Abstract
Introduction
Renovascular hypertension (RVH) remains underdiagnosed despite its significant cardiovascular and renal morbidity.
Aim
This survey investigated screening and management practices for RVH among hypertensive patients in Italian hypertension centres in a real-life setting. Secondary, we analysed the current spread of renal denervation (RDN) and the criteria used for its eligibility.
Methods
A 12 item-questionnaire was sent to hypertension centres belonging to the European Society of Hypertension and to the Italian Society of Hypertension (SIIA) in Italy. Data concerning the screening and management of RVH and of RDN were analysed according to the type of centre (excellence vs non-excellence centres), geographical area and medical specialty.
Results
Eighty-two centres participated to the survey. The number of patients diagnosed in each centre with RVH and fibromuscular dysplasia during the last five years was 3 [1;6] and 1 [0;2], respectively. Despite higher rates of RVH diagnosis in excellence centres (p = 0.017), overall numbers remained unacceptably low, when compared to expected prevalence estimates. Screening rates were inadequate, particularly among young hypertensive patients, with only 28% of the centres screening for RVH in such population. Renal duplex ultrasound was underused, with computed tomographic angiography or magnetic resonance angiography reserved for confirming a RVH diagnosis (76.8%) rather than for screening (1.9–32.7%, according to patients’ characteristics). Scepticism and logistical challenges limited RDN widespread adoption.
Conclusions
These findings underscore the need for improving RVH screening strategies and for a wider use of related diagnostic tools. Enhanced awareness and adherence to guidelines are crucial to identifying renovascular hypertension and mitigating associated cardiovascular and renal risks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40292-024-00668-8.
Keywords: Atherosclerotic renovascular disease, Fibromuscular dysplasia, Hypertension, Renal denervation, Survey
Introduction
Renovascular hypertension (RVH) is a secondary form of hypertension resulting from a reduced renal blood flow, typically due to a renal artery stenosis (RAS) leading to un upregulation of the renin-angiotensin-aldosterone system. In adults, RVH is caused by atherosclerotic renovascular disease (ARVD) in most of the patients [1], despite differential diagnosis should consider fibromuscular dysplasia (FMD), a non-inflammatory non-atherosclerotic idiopathic and segmental disease leading to stenosis of medium sized arteries due to intima hyperplasia, especially in specific subsets of patients (i.e. young women with hypertension) [2], and other more rare causes [3]. ARVD is the second most common cause of secondary hypertension, with a prevalence ranging from 1 to 14-24% according to hypertension severity [4, 5] and the coexistence of other form of arteriopathies, such as atherosclerotic peripheral or coronary artery disease [6–8]. FMD has been considered a rare disease, often asymptomatic and diagnosed incidentally, with a prevalence of < 1% [9], which may be responsible for severe and life-threatening vascular complications or severe and refractory form of hypertension, especially among the youngest [10, 11]. However, recent study suggested that its prevalence may be higher, reaching 3.5% among female potential renal donor candidates [12–14] and 5-10% in patients with hypertension [2].
ARVD is commonly diagnosed in patients presenting with hypertension, impaired renal function and, less frequently, with heart failure and has been repeatedly associated with left ventricular hypertrophy and slowly or rapidly progressing chronic kidney disease (CKD) leading to an increased risk for overall cardiovascular and renal morbidity and mortality due to congestive heart failure, end-stage CKD, atherosclerotic coronary disease or cerebrovascular events [15–17]. Similarly, hypertension is the most frequent presenting symptom of renal FMD [2], alongside with progressing CKD and/or hypertension-related pregnancy disorders, such as gestational hypertension or preeclampsia [18], interesting more frequently younger patients [2].
Several studies have underlined that an early diagnosis of RVH is crucial to optimize therapeutic strategy, to reduce overall vascular and renal risk, which frequently may remain high due to the comorbidities and the already superimposed organ damages, and to set-up a proper and patient-based follow-up. Nevertheless, RVH diagnosis, both in terms of ARVD and, especially, of FMD diagnosis, is still adversely affected by a large diagnostic delay, affecting effectiveness of treatment strategies, especially of endovascular ones. As such, the missed diagnosis or the diagnostic delays of secondary form of hypertension have been advocated among the main reasons for poor blood pressure control by the Lancet Commission [19].
Renal denervation (RDN), through targeted modulation of sympathetic nerve activity, offers an effective mechanism for blood pressure reduction and represents a valuable therapeutic option for patients with treatment-resistant hypertension (RHTN) and intolerance to multiple medications. However, despite the positive results of the recent trials and the indication in the current 2023 ESH guideline, RDN diffusion still appears to be limited [20, 21].
The aim of this study was to investigate the current rate of screening and management of ARVD and FMD among hypertensive patients evaluated at referral centres for hypertension in Italy, belonging to the European Society of Hypertension (ESH) and the Italian Society of Hypertension (SIIA). Finally, despite RDN is not recommended as a treatment for RVH, we aimed to implement this survey by investigating also the current spread of RDN and the criteria used for its eligibility among the same centres, since this procedure may often involve the same figures and specialists dealing with the diagnosis and treatment of RVH.
Methods
We sent the invitation to participate to this nationwide survey to the hypertension specialists belonging to the Hypertension centres certified by the SIIA in Italy, with a rate response of circa 56%. Participating centres were divided into excellence centres (if certified by the Societies as reference centre for care of hypertension) and non-excellence centres. All physicians were interviewed concerning the screening and management of RVH trough a designed self-reported questionnaire consisting of 12 items (see Table S1 in the supplementary file) which was sent via institutional communication channels of the Societies and with the help of the regional sections and the ARCA (Associazioni Regionali Cardiologi Ambulatoriali) Piemonte.
Data were descriptively analysed for the whole cohort and then after stratification according to geographical area (North versus Centre-South Italy), type of the centres (excellence versus non-excellence centres) and medical specialty (cardiology, internal medicine and others). All participating centres were included. Centres were excluded only if no response/filled questionnaire was provided within the fixed deadline.
Statistical analysis was performed by IBM SPSS Statistics 26 (IBM Corp, Armonk, NY) and GraphPad Prism 9.0 (GraphPad, La Jolla, CA). Data are presented as median and interquartile range or frequencies as appropriated. Categorical variables were compared using the Chi-square or Fisher’s test. Scalar variables were compared using the Kruskal-Wallis or Mann-Whitney tests. A P < 0.05 was considered to be statistically significant.
Results
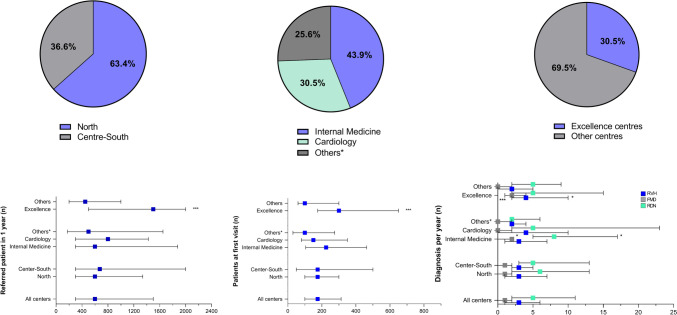
A total of 82 centers in 14 Italian regions were involved, of which 63% located in the north of Italy and 37% in the center-south of Italy, as already shown in the study of Di Dalmazi G et al. [22]. One third of the centers were labelled as excellence centers and concerning on the medical specialty of the physicians referring to each hypertension center, internal medicine was the most frequent one (44%), followed by cardiology (31%), while other medical specialties, such as endocrinology and nephrology, represented the remaining 25% (Fig. 1). The resulting geographical distribution of the involved centres largely reflects the geographical distribution of all Hypertension Centres belonging to the SIIA and, especially, the geographical distribution of the Italian ESH Excellence Hypertension Centres.
Fig. 1.
Response to questions 2, 3 and 5 of the questionnaire. The pie charts report frequencies (%) while histograms report distribution according to geographical areas, prevalent medical specialty, and type of the centre. *P < 0.05; **P < 0.01; *P < 0.001
The total number of patients evaluated by all the centers during the year before the response was 78,790, of which 21,890 as first evaluations. The average number of patients referred in 1 year to each hypertension center was 600 [300;1500], of which 175 patients [100;313] as first evaluation and 10% (5–25%) with RHTN. The median number of patients diagnosed in each center with RVH and FMD during the last five years was 3 [1;6] and 1 [0;2], respectively, irrespectively of the geographical area (North vs Center-south: RVH P = 0.804 and FMD P = 0.812, respectively). When compared to the other groups, cardiologists were less likely to be referred with patients with RHTN (P = 0.048), and newly diagnosed FMD patients during the last five years were more likely to be recorded among internal medicine specialists (P = 0.013), rather than among cardiologists or other specialists. When compared to non-excellence centers, excellence centers had a higher average of patients per year (P < 0.001) and reported a higher number of patients newly diagnosed with RVH (P = 0.017) and FMD (P < 0.001) during the last five years.
Concerning on RDN, the median number of patients undergoing RDN for each center was 5 [2;11] without significant differences when considering the geographical area or the type of the center, but with internal medicine specialists reporting a higher number of patients in the last five years compared to other groups (P = 0.021) (Table 1).
Table 1.
Sub-analysis on geographical areas, prevalent specialty, and excellence centres concerning numbers of patients for each centre
| Geographical areas | North (n = 52) | Centre-South (n = 30) | P-value |
|---|---|---|---|
| Average number of referred patient in 1 year (n) | 600 [300; 1338] | 675 [300; 2000] | 0.585 |
| Patients evaluated as first visit (n) | 175 [100; 300] | 175 [50; 500] | 0.828 |
| Patients with resistant hypertension (%) | 10.0 [5.0; 28.8] | 12.5 [5.0; 20.0] | 0.919 |
| Patients with RVH diagnosed per year in the last 5 years (n) | 3 [1; 7] | 3 [2; 5] | 0.804 |
| Patients with FMD diagnosed in the last 5 years (n) | 1 [0; 2] | 1 [0; 2] | 0.812 |
| Patients undergoing RDN followed per center (n) | 6 [2; 13] | 5 [3; 13] | 0.967 |
| Prevalent specialty | Internal medicine (n = 36) | Cardiology (n = 25) | Others (n = 21) | P-value |
|---|---|---|---|---|
| Average number of referred patient in 1 year (n) | 600 [300; 1876] | 800 [300; 1425] | 500 [175; 1650] | 0.731 |
| Patients evaluated as first visit (n) | 225 [105; 463] | 150 [80; 350] | 100 [30; 275] | 0.064 |
| Patients with resistant hypertension (%) | 12.5 [6.3; 30.0] | 10.0 [5.0; 12.5] | 15.0 [10.0; 45.0] | 0.048 |
| Patients with RVH diagnosed per year in the last 5 years (n) | 3 [1; 7] | 4 [0; 10] | 2 [2; 4] | 0.760 |
| Patients with FMD diagnosed in the last 5 years (n) | 2 [0; 2] | 0 [0; 2] | 0 [0; 2] | 0.013 |
| Patients undergoing RDN followed per center (n) | 8 [5; 17] | 5 [2; 23] | 2 [2; 6] | 0.021 |
| Excellence centres | Yes (n = 25) | No (n = 57) | P-value |
|---|---|---|---|
| Average number of referred patient in 1 year (n) | 1500 [500; 2000] | 450 [200; 1000] | < 0.001 |
| Patients evaluated as first visit (n) | 300 [175; 650] | 100 [60; 300] | < 0.001 |
| Patients with resistant hypertension (%) | 15.0 [6.5; 30.0] | 10.0 [5.0; 22.5] | 0.895 |
| Patients with diagnosed per year in the last 5 years (n) | 4 [2; 10] | 2 [0; 5] | 0.017 |
| Patients with diagnosed in the last 5 years (n) | 2 [1; 4] | 0 [0; 2] | < 0.001 |
| Patients undergoing RDN followed per center (n) | 5 [2; 15] | 5 [2; 9] | 0.641 |
P-value in bold are statistically significant (P-value < 0.05)
RVH renovascular hypertension, FMD fibromuscular dysplasia, RDN renal denervation
Diagnosis and Management of RVH
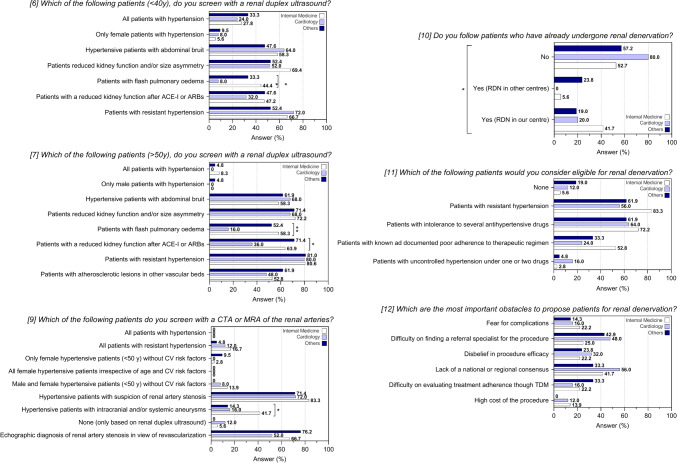
Table 2 and Fig. 2 report the results of the questions on the diagnosis and the management of RVH and RDN. Among patients younger than 40 years old, only 28% (23/82) of the centers would screen with a renal duplex ultrasound (US) in the case of hypertension with no other symptoms, whereas the screening percentage would increase in the presence of hypertension and another sign/symptom such as abdominal bruit (57.3%), CKD or renal size asymmetry (59.8%), reduction of kidney function after therapy with a renin-angiotensin system inhibitor (42.7%) (angiotensin I-converting enzyme inhibitors—ACE-Is, or angiotensin II receptor blockers—ARBs) and in the case of RHTN (64.6%). When considering patients older than 50 years old, screening with renal duplex US would be performed in the presence of RHTN in 80.5% of the centers, of hypertension and reduced kidney function after therapy with ACE-Is/ARBs in 57.3% of the centers, of CKD and/or renal size asymmetry in 70.7% of the centers, of abdominal bruit in 62.2% of the centers and in the presence of atherosclerotic lesions in other vascular beds in the 53.7% of the centers. Only 4.9% of the centers would screen with a renal duplex US routinely hypertensive subjects with no other symptoms when older than 50 years old.
Table 2.
Analysis on screening and management of renovascular hypertension (RVH), fibromuscular dysplasia (FMD) and renal denervation (RDN) for all centres
| All centers (n = 82) | |
|---|---|
| (6) Which of the following patients, younger than 40 years, do you screen with a renal duplex ultrasound? | |
| a. All patients with hypertension | 23 (28.0) |
| b. Only female patients with hypertension | 6 (7.3) |
| c. Hypertensive patients with abdominal bruit | 47 (57.3) |
| d. Patients with reduced kidney function and/or renal size asymmetry | 49 (59.8) |
| e. Patients with flash pulmonary oedema | 25 (30.5) |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 35 (42.7) |
| g. Patients with resistant hypertension | 53 (64.6) |
| (7) Which of the following patients, older than 50 years, do you screen with a renal duplex ultrasound? | |
| a. All patients with hypertension | 4 (4.9) |
| b. Only male patients with hypertension | 1 (1.2) |
| c. Hypertensive patients with abdominal bruit | 51 (62.2) |
| d. Patients with reduced kidney function and/or renal size asymmetry | 58 (70.7) |
| e. Patients with flash pulmonary oedema | 36 (43.9) |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 47 (57.3) |
| g. Patients with resistant hypertension | 66 (80.5) |
| h. Patients with atherosclerotic lesions in other vascular beds (carotid, coronary …) | 44 (53.7) |
| (9) Which of the following patients do you screen with a CTA or MRA of the renal arteries? | |
| a. All patients with hypertension | 0 (0.0) |
| b. All patients with resistant hypertension | 10 (12.2) |
| c. Only female hypertensive patients (< 50 years) without cardiovascular risk factors | 3 (3.7) |
| d. All female hypertensive patients irrespective of age and of cardiovascular risk factors | 0 (0.0) |
| e. Male and female hypertensive patients (< 50 years) without cardiovascular risk factors | 7 (8.5) |
| f. Hypertensive patients with suspicion of renal artery stenosis | 63 (76.8) |
| g. Hypertensive patients with intracranial and/or systemic aneurysms | 22 (26.8) |
| h. None (only based on renal duplex ultrasound) | 5 (6.1) |
| i. Echographic diagnosis of renal artery stenosis in view of endovascular revascularization | 53 (64.6) |
| (10) Do you follow patients who have already undergone renal denervation (RDN)? | |
| a. No | 51 (62.2) |
| b. Yes (patients who underwent renal denervation in other centres) | 7 (8.5) |
| c. Yes (patients who underwent renal denervation in our centre) | 24 (29.3) |
| (11) Which of the following patients would you consider eligible for renal denervation? | |
| a. None | 9 (11.0) |
| b. Patients with resistant hypertension | 57 (69.5) |
| c. Patients with intolerance to several antihypertensive drugs | 55 (67.1) |
| d. Patients with known ad documented poor adherence to therapeutic regimen | 32 (39.0) |
| e. Patients with uncontrolled hypertension under one or two drugs | 6 (7.3) |
| (12) Which are the most important obstacles in your centre in order to propose patients for renal denervation? | |
| a. Fear for complications | 15 (18.3) |
| b. Difficulty on finding a referral specialist for the procedure | 30 (36.6) |
| c. Disbelief in procedure efficacy | 21 (25.6) |
| d. Lack of a national or regional consensus which standardize algorithm of choice and treatment | 36 (43.9) |
| e. Difficulty on evaluating treatment adherence though therapeutic drug monitoring | 19 (23.2) |
| f. High cost of the procedure | 8 (9.8) |
ACE-Is angiotensin I converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CTA computed tomography angiography, MRA magnetic resonance angiography
Fig. 2.
Response to questions 6-to-12 of the questionnaire after stratification for medical specialty. Data are reported as frequencies. *P < 0.05; **P < 0.01. ACE-Is angiotensin I converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CTA computed tomography angiography, MRA magnetic resonance angiography
Computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) of the renal arteries would be performed predominantly in case of patients with hypertension and suspicion of RAS in the 76.8% of the centers or in patients with a diagnosis of RAS based on renal duplex US in view of endovascular revascularization in 64.6% of the centers. Interestingly, only 26.8% and 8.5% of the centers would screen with a CTA or a MRA of the renal arteries respectively patients with RHTN or mild to moderate form of hypertension without CV risk factors, irrespective of the gender (see Figure S1 in the supplementary file). The analysis by geographical area and type of centers did not highlight significant differences in the management and screening of patients with RVH, with the only exception of excellence centers being more likely to screen patients with RHTN for RVH with a CTA or MRA of renal arteries rather than non-excellence centers (28% vs 5.3% P=0.007) (see Table S2 and Figure S2 in the supplementary file and Fig. 3). Conversely, the analysis according to the medical specialty revealed a lower proportion of screening for RVH among cardiologists than other specialists for patients with flash pulmonary edema, irrespective of the age (< 40 years old: P=0.006; > 50 years old: P = 0.002), and for patient with a reduction of kidney function after ACE-Is/ARBs when older than 50 years old (P = 0.030). Furthermore, specialists in internal medicine would screen more frequently than all other specialists for RVH with a CTA or MRA in case of patients with hypertension and intracranial and/or systemic aneurysms (P = 0.027) (Table 3).
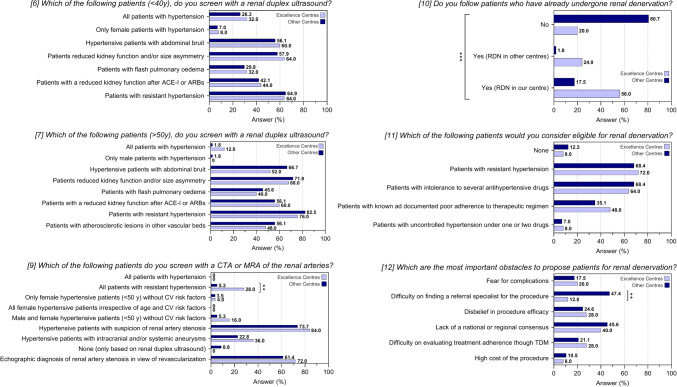
Fig. 3.
Response to questions 6-to-12 of the questionnaire after stratification for type of centre. Data are reported as frequencies. *P < 0.05. ACE-Is angiotensin I converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CTA computed tomography angiography, MRA magnetic resonance angiography
Table 3.
Sub-analysis on medical specialty concerning screening and management of renovascular hypertension (RVH), fibromuscular dysplasia (FMD) and renal denervation (RDN)
| Prevalent specialty | Internal medicine (n = 36) | Cardiology (n = 25) | Others (n = 21) | P-value |
|---|---|---|---|---|
| (6) Which of the following patients, younger than 40 years, do you screen with a renal duplex ultrasound? | ||||
| a. All patients with hypertension | 10 (27.8) | 6 (24.0) | 7 (33.3) | 0.783 |
| b. Only female patients with hypertension | 2 (5.6) | 2 (8.0) | 2 (9.5) | 0.872 |
| c. Hypertensive patients with abdominal bruit | 21 (58.3) | 16 (64.0) | 10 (47.6) | 0.527 |
| d. Patients with reduced kidney function and/or renal size asymmetry | 25 (69.4) | 13 (52.0) | 11 (52.4) | 0.285 |
| e. Patients with flash pulmonary oedema | 16 (44.4) | 2 (8.0) | 7 (33.3) | 0.006 |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 17 (47.2) | 8 (32.0) | 10 (47.6) | 0.432 |
| g. Patients with resistant hypertension | 24 (66.7) | 18 (72.0) | 11 (52.4) | 0.361 |
| (7) Which of the following patients, older than 50 years, do you screen with a renal duplex ultrasound? | ||||
| a. All patients with hypertension | 3 (8.3) | 0 (0.0) | 1 (4.8) | 0.363 |
| b. Only male patients with hypertension | 0 (0.0) | 0 (0.0) | 1 (4.8) | 0.256 |
| c. Hypertensive patients with abdominal bruit | 21 (58.3) | 17 (68.0) | 13 (61.9) | 0.745 |
| d. Patients with reduced kidney function and/or renal size asymmetry | 26 (72.2) | 17 (68.0) | 15 (71.4) | 0.937 |
| e. Patients with flash pulmonary oedema | 21 (58.3) | 4 (16.0) | 11 (52.4) | 0.002 |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 23 (63.9) | 9 (36.0) | 15 (71.4) | 0.030 |
| g. Patients with resistant hypertension | 29 (80.6) | 20 (80.0) | 17 (81.0) | 1.000 |
| h. Patients with atherosclerotic lesions in other vascular beds (carotid, coronary …) | 19 (52.8) | 12 (48.0) | 13 (61.9) | 0.634 |
| (9) Which of the following patients do you screen with a CTA or MRA of the renal arteries? | ||||
| a. All patients with hypertension | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| b. All patients with resistant hypertension | 6 (16.7) | 3 (12.0) | 1 (4.8) | 0.431 |
| c. Only female hypertensive patients (< 50 years) without cardiovascular risk factors | 1 (2.8) | 0 (0.0) | 2 (9.5) | 0.337 |
| d. All female hypertensive patients irrespective of age and of cardiovascular risk factors | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| e. Male and female hypertensive patients (< 50 years) without cardiovascular risk factors | 5 (13.9) | 2 (8.0) | 0 (0.0) | 0.272 |
| f. Hypertensive patients with suspicion of renal artery stenosis | 30 (83.3) | 18 (72.0) | 15 (71.4) | 0.465 |
| g. Hypertensive patients with intracranial and/or systemic aneurysms | 15 (41.7) | 4 (16.0) | 3 (14.3) | 0.027 |
| h. None (only based on renal duplex ultrasound) | 2 (5.6) | 3 (12.0) | 0 (0.0) | 0.262 |
| i. Echographic diagnosis of renal artery stenosis in view of endovascular revascularization | 24 (66.7) | 13 (52.0) | 16 (76.2) | 0.219 |
| (10) Do you follow patients who have already undergone renal denervation (RDN)? | ||||
| a. No | 19 (52.7) | 20 (80.0) | 12 (57.2) | 0.017 |
| b. Yes (patients who underwent renal denervation in other centres) | 2 (5.6) | 0 (0.0) | 5 (23.8) | |
| c. Yes (patients who underwent renal denervation in our centre) | 15 (41.7) | 5 (20.0) | 4 (19.0) | |
| (11) Which of the following patients would you consider eligible for renal denervation? | ||||
| a. None | 2 (5.6) | 3 (12.0) | 4 (19.0) | 0.228 |
| b. Patients with resistant hypertension | 30 (83.3) | 14 (56.0) | 13 (61.9) | 0.051 |
| c. Patients with intolerance to several antihypertensive drugs | 26 (72.2) | 16 (64.0) | 13 (61.9) | 0.674 |
| d. Patients with known ad documented poor adherence to therapeutic regimen | 19 (52.8) | 6 (24.0) | 7 (33.3) | 0.063 |
| e. Patients with uncontrolled hypertension under one or two drugs | 1 (2.8) | 4 (16.0) | 1 (4.8) | 0.193 |
| (12) Which are the most important obstacles in your centre in order to propose patients for renal denervation? | ||||
| a. Fear for complications | 8 (22.2) | 4 (16.0) | 3 (14.3) | 0.815 |
| b. Difficulty on finding a referral specialist for the procedure | 9 (25.0) | 12 (48.0) | 9 (42.9) | 0.146 |
| c. Disbelief in procedure efficacy | 8 (22.2) | 8 (32.0) | 5 (23.8) | 0.673 |
| d. Lack of a national or regional consensus which standardize algorithm of choice and treatment | 15 (41.7) | 14 (56.0) | 7 (33.3) | 0.285 |
| e. Difficulty on evaluating treatment adherence though therapeutic drug monitoring | 8 (22.2) | 4 (16.0) | 7 (33.3) | 0.402 |
| f. High cost of the procedure | 5 (13.9) | 3 (12.0) | 0 (0.0) | 0.218 |
P-value in bold are statistically significant (P-value < 0.05)
ACE-Is angiotensin I converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CTA computed tomography angiography, MRA magnetic resonance angiography
Diffusion of RDN
Concerning on RDN, most of the centers involved (62.2%) declared not to follow patients who had already undergone RDN. Reasons for reduced eligibility to RDN, without significant differences among the centers, were lack of standardized national or regional consensus protocols for treatment (43.9%), lack of referral specialists for the procedure (36.6%), difficulty on evaluating treatment adherence before the procedure (23.2%), fear for complications (18.3%) and costs of the procedures (9.8%). Finally, a high proportion of the interviewed centers remains skeptical on the effectiveness of the procedure (25.6%). Patients were considered eligible for RDN more frequently in case of RHTN (69.5%) or difficult-to-control hypertension due to multiple intolerance to antihypertensive drugs (67.1%), rather than in case of poor adherence to therapeutic regimen (39%) or of uncontrolled BP values under one or two antihypertensive drugs (7.3%). While the analysis by geographical area and type of specialty did not highlight significant differences in terms of RDN spread and management, analysis by type of center showed that excellence centers were more likely to follow patients who underwent RDN (P < 0.001), irrespective of the centers where the procedure was initially performed, probably mostly because of the difficulty to find a referral specialist for the procedure experienced by the non-excellence centers (P = 0.002) (Table 4).
Table 4.
Sub-analysis on type of hypertension centre concerning screening and management of renovascular hypertension (RVH), fibromuscular dysplasia (FMD) and renal denervation (RDN)
| Excellence centres | Yes (n = 25) | No (n = 57) | P-value |
|---|---|---|---|
| (6) Which of the following patients, younger than 40 years, do you screen with a renal duplex ultrasound? | |||
| a. All patients with hypertension | 8 (32.0) | 15 (26.3) | 0.597 |
| b. Only female patients with hypertension | 2 (8.0) | 4 (7.0) | 1.000 |
| c. Hypertensive patients with abdominal bruit | 15 (60.0) | 32 (56.1) | 0.740 |
| d. Patients with reduced kidney function and/or renal size asymmetry | 16 (64.0) | 33 (57.9) | 0.603 |
| e. Patients with flash pulmonary oedema | 8 (32.0) | 17 (29.8) | 0.841 |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 11 (44.0) | 24 (42.1) | 0.862 |
| g. Patients with resistant hypertension | 16 (64.0) | 37 (64.9) | 0.920 |
| (7) Which of the following patients, older than 50 years, do you screen with a renal duplex ultrasound? | |||
| a. All patients with hypertension | 3 (12.0) | 1 (1.8) | 0.082 |
| b. Only male patients with hypertension | 0 (0.0) | 1 (1.8) | 1.000 |
| c. Hypertensive patients with abdominal bruit | 13 (52.0) | 38 (66.7) | 0.207 |
| d. Patients with reduced kidney function and/or renal size asymmetry | 17 (68.0) | 41 (71.9) | 0.718 |
| e. Patients with flash pulmonary oedema | 10 (40.0) | 26 (45.6) | 0.639 |
| f. Patients with a reduction in kidney function after therapy with ACE-I or ARBs | 15 (60.0) | 32 (56.1) | 0.740 |
| g. Patients with resistant hypertension | 19 (76.0) | 47 (82.5) | 0.551 |
| h. Patients with atherosclerotic lesions in other vascular beds (carotid, coronary …) | 12 (48.0) | 32 (56.1) | 0.498 |
| (9) Which of the following patients do you screen with a CTA or MRA of the renal arteries? | |||
| a. All patients with hypertension | 0 (0.0) | 0 (0.0) | 1.000 |
| b. All patients with resistant hypertension | 7 (28.0) | 3 (5.3) | 0.007 |
| c. Only female hypertensive patients (< 50 years) without cardiovascular risk factors | 1 (4.0) | 2 (3.5) | 1.000 |
| d. All female hypertensive patients irrespective of age and of cardiovascular risk factors | 0 (0.0) | 0 (0.0) | 1.000 |
| e. Male and female hypertensive patients (< 50 years) without cardiovascular risk factors | 4 (16.0) | 3 (5.3) | 0.192 |
| f. Hypertensive patients with suspicion of renal artery stenosis | 21 (84.0) | 42 (73.7) | 0.308 |
| g. Hypertensive patients with intracranial and/or systemic aneurysms | 9 (36.0) | 13 (22.8) | 0.214 |
| h. None (only based on renal duplex ultrasound) | 0 (0.0) | 5 (8.8) | 0.182 |
| i. Echographic diagnosis of renal artery stenosis in view of endovascular revascularization | 18 (72.0) | 35 (61.4) | 0.357 |
| (10) Do you follow patients who have already undergone renal denervation (RDN)? | |||
| a. No | 5 (20.0) | 46 (80.7) | < 0.001 |
| b. Yes (patients who underwent renal denervation in other centres) | 6 (24.0) | 1 (1.8) | |
| c. Yes (patients who underwent renal denervation in our centre) | 14 (56.0) | 10 (17.5) | |
| (11) Which of the following patients would you consider eligible for renal denervation? | |||
| a. None | 2 (8.0) | 7 (12.3) | 0.715 |
| b. Patients with resistant hypertension | 18 (72.0) | 39 (68.4) | 0.740 |
| c. Patients with intolerance to several antihypertensive drugs | 16 (64.0) | 39 (68.4) | 0.699 |
| d. Patients with known ad documented poor adherence to therapeutic regimen | 12 (48.0) | 20 (35.1) | 0.269 |
| e. Patients with uncontrolled hypertension under one or two drugs | 2 (8.0) | 4 (7.0) | 1.000 |
| (12) Which are the most important obstacles in your centre in order to propose patients for renal denervation? | |||
| a. Fear for complications | 5 (20.0) | 10 (17.5) | 1.000 |
| b. Difficulty on finding a referral specialist for the procedure | 3 (12.0) | 27 (47.4) | 0.002 |
| c. Disbelief in procedure efficacy | 7 (28.0) | 14 (24.6) | 0.740 |
| d. Lack of a national or regional consensus which standardize algorithm of choice and treatment | 10 (40.0) | 26 (45.6) | 0.639 |
| e. Difficulty on evaluating treatment adherence though therapeutic drug monitoring | 7 (28.0) | 12 (21.1) | 0.493 |
| f. High cost of the procedure | 2 (8.0) | 6 (10.5) | 1.000 |
P-value in bold are statistically significant (P-value < 0.05)
ACE-Is angiotensin I converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CTA computed tomography angiography, MRA magnetic resonance angiography
Discussion
This study provides the results of a large-scale survey on the screening and management of renovascular hypertension and RDN among hypertension centres affiliated with SIIA and ESH and distributed in all the Italian territory. The aim was to provide an insight on the current common practice on RVH management in a real-life setting.
The survey highlighted that screening of RVH remains underperformed irrespective of the medical specialty, and the type or the geographical location of the hypertension centres. Despite data collection was not planned for calculating the prevalence of ARVD and FMD, we may estimate from this report that the prevalence of new diagnosis is 0.3% (average of 3 patients in the last five years/175 new patients/year) for ARVD and 0.1% (average of 1 patient in the last five years/175 new patients/year) for FMD, in patients at first evaluations and even lower in the whole cohort of patients referred in 1 year (average of 600 patients/year). These numbers are lower than expected, since the prevalence of ARVD and FMD among patients with hypertension has been reported to range from 1 to 24% and from 5 to 10% for ARVD and FMD, respectively, according to hypertension severity [2, 4, 5]. Furthermore, despite excellence centres reported a higher number of new diagnoses of ARVD and FMD when compared with non-excellence centres, the estimated numbers remain too small compared with the expected prevalences. The present data are of concern considering that 10% of the patients (5–25%) were referred for RHTN, a condition in which the prevalence of secondary form of hypertension is particularly high [5, 15, 23].
On average, less than one third of the centres would screen routinely for RVH patients with hypertension younger than 40 years using a renal duplex US. Despite the rate of screening reported is higher in case of hypertension associated with specific symptoms suspicious for RVH the proportion of screening for RVH among young patients with hypertension remains unacceptably low. In fact, roughly 40–50% of the centres do not to screen for RVH with a renal duplex US even in case of abdominal bruit, CKD or asymmetry in renal diameters, reduction in kidney function after therapy with a renin-angiotensin system inhibitor or in case of RHTN, independently of the type and geographic distribution of the centres and of the medical specialty. This low screening rate of RVH among young patients with hypertension is worrying since secondary forms of hypertension are more frequent in this group of patients, where hypertension work-up should be deepened also to reduce development of hypertension-mediated organ damages (HMODs).
In older patients, the screening rate for RVH in the presence of hypertension alone, independently of the gender, is even lower (4.9%) and increases in case of RHTN or when hypertension coexists with a reduced kidney function. Importantly, roughly 40–55% of the hypertension centres would not screen for RVH in case of hypertension and a reduction in kidney function after a therapy with a renin-angiotensin system inhibitor or in case of recurrent flash pulmonary oedema, with the highest proportion of underscreening among cardiologists.
This is in contrast with the current guideline that recommend screening for secondary hypertension, including RVH, all young patients with moderate-to-severe hypertension, patients with severe or malignant hypertension, true RHTN, patients with acute worsening of blood pressure control when previously normotensive or with controlled hypertension, and patients with a severe or disproportioned HMOD in respect of the duration and severity of hypertension [20]. Thus, ARVD should be suspected in old patients (> 65 years old) with RHTN, flash pulmonary oedema, rapidly decline in kidney function, especially under the use of ACE-Is or ARBs, or generalized atherosclerosis. Screening should be performed with a renal artery duplex US and deepened with a CTA or MRA. Similarly, renal FMD should be considered in case of young or middle-aged patients with hypertension, especially women, with the clinical characteristics described above and in case of history of migraine and pulsatile tinnitus or other vascular abnormalities, such as arterial aneurysms or spontaneous dissection [2, 20].
If RVH screening with a renal duplex US resulted scarce, the use of CTA or MRA of the renal arteries for screening was even lower. Rather than improving RVH diagnosis in case of high suspicion of renovascular disease without clear ultrasonographic findings, CTA and MRA of the renal arteries are used for a confirmatory diagnosis or in view of an endovascular treatment. These results hold true irrespective of the geographic distribution of the centres and of the medical specialty. In contrary, excellence centres were more likely to screen for RVH with CTA or MRA of the renal arteries compared to non-excellence centres, probably because of a wider and easiest access to these diagnostic tools.
Due to its low cost and widespread availability, implementing the use of renal duplex US together with non-contrast renal US for HMOD assessment and RVH screening in all patients with hypertension, would be already a good starting point, as suggested by current recommendation [20]. Furthermore, since renal duplex US has several limitations [24], the use of CTA and MRA of the renal arteries should be also considered to deeply characterize the renovascular system in patients with high suspicion of RVH but negative ultrasonographic findings, as it may occur in case of FMD, since the lack of validated FMD-related duplex US parameter and the difficulty of visualizing the middle and distal segments of the renal arteries [24].
These recommendations are valid for all specialists, especially for cardiologists, who were less likely to screen for RVH even in case of cardiac complications such as recurrent flash pulmonary oedema [25].
According to the results of this survey, the spread of RDN is still limited. More than half of the centres did not to follow any patient who had previously undergone RDN and reserved the procedure mostly for patients with RHTN or difficult-to-control hypertension, as a sort of last-resort after multiple therapeutic attempts.
The exiguity of the numbers has several reasons. Some centres have still concerns about its efficacy and procedure-related complications, while others complain about the absence of standardized protocols and of referral specialists, highlighting that RDN is not easily accessible for everyone.
Despite these results may partly be an echo of previous negative findings [26, 27], recent clinical trials showed favourable effects of RDN on reducing blood pressure both in the absence or in presence of antihypertensive medications [28, 29]. For these reasons in the 2023 ESH guidelines [20] the efficacy and safety of RDN has been fully recognized. The new guideline also gave a special emphasis on the phenotypes of patients who are candidate for the procedure, expanding this procedure to patients with uncontrolled hypertension and with multiple drug intolerance syndrome [30].
The major limitation of this study is that the results were based on a self-reported not validated survey questionnaire. However, this study involved a large number of centres with a balanced representation of excellence and non-excellence centres.
Conclusions
The results of this survey revealed that the current screening of RVH, both for ARVD and FMD, among patients with hypertension is still unsatisfactory. As such, these results may partially justify the delay in the diagnosis of RVH, the high CV and overall risk of these patients and, secondary, some of the negative findings of the big trials evaluating the efficacy of targeted therapies in such populations.
The results of this survey should lay the basis to increase the awareness on these conditions among all specialists. Furthermore, these results should reinforce the current recommendation in accordance with screening for RVH should become of routine practice to identify secondary cause of hypertension, reduce the percentage of pseudo-resistant hypertension, and minimize HMODs, whose onset may reduce the effectiveness of targeted therapies.
Similarly, the negative results of this survey on the use of RDN may led to promote initiative aiming to increase awareness on his safety and efficacy and to broadcast it once and for all, in line with the current recommendation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the participants to this survey from the Italian Society of Hypertension (SIIA) and the cardiologists of the Associazione Regionale Cardiologi Ambulatoriali (ARCA) Piemonte
Author contributions
P.M., C.B., and M.L.M designed the study and directed the survey. J.G. and J.B. performed the statistic analysis. M.P. wrote the manuscript. L.P. contributed with figures and tables. G.D.D., A.F.G.C., C.M., E.C.M., G.I., C.B., M.L.M., F.R. and P.M. contributed clinically and critically revised the contents of the manuscript.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Data availability
Data are available on request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–42. 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 2.Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, Bruno RM, de Leeuw P, Fendrikova-Mahlay N, Froehlich J, Ganesh SK, Gray BH, Jamison C, Januszewicz A, Jeunemaitre X, Kadian-Dodov D, Kim ES, Kovacic JC, Mace P, Morganti A, Sharma A, Southerland AM, Touzé E, van der Niepen P, Wang J, Weinberg I, Wilson S, Olin JW, Plouin PF. First International consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2019;37:229–52. 10.1097/HJH.0000000000002019. (Vasc Med. 2019 Apr;24(2):164-189. 10.1177/1358863X18821816). [DOI] [PubMed] [Google Scholar]
- 3.Persu A, Canning C, Prejbisz A, Dobrowolski P, Amar L, Chrysochou C, Kądziela J, Litwin M, van Twist D, Van der Niepen P, Wuerzner G, de Leeuw P, Azizi M, Januszewicz M, Januszewicz A. Beyond atherosclerosis and fibromuscular dysplasia: rare causes of renovascular hypertension. Hypertension. 2021;78:898–911. 10.1161/HYPERTENSIONAHA.121.17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund G, Andersson O, Wilhelmsen L. Prevalence of primary and secondary hypertension: studies in a random population sample. BMJ. 1976;2:554–6. 10.1136/bmj.2.6035.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol. 2014;113(4):687–90. 10.1016/j.amjcard.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 6.Khosla S, Kunjummen B, Manda R, Khaleel R, Kular R, Gladson M, Razminia M, Guerrero M, Trivedi A, Vidyarthi V, Elbzour M, Ahmed A. Prevalence of renal artery stenosis requiring revascularization in patients initially referred for coronary angiography. Catheter Cardiovasc Interv. 2003;58(3):400–3. 10.1002/ccd.10387. [DOI] [PubMed] [Google Scholar]
- 7.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333–40. 10.1097/HJH.0b013e328329bbf4. [DOI] [PubMed] [Google Scholar]
- 8.de Silva R, Loh H, Rigby AS, Nikitin NP, Witte KK, Goode K, Bhandari S, Nicholson A, Clark AL, Cleland JG. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100(2):273–9. 10.1016/j.amjcard.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 9.Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007;2:28. 10.1186/1750-1172-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliot WJ. Renovascular hypertension: an update. J Clin Hypertens. 2008;10(7):522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon MJ. The diagnosis of renovascular disease. Pediatr Nephrol. 1997;11(3):366–72. [DOI] [PubMed] [Google Scholar]
- 12.Hendricks NJ, Matsumoto AH, Angle JF, Baheti A, Sabri SS, Park AW, Stone JR, Patrie JT, Dworkin L, Cooper CJ, Murphy TP, Cutlip DE. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates. Vasc Med. 2014;19(5):363–7. 10.1177/1358863X14544715. [DOI] [PubMed] [Google Scholar]
- 13.Cragg AH, Smith TP, Thompson BH, Maroney TP, Stanson AW, Shaw GT, Hunter DW, Cochran ST. Incidental Fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology. 1989;172(1):145–7. 10.1148/radiology.172.1.2662248. [DOI] [PubMed] [Google Scholar]
- 14.Andreoni KA, Weeks SM, Gerber DA, Fair JH, Mauro MA, McCoy L, Scott L, Johnson MW. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation. 2002;73(7):1112–6. 10.1097/00007890-200204150-00018. [DOI] [PubMed] [Google Scholar]
- 15.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68(1):293–301. 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie J, Green D, Alderson HV, Chiu D, Sinha S, Kalra PA. Risks for mortality and renal replacement therapy in atherosclerotic renovascular disease compared with other causes of chronic kidney disease. Nephrology (Carlton). 2015;20(10):688–96. 10.1111/nep.12501. [DOI] [PubMed] [Google Scholar]
- 17.Pappaccogli M, Robberechts T, Lengelé JP, Van der Niepen P, Sarafidis P, Rabbia F, Persu A. Endovascular versus medical management of atherosclerotic renovascular disease: update and emerging concepts. Hypertension. 2023;80(6):1150–61. 10.1161/HYPERTENSIONAHA.122.17965. [DOI] [PubMed] [Google Scholar]
- 18.Pappaccogli M, Prejbisz A, Ciurică S, Bruno RM, Aniszczuk-Hybiak A, Bracalente I, De Backer T, Debiève F, Delmotte P, Di Monaco S, Jarraya F, Gordin D, Kosiński P, Kroon AA, Maas AHEM, Marcon D, Minuz P, Montagud-Marrahi E, Pasquet A, Poch E, Rabbia F, Stergiou GS, Tikkanen I, Toubiana L, Vinck W, Warchoł-Celińska E, Van der Niepen P, de Leeuw P, Januszewicz A, Persu A, European/International Fibromuscular Dysplasia Registry and Initiative (FEIRI) and the Working Group “Hypertension and the Kidney” of the ESH. Pregnancy-related complications in patients with fibromuscular dysplasia: a report from the European/internation fibromuscolare dysplasia registry. Hypertension. 2020;76(2):545–553. 10.1161/HYPERTENSIONAHA.120.15349. [DOI] [PubMed]
- 19.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388(10060):2665–712. 10.1016/S0140-6736(16)31134-5. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi JM, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang ZY, Kjeldsen SE. 2023 ESH guidelines for the management ofd arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071. 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 21.Stabile E, Muiesan ML, Ribichini FL, Sangiorgi G, Taddei S, Versaci F, Villari B, Bacca A, Benedetto D, Fioretti V, Laurenzano E, Scapaticci M, Saia F, Tarantini G, Grassi G, Esposito G. Italian Society of Interventional Cardiology (GISE) and Italian Society of Arterial Hypertension (SIIA) Position Paper on the role of renal denervation in the management of the difficult-to-treat hypertension. Minerva Cardiol Angiol. 2024. 10.23736/S2724-5683.23.06433-5. [DOI] [PubMed] [Google Scholar]
- 22.Di Dalmazi G, Goi J, Burrello J, Tucci L, Cicero AFG, Mancusi C, Coletti Moia E, Iaccarino G, Borghi C, Muiesan ML, Ferri C, Mulatero P. Screening of hyperticolisolism among patients with hypertension: an Italian nationwide survey. J Endocrinol Investig. 2024. 10.1007/s40618-024-02387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Genomic and Precision Medicine, Council on Peripheral Vascular Disease, Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90. 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed]
- 24.Schaberle W, Leyerer L, Schierling W, Pfister K. Ultrasound diagnostics of renal artery stenosis: stenosis criteria. CEUS and recurrent in-stent stenosis. Gefasschirurgie. 2016;21:4–13. 10.1007/s00772-015-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanga V, Bertoli E, Crimì F, Barbiero G, Battistel M, Seccia TM, Rossi GP. Pickering syndrome: an overlooked renovascular cause of recurrent heart failure. J Am Heart Assoc. 2023;12(19): e030474. 10.1161/JAHA.123.030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. 10.1056/NEJMoa1402670.
- 27.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 28.Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D’Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR, SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–51. 10.1016/S0140-6736(20)30554-7. [DOI] [PubMed] [Google Scholar]
- 29.Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Dimitriadis K, Choi JW, East C, D’Souza R, Sharp ASP, Ewen S, Walton A, Hopper I, Brar S, McKenna P, Fahy M, Böhm M. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399(10333):1401–10. 10.1016/S0140-6736(22)00455-X. [DOI] [PubMed] [Google Scholar]
- 30.Palermiti A, Pappaccogli M, Rabbia F, D’Avolio A, Veglio F. Multiple drug intolerance (MDI) in antihypertensive patients: what is known and what is missing. J Hypertens. 2024. 10.1097/HJH.0000000000003737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.