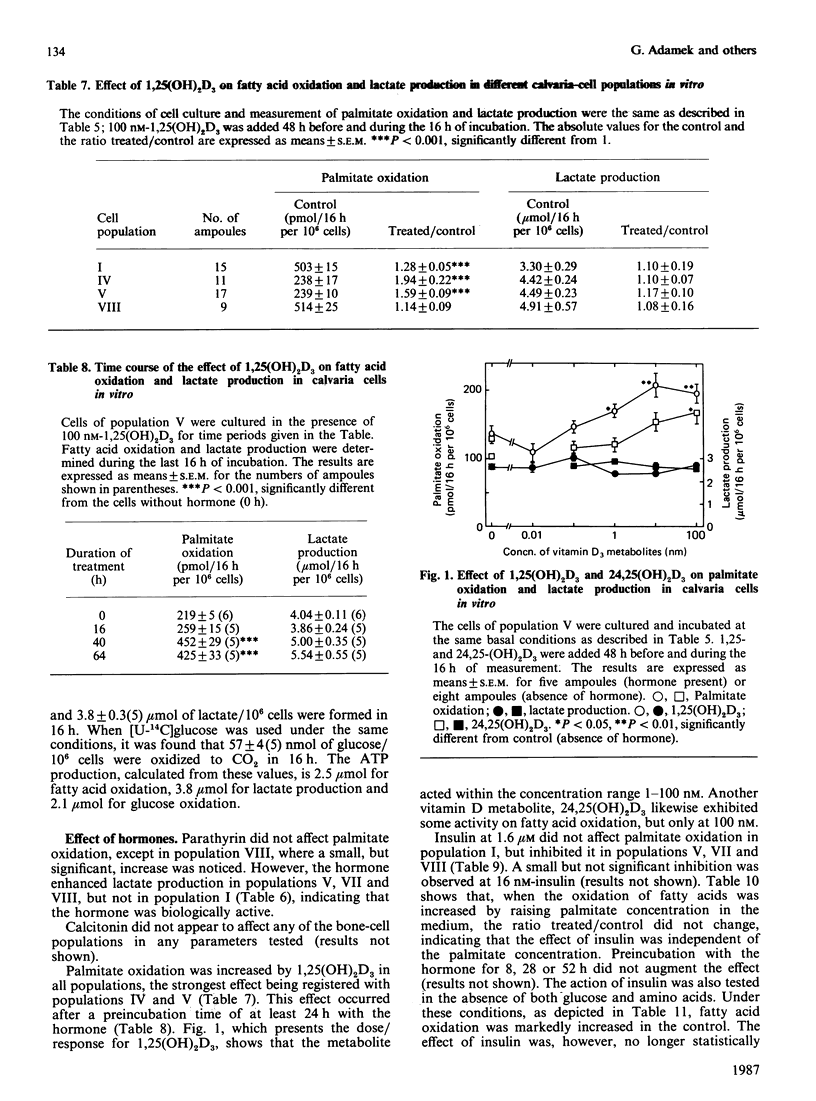
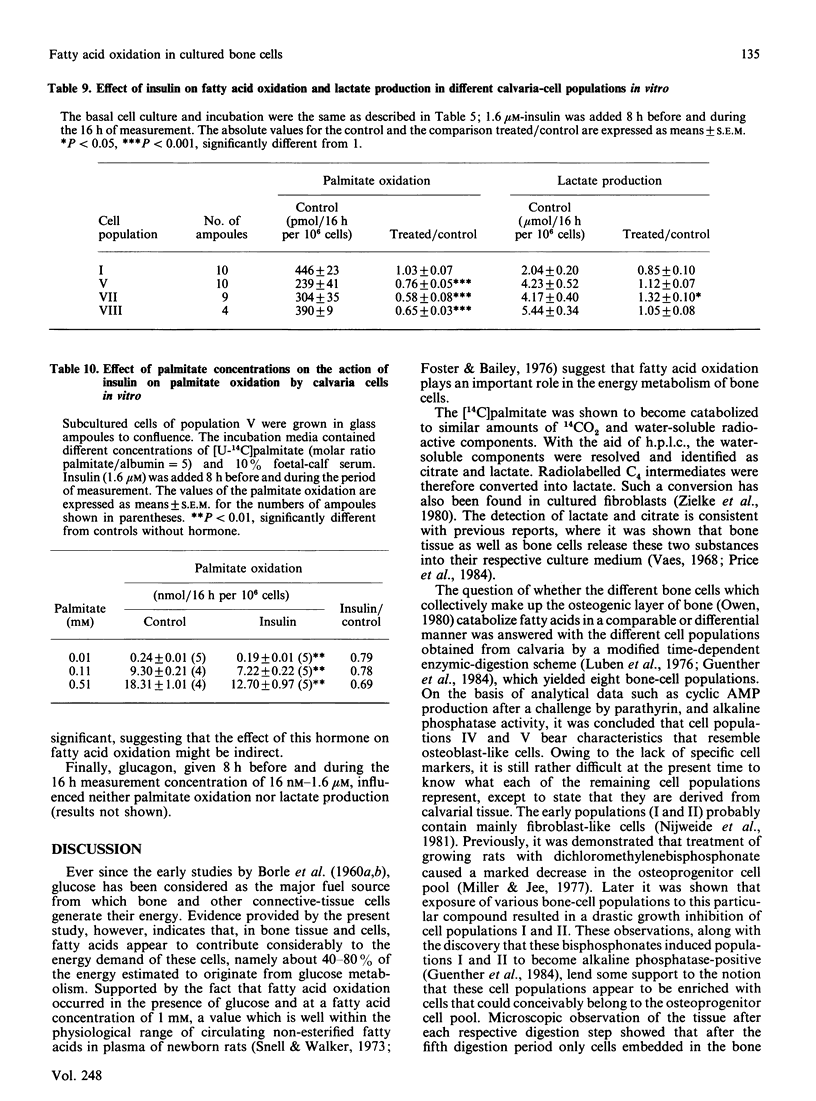
Fatty acid oxidation and its hormonal modulation were investigated in cultured rat calvaria and in cultivated cell populations. The latter were obtained from calvaria of newborn rats by sequential time-dependent digestion with collagenase, yielding eight cell populations: the early ones containing mainly fibroblasts, the middle ones being osteoblast-like, and late ones osteoblast-osteocyte-like. In calvaria, fatty acid oxidation was increased by adding 0.1 mM- and 1.0 mM-palmitate to the medium, containing 10% (v/v) fetal-calf serum. No effect was found after parathyrin addition in vitro or when injected in vivo. All cell populations obtained by sequential digestion were found to oxidize palmitate, whereby the osteoblast-like cells showed a lower oxidation rate than the other populations. Both parathyrin and calcitonin had no effect on fatty acid oxidation. 1,25-Dihydroxycholecalciferol at 1-100 nM and 24,25-dihydroxycholecalciferol at 100 nM increased oxidation primarily in the population enriched with osteoblast-like cells. Insulin at 1.6 microM diminished it in the cell populations enriched with osteoblast-like cells and in the late bone-cell fraction. However, glucagon had no effect. The energy provided by fatty acid oxidation in this system is approx. 40-80% of glucose metabolism, suggesting that this event may be of importance in the energy metabolism of bone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORLE A. B., NICHOLS N., NICHOLS G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960 Apr;235:1206–1210. [PubMed] [Google Scholar]
- BORLE A. B., NICHOLS N., NICHOLS G., Jr Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960 Apr;235:1211–1214. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN D. V., FORSCHER B. K. Effect of parathyroid extract on the oxidation in vitro of glucose and the production of 14CO-2 by bone and kidney. Biochim Biophys Acta. 1962 Nov 19;65:20–26. doi: 10.1016/0006-3002(62)90145-2. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Moore A. The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab. 1983 Oct;57(4):819–824. doi: 10.1210/jcem-57-4-819. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Cone C. M., Morey-Holton E., Feldman D. 1 alpha,25-dihydroxyvitamin D3 receptors in cultured rat osteoblast-like cells. Glucocorticoid treatment increases receptor content. J Biol Chem. 1983 Apr 10;258(7):4350–4355. [PubMed] [Google Scholar]
- Chen T. L., Hauschka P. V., Feldman D. Dexamethasone increases 1,25-dihydroxyvitamin D3 receptor levels and augments bioresponses in rat osteoblast-like cells. Endocrinology. 1986 Mar;118(3):1119–1126. doi: 10.1210/endo-118-3-1119. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Jones J. B. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal Biochem. 1978 Aug 1;88(2):475–484. doi: 10.1016/0003-2697(78)90447-5. [DOI] [PubMed] [Google Scholar]
- Fast D. K., Felix R., Dowse C., Neuman W. F., Fleisch H. The effects of diphosphonates on the growth and glycolysis of connective-tissue cells in culture. Biochem J. 1978 Apr 15;172(1):97–107. doi: 10.1042/bj1720097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R., Fleisch H. Increase in alkaline phosphatase activity in calvaria cells cultured with diphosphonates. Biochem J. 1979 Oct 1;183(1):73–81. doi: 10.1042/bj1830073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R., Fleisch H. Increase in fatty acid oxidation in calvaria cells cultured with diphosphonates. Biochem J. 1981 Apr 15;196(1):237–245. doi: 10.1042/bj1960237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R., Fleisch H., Schenk R. Effect of halogenmethylenebisphosphonates on bone cells in culture and on bone resorption in vivo. Experientia. 1986 Mar 15;42(3):302–304. doi: 10.1007/BF01942514. [DOI] [PubMed] [Google Scholar]
- Felix R., Fleisch H. The effect of diphosphonates on periosteal and bone cells in culture. Experientia. 1981;37(8):817–819. doi: 10.1007/BF01985657. [DOI] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in hepatic fatty acid degradation and blood lipid and ketone body content during development of the rat. Enzyme. 1976;21(5):397–407. doi: 10.1159/000458889. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Kopple J. D. Evidence that dog kidney is an endogenous source of histidine. Am J Physiol. 1979 Jul;237(1):E1–E5. doi: 10.1152/ajpendo.1979.237.1.E1. [DOI] [PubMed] [Google Scholar]
- Guenther H. L., Gallagher J. A., Fleisch H. Differential response of bone cells isolated by sequential digestion to dichloromethylenebisphonate in culture. Calcif Tissue Int. 1984 Sep;36(5):568–575. doi: 10.1007/BF02405368. [DOI] [PubMed] [Google Scholar]
- Guenther H. L., Guenther H. E., Fleisch H. Effects of 1-hydroxyethane-1,1-diphosphonate and dichloromethanediphosphonate on rabbit articular chondrocytes in culture. Biochem J. 1979 Nov 15;184(2):203–214. doi: 10.1042/bj1840203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T. J., Downing S. J., Phang J. M. Insulin effect on amino acid transport in bone: dependence on protein synthesis and Na+. Am J Physiol. 1971 Jun;220(6):1717–1723. doi: 10.1152/ajplegacy.1971.220.6.1717. [DOI] [PubMed] [Google Scholar]
- Lorch E., Gey K. F. Photometric "titration" of free fatty acids with the Technicon AutoAnalyzer. Anal Biochem. 1966 Aug;16(2):244–252. doi: 10.1016/0003-2697(66)90152-7. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976 Aug;99(2):526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- Miller S. C., Jee W. S. The comparative effects of dichloromethylene diphosphonate (C12MDP) and ethane-1-hydroxy-1,1-diphosphonate (EHDP) on growth and modeling of the rat tibia. Calcif Tissue Res. 1977 Oct 20;23(3):207–214. doi: 10.1007/BF02012787. [DOI] [PubMed] [Google Scholar]
- Neuman W. F., Neuman M. W., Brommage R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am J Physiol. 1978 Jan;234(1):C41–C50. doi: 10.1152/ajpcell.1978.234.1.C41. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., Moseley J. M., Sexton P. M., Mendelsohn F. A., Martin T. J. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest. 1986 Aug;78(2):355–360. doi: 10.1172/JCI112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijweide P. J., van der Plas A., Scherft J. P. Biochemical and histological studies on various bone cell preparations. Calcif Tissue Int. 1981;33(5):529–540. doi: 10.1007/BF02409485. [DOI] [PubMed] [Google Scholar]
- Owen M. The origin of bone cells in the postnatal organism. Arthritis Rheum. 1980 Oct;23(10):1073–1080. doi: 10.1002/art.1780231002. [DOI] [PubMed] [Google Scholar]
- Price P. A., Williamson M. K., Sloper S. A. 1,25-Dihydroxyvitamin D3 increases citrate secretion from osteosarcoma cells. J Biol Chem. 1984 Feb 25;259(4):2537–2540. [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Insulin-like growth factors stimulate synthesis of nucleic acids and glycogen in cultured calvaria cells. Calcif Tissue Int. 1983 Jul;35(4-5):578–585. doi: 10.1007/BF02405097. [DOI] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Parathormone promotes glycogen formation from [14C]glucose in cultured osteoblast-like cells. FEBS Lett. 1982 Nov 1;148(1):31–34. doi: 10.1016/0014-5793(82)81236-2. [DOI] [PubMed] [Google Scholar]
- Shinoda H., Adamek G., Felix R., Fleisch H., Schenk R., Hagan P. Structure-activity relationships of various bisphosphonates. Calcif Tissue Int. 1983;35(1):87–99. doi: 10.1007/BF02405012. [DOI] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Glucose metabolism in the newborn rat. Temporal studies in vivo. Biochem J. 1973 Apr;132(4):739–752. doi: 10.1042/bj1320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. H., DeLuca H. F., Ikekawa N. Bone resorbing activities of 24-hydroxy stereoisomers of 24-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. Biochem Biophys Res Commun. 1975 Dec 1;67(3):965–971. doi: 10.1016/0006-291x(75)90769-x. [DOI] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. L., Luben R. A., Cohn D. V. 1,25-dihydroxycholecalciferol and parathormone: effects on isolated osteoclast-like and osteoblast-like cells. Science. 1977 Aug 12;197(4304):663–665. doi: 10.1126/science.195343. [DOI] [PubMed] [Google Scholar]
- Zielke H. R., Sumbilla C. M., Sevdalian D. A., Hawkins R. L., Ozand P. T. Lactate: a major product of glutamine metabolism by human diploid fibroblasts. J Cell Physiol. 1980 Sep;104(3):433–441. doi: 10.1002/jcp.1041040316. [DOI] [PubMed] [Google Scholar]


