Figure 9.
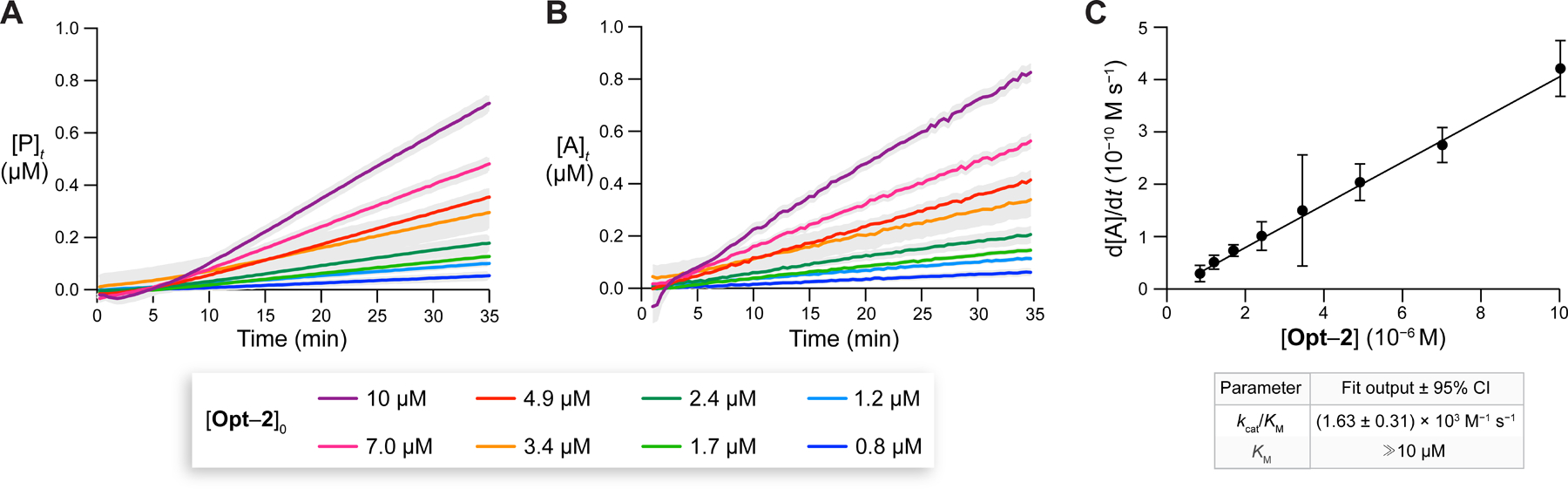
(A) Time-dependence of [P] from Opt–2 cleavage by PLE (25 nM) at 37 °C. (B) Corresponding time-dependence of . (C) Michaelis–Menten plot for the initial rates of formation as a function of [Opt–2]0. A linear fit to eq 5 enabled the determination of the values of (±95% CI) and a lower limit for . Assays were performed in 10 mM HEPES–NaOH buffer, pH 7.4, containing DMF (1.5% v/v) and Triton X-100 (0.8% w/v). Gray areas represent the SD; n = 2 independent replicates, n = 3 technical replicates.
