Abstract
Hepatitis delta virus (HDV) infection and spread in vivo are dependent upon coinfection by hepatitis B virus (HBV), and dual HDV/HBV infection is frequently more severe than HBV infection alone, raising the possibility that HDV infection may be deleterious to cells. Here we have examined the effects of HDV replication on the long-term growth of cultured cells. Our results show that most cells transfected with HDV cDNA do not give rise to stable cell lines expressing viral antigens or replicative intermediates; in addition, cotransfection of HDV replicons with a plasmid vector expressing a hygromycin resistance marker results in a dose-dependent impairment of hygromycin-resistant colony formation. When cells transfected with replication-competent HDV cDNA are followed prospectively, a progressive decline in viral RNA replication and a steady decrease in the proportion of cells expressing delta antigen are observed. However, in transient transfection assays, no evidence was found to link HDV replication to apoptosis or to cell cycle arrest, nor did HDV replication confer on host cells enhanced sensitivity to inducers of apoptosis. Thus, HDV replication does not appear to be acutely cytotoxic. However, in dividing cells HDV replication is associated with a subtler growth disadvantage, leading to selection in culture for cells displaying diminished HDV expression. This effect would not be expected to cause hepatitis in vivo but might contribute to impaired liver regeneration in the setting of ongoing hepatocellular injury.
Hepatitis delta virus (HDV) is a single-stranded circular RNA virus that concurrently infects humans with hepatitis B and generally exacerbates the resulting hepatitis. HDV encodes a single protein, the hepatitis delta antigen (HDAg), which is expressed in two isoforms: a 24-kDa small form (S-HDAg) that is required for viral replication and a 27-kDa large form (L-HDAg) that is produced late in infection as a consequence of an RNA editing event (2). Replication of the virus is believed to proceed via a double rolling circle mechanism, leading to the synthesis of single-stranded HDV species of both genomic and antigenomic polarities in a process that is likely to be mediated by a cellular polymerase. Although it is not presently possible to infect cell lines with HDV virions, the study of HDV has been facilitated by the development of cell culture systems that utilize HDV cDNA templates to initiate viral RNA production (7).
The mechanisms by which viral infection contribute to clinical hepatitis are poorly understood. As a first step towards addressing this issue, a number of experiments have been carried out in model systems examining the potential impact of HDAg expression and HDV replication on the phenotype of cells in culture. These studies have yielded conflicting results. One early study indicated that overexpression of HDAg alone (i.e., in the absence of HDV RNA replication) can induce cytotoxicity (11); in another study, baculovirus-mediated HDAg expression led to cell cycle arrest in insect cells (6). The relevance of these observations to authentic HDV replication is, however, uncertain. When replicating HDV constructs were utilized, one study found that HDV replication in a single stably transformed clone slowly diminished over time, while another report identified several stable clones in which HDV RNA appeared to accumulate without consequence to the cell (3, 10). Furthermore, transgenic mice expressing either HDAg alone or intact HDV genomes show little cellular injury, indicating that HDV itself may be noncytopathic (5, 13); this in turn has suggested that HDV-induced liver injury might arise from immune responses to HDV antigens displayed by infected cells, as is the case for hepatitis B virus (4). Here we have reexamined the issue of cytotoxicity associated with HDV replication, in an effort to better understand the role of direct versus indirect mechanisms by which HDV may contribute to liver disease.
MATERIALS AND METHODS
Plasmids.
Plasmid D452, which expresses a replication-competent HDV 1.1-mer, and D449, which expresses a nonreplicating mutant HDV construct with a 2-bp deletion, were kindly provided by D. Lazinski (Tufts University). D731 contains the 1.1-mer HDV fragment from D452 subcloned into pCEP4 (Invitrogen). D393 and D669 express S-HDAg and L-HDAg from pCEP4 vectors.
Cell culture and transfections.
The human HeLa or monkey CV1 cell line was grown at 37°C in 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum and antibiotics. For all transfections, Fugene (Roche) was used according to the manufacturer's instructions. For the establishment of stable cell lines, HeLa cells were transfected with a 10:1 ratio of D452 or of D449 to pcDNA Neo and selected in 1.5 mg of G418/ml and individual colonies were picked and expanded. For colony formation assays, HeLa and CV1 cells were transfected with 25 ng of pcDNA Hygro, along with 0 to 2 μg of various HDV plasmids. Twenty-four hours posttransfection, cells were trypsinized and plated into 10-cm-diameter dishes in the presence of 300 μg of hygromycin/ml. Between 14 and 20 days later, cells were fixed with 1% glutaraldehyde in phosphate-buffered saline and stained with crystal violet. For time courses, transfected cells were grown continuously in 300 μg of hygromycin/ml. Every 4 to 5 days, cells were pooled and passaged and samples were taken for fluorescence-activated cell sorter (FACS) and Northern blot analyses. In all transient transfection experiments, cells were analyzed 3 or 4 days posttransfection, when HDV replication had reached high levels.
Flow cytometry analysis.
For HDAg staining, cells were fixed with 4% formaldehyde, permeabilized with 0.02% saponin, incubated with a rabbit polyclonal antibody against HDAg, washed, and stained with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. At least 10,000 cells were analyzed for each sample using a Becton Dickinson FACSCalibur. For cell cycle analysis, fixed and permeabilized cells were stained with 50 μg of propidium iodide/ml. For apoptosis assays, cells were stained with annexin 5-FITC (Clontech) or annexin 5-Cy3 (Coulter Immunotech). In these experiments, a marker plasmid (expressing either green fluorescent protein [GFP] or CD8) was cotransfected with HDV constructs in order to gate on transfected cell populations.
Northern blotting.
RNA was isolated using RNAzol (Teltest), electrophoresed on 1% formaldehyde denaturing agarose gels, transferred to nylon membranes, and probed with a riboprobe uniformly labeled with digoxigenin as described previously (1).
RESULTS AND DISCUSSION
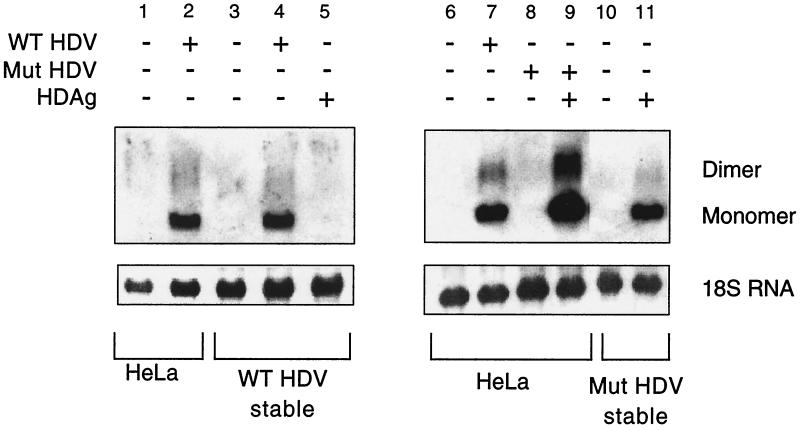
In order to examine the effects of viral replication on cell viability, we attempted to establish stable HeLa cell lines harboring integrated copies of either a replication-competent 1.1-mer HDV cDNA (WT) or a replication-incompetent HDV mutant (MUT) that bears a 2-bp deletion in the delta antigen coding region. Twelve WT and 12 MUT colonies were isolated after G418 selection and were determined by PCR to contain integrated HDV sequences (data not shown). However, Northern blot analysis revealed that none of the stable cell lines established using WT HDV cDNA displayed HDV replication (Fig. 1, lane 3 shows results from a representative clone). As expected, the replication-incompetent MUT stable cell lines did not replicate HDV either (Fig. 1, lane 10), but replication could be rescued by transient transfection of a vector encoding S-HDAg in trans (Fig. 1, lane 11).
FIG. 1.
Northern blot analysis to detect HDV replication in HeLa, WT HDV stable, or MUT HDV stable cell line. Representative clones from the WT HDV and MUT HDV stable cell line selections are shown. The blots were probed for HDV antigenomic RNA using a strand-specific riboprobe. Monomeric and dimeric HDV species are shown as indicated. Transient transfections with the indicated plasmids were cultured for 3 days prior to RNA isolation. −, absence of virus or antigen; +, presence of virus or antigen.
In order to determine if the WT stable cell lines had undergone some type of genomic alteration that rendered the cells nonpermissive for HDV replication, the WT stable cell lines were transiently transfected with the WT HDV 1.1-mer cDNA (D452). The presence of HDV replicative intermediates (Fig. 1, lane 4) 3 days posttransfection indicated that the cells remained permissive for viral replication, at least from such a template. In view of this finding, it is unclear why the endogenous template did not support HDV replication. One possibility is that replication from the WT HDV templates is deleterious to cell growth; if so, then selection might occur for clones which acquired HDV mutations or for clones in which HDV DNA integrated into transcriptionally silent regions of the chromosome. Such clones would be defective for replication from the integrated DNA but would be able to support replication from a newly introduced WT HDV genome. Consistent with this, transfection with the MUT version of HDV gives rise to stable integrants in which replication can be rescued simply by providing HDAg in trans (Fig. 1, lane 11); this indicates that in the absence of viral replication there is no selection for transcriptional silencing of the integrated copy of HDV.
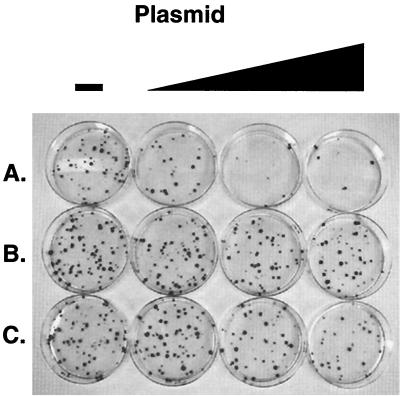
Accordingly, we asked more directly whether HDV replication could be shown to be deleterious to cell survival or growth. To do this, we employed a colony inhibition assay. Briefly, a fixed amount of plasmid pcDNA Hygro was cotransfected with graded amounts of HDV 1.1-mer plasmids expressing WT HDV or MUT HDV; in parallel, graded doses of an expression vector for S-HDAg alone were also cotransfected with the selectable marker plasmid (Fig. 2). A striking inhibition of colony formation in CV1 cells was observed in the presence of WT HDV plasmid (Fig. 2A). In sharp contrast, the nonreplicating MUT construct had no effect on colony formation (Fig. 2B), whereas S-HDAg had only a modest effect, clearly smaller than that observed with WT HDV (Fig. 2C). Similar results were observed with HeLa cells and mouse fibroblasts (data not shown).
FIG. 2.
Colony suppression assay in CV1 cells. Cells were transfected with 25 ng of plasmid expressing hygromycin resistance and 0.0, 0.1, 0.2, or 2 μg of plasmid expressing WT HDV (A), MUT HDV (B), and S-HDAg (C). Cells were grown in the presence of 300 μg of hygromycin/ml, and colonies were identified by crystal violet staining 14 days posttransfection.
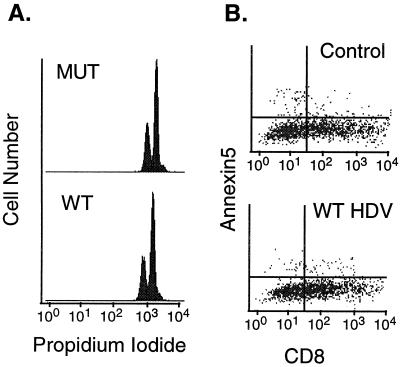
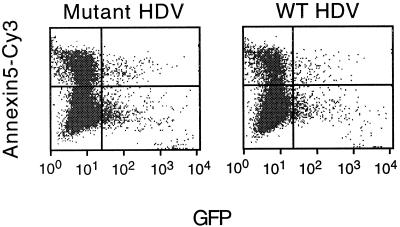
The simplest explanation for the observed colony suppression results is that WT HDV replication could either inhibit cellular proliferation or induce cell death. We therefore directly examined the ability of cells transiently transfected with WT HDV constructs to traverse the cell cycle. WT or MUT HDV genomes were transfected into CV1 cells together with a GFP marker and were stained with propidium iodide, and GFP-positive (transfected) cells were analyzed by flow cytometry for their DNA content. As shown in Fig. 3A, no difference was observed between WT- and MUT-transfected cells, indicating that no cell cycle arrest was observed in most cells. Similarly, we did not find any evidence of an increase in apoptosis in HDV-infected cells: WT- or vector-transfected HeLa cells displayed similar staining with annexin 5, binding of which is a specific and sensitive marker of apoptosis (12) (Fig. 3B). Next, we considered the possibility that HDV replication might sensitize cells to killing by known inducers of apoptosis, even if it did not directly induce apoptosis by itself. In this context we were particularly interested in apoptosis induced via the tumor necrosis factor (TNF) or Fas pathway, since these pathways are engaged by molecules typically found in inflammatory foci in chronic hepatitis. However, Fig. 4 shows no observable change in sensitivity of HDV-infected NIH 3T3 cells to apoptosis induced by TNF alpha (TNF-α) (3T3 cells were chosen because of their known sensitivity to TNF). Similarly, HDV replication did not sensitize BJAB cells to Fas-mediated killing or several fibroblastic cell lines to killing by staurosporine (data not shown).
FIG. 3.
(A) Cell cycle analysis of transiently transfected cells. Control MUT HDV- and WT HDV-transfected CV1 cells were stained with propidium iodide for total DNA content. Using a GFP marker to gate on transfected cells, the distribution of DNA content in MUT HDV- and WT HDV-transfected cells is shown. (B) Apoptosis assay in transiently transfected cells. Cells transfected with either vector alone (control) or WT HDV were stained with annexin 5-FITC.
FIG. 4.
Sensitivity of NIH 3T3 cells, transiently transfected with either MUT or WT HDV, to TNF-α. Cells were cotransfected with GFP and were stained with annexin 5-Cy3 5 h after treatment with 10 ng of TNF-α/ml and 50 ng of actinomycin D/ml.
Since cells transiently transfected with HDV cDNA efficiently support viral replication, while stable clones derived from such transfections do not (Fig. 1), we sought to characterize in more detail the events transpiring distal to the introduction of HDV sequences. This is difficult to do with conventional plasmid vectors, since only a small fraction (0.1% or less) of transfected cells will experience an integration event that allows stable colony formation. Therefore, cells were transfected with derivatives of the hygromycin resistance vector pCEP4 into which either WT HDV 1.1-mer cDNA or the isolated S-HDAg or L-HDAg open reading frames had been cloned. pCEP4 carries the Epstein-Barr virus EBNA-1 and oriP functions, allowing its efficient stable maintenance as a nuclear episome. Since CV1 cells are highly transfectable, this strategy ensures that 20 to 35% of the exposed cells will become stably transformed with the vectors. Transfected mass cultures were then followed over 6 weeks, with periodic assays for viral RNA (by Northern blotting) and HDAg expression (by flow cytometry).
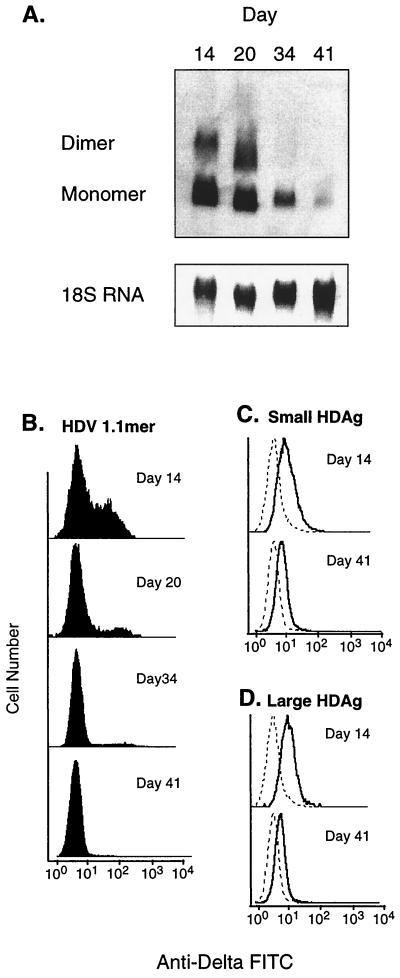
Northern blot analysis (Fig. 5A) revealed a dramatic loss of HDV replication over time in cells transfected with WT HDV cDNA. Similarly, FACS analysis of the same cells revealed a progressive decline in the proportion of delta antigen-positive cells (Fig. 5B). Even at the first time point examined, 14 days posttransfection, only approximately 35% of the hygromycin-resistant cells expressed HDAg; by day 41, less than 1% of the cells continued to express this antigen. In contrast, the profiles for cells bearing the S-HDAg and L-HDAg expression vectors were qualitatively different. They showed that the population as a whole expressed high levels of HDAg at day 14; by day 41 there was a modest downregulation of the mean level of HDAg expression, but this decrease appeared to be uniform across the whole population, rather than reflecting the overgrowth of cells lacking HDAg altogether (Fig. 5C). The observed loss of delta antigen expression and the concomitant decrease in viral RNA in WT HDV-transfected cells suggest that CV1 and HeLa cells do not tolerate viral replication and are in some fashion able to extinguish such activity.
FIG. 5.
Time course of HDV replication and delta antigen expression using pCEP expression vectors. Cells were continuously cultured for 6 weeks in 300 μg of hygromycin/ml, and samples were analyzed periodically for replication level and protein expression levels. (A) Northern blot for HDV replication in cells transfected with D731. (B to D) FACS analysis of delta antigen expression in cells transfected with D731, S-HDAg, and L-HDAg, respectively. Dashed lines indicate background staining of untransfected cells.
In contrast to reports by others (3, 9, 13) who have noted the establishment of stable cell lines or transgenic mice which continuously replicate HDV, we have been unable to observe persistent HDV replication following introduction of a 1.1-mer HDV construct. It seems unlikely that these differences result from variations in the strains of HDV used or differences in the cell lines, though this cannot be formally excluded. Rather, it seems likelier that the isolated clones previously reported may have acquired host mutations that render the cells able to tolerate ongoing replication or viral mutations that abrogate the growth-retarding effects of such replication. As evidenced from our experiments, the ability to continually replicate HDV is not a general property of the vast majority of cells in culture.
In sum, our observations are suggestive of a negative effect of HDV infection on cellular growth or viability. A clear, dose-related suppression of colony formation was observed in cells replicating HDV; this could not be attributed to the direct induction of apoptosis by HDV or to a detectable arrest of the cell cycle. The time-dependent loss of viral replication, coupled with a steady decline in the proportion of cells expressing delta antigen, suggests that cells supporting HDV replication suffer from a subtler form of growth disadvantage than do cells lacking HDV replication. Although we are unable to determine the precise mechanism underlying this growth impairment, multiple possibilities can be envisioned. For example, the robustness of the viral replication cycle could titrate essential cellular transcription factors away from their cognate promoters. In addition, the delta antigen has been reported to inhibit RNA polymerase (8), which could similarly compromise cellular growth. Other endogenous pathways, such as tRNA splicing and ligation, could also be disrupted by viral infection. While such a growth disadvantage seems unlikely to be able to generate hepatitis by itself, it would be expected to compromise the ability of an HDV-infected cell to participate in liver regeneration, a key response of the liver to injury. We propose this as one possible mechanism by which HDV may contribute to liver disease in vivo.
REFERENCES
- 1.Brazas R, Ganem D. A cellular homolog of hepatitis delta antigen: implications for viral replication and evolution. Science. 1996;274:90–94. doi: 10.1126/science.274.5284.90. [DOI] [PubMed] [Google Scholar]
- 2.Casey J L, Gerin J L. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593–7600. doi: 10.1128/jvi.69.12.7593-7600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P J, Kuo M Y, Chen M L, Tu S J, Chiu M N, Wu H L, Hsu H C, Chen D S. Continuous expression and replication of the hepatitis delta virus genome in Hep G2 hepatoblastoma cells transfected with cloned viral DNA. Proc Natl Acad Sci USA. 1990;87:5253–5257. doi: 10.1073/pnas.87.14.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Guilhot S, Huang S N, Xia Y P, La Monica N, Lai M M, Chisari F V. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol. 1994;68:1052–1058. doi: 10.1128/jvi.68.2.1052-1058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang S B, Park K J. Cell cycle arrest mediated by hepatitis delta antigen. FEBS Lett. 1999;449:41–44. doi: 10.1016/s0014-5793(99)00394-4. [DOI] [PubMed] [Google Scholar]
- 7.Kuo Y M, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo K, Sheu G T, Lai M M. Inhibition of cellular RNA polymerase II transcription by delta antigen of hepatitis delta virus. Virology. 1998;247:178–188. doi: 10.1006/viro.1998.9253. [DOI] [PubMed] [Google Scholar]
- 9.Macnaughton T B, Beard M R, Chao M, Gowans E J, Lai M M. Endogenous promoters can direct the transcription of hepatitis delta virus RNA from a recircularized cDNA template. Virology. 1993;196:629–636. doi: 10.1006/viro.1993.1519. [DOI] [PubMed] [Google Scholar]
- 10.Macnaughton T B, Gowans E J, Jilbert A R, Burrell C J. Hepatitis delta virus RNA, protein synthesis and associated cytotoxicity in a stably transfected cell line. Virology. 1990;177:692–698. doi: 10.1016/0042-6822(90)90535-y. [DOI] [PubMed] [Google Scholar]
- 11.Macnaughton T B, Gowans E J, Reinboth B, Jilbert A R, Burrell C J. Stable expression of hepatitis delta virus antigen in a eukaryotic cell line. J Gen Virol. 1990;71:1339–1345. doi: 10.1099/0022-1317-71-6-1339. [DOI] [PubMed] [Google Scholar]
- 12.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polo J M, Jeng K-S, Lim B, Govindarajan S, Hofman F, Sangiorgi F, Lai M M C. Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol. 1995;69:4880–4887. doi: 10.1128/jvi.69.8.4880-4887.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]