Abstract
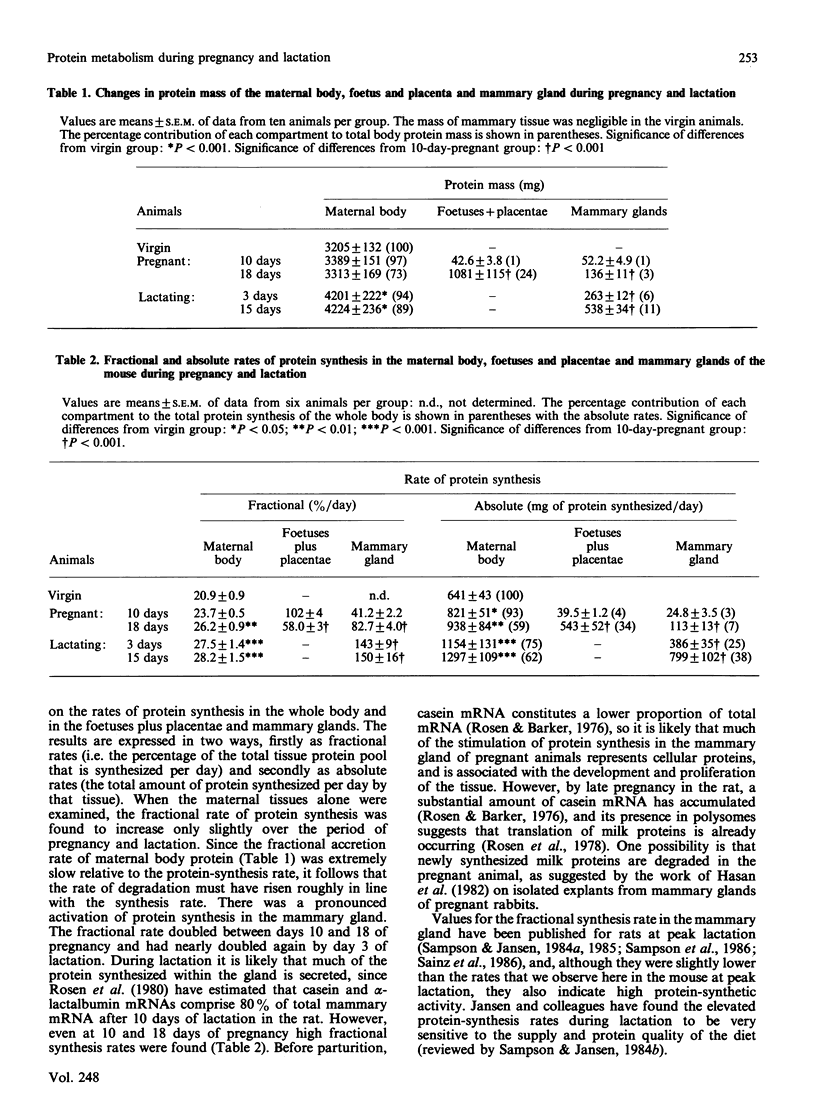
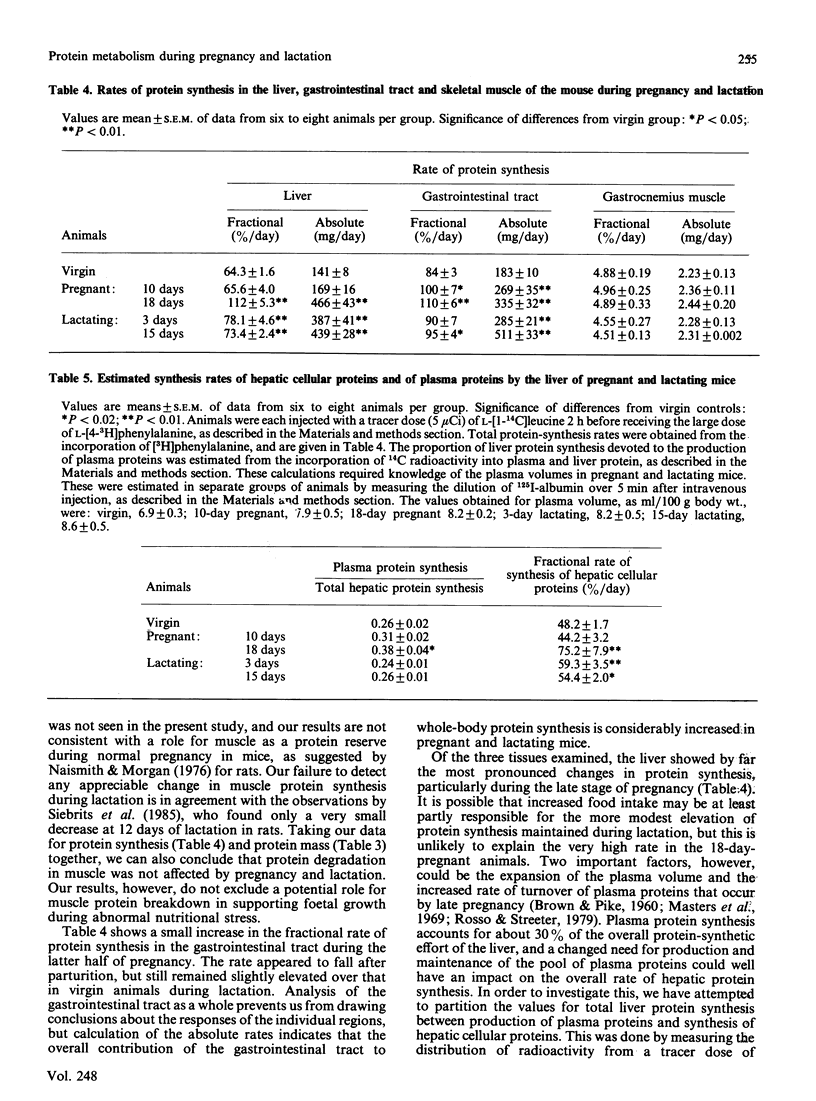
Protein synthesis was measured in vivo in the whole body and in a number of individual tissues in mice at various stages of pregnancy and lactation. The absolute rate of protein synthesis in the whole body increased from 640 mg/day in virgin mice to 1590 mg/day by day 18 of pregnancy, and to 2100 mg/day by day 15 of lactation. Large proportions of these increments were contributed by the rapidly growing foetuses and placentae in the pregnant animals and by protein synthesis in the mammary glands during lactation. In addition, a substantial stimulation of growth and protein synthesis was also observed in the liver and the gastrointestinal tract. Gastrocnemius muscle showed no changes in protein metabolism, indicating that in the well-fed mouse this tissue is not required to play a role as a protein reserve during pregnancy and lactation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEATON G. H., BEARE J., RYU M. H., McHENRY E. W. Protein metabolism in the pregnant rat. J Nutr. 1954 Oct 11;54(2):291–304. doi: 10.1093/jn/54.2.291. [DOI] [PubMed] [Google Scholar]
- BROWN M. L., PIKE R. L. Blood volume and serum protein in the deoxypyridoxine-fed rat during pregnancy. J Nutr. 1960 Jun;71:191–199. doi: 10.1093/jn/71.2.191. [DOI] [PubMed] [Google Scholar]
- Burdett K., Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem J. 1979 Nov 15;184(2):245–251. doi: 10.1042/bj1840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL R. M., FELL B. F. GASTRO-INTESTINAL HYPERTROPHY IN THE LACTATING RAT AND ITS RELATION TO FOOD INTAKE. J Physiol. 1964 May;171:90–97. doi: 10.1113/jphysiol.1964.sp007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. M., Fell B. F., Mackie W. S. Ornithine decarboxylase activity, nucleic acids and cell turnover in the livers of pregnant rats. J Physiol. 1974 Sep;241(3):699–713. doi: 10.1113/jphysiol.1974.sp010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatwin A. L., Linzell J. L., Setchell B. P. Cardiovascular changes during lactation in the rat. J Endocrinol. 1969 Jun;44(2):247–254. doi: 10.1677/joe.0.0440247. [DOI] [PubMed] [Google Scholar]
- Craft I. L. The influence of pregnancy and lactation on the morphology and absorptive capacity of the rat small intestine. Clin Sci. 1970 Mar;38(3):287–295. doi: 10.1042/cs0380287. [DOI] [PubMed] [Google Scholar]
- FELL B. F., SMITH K. A., CAMPBELL R. M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J Pathol Bacteriol. 1963 Jan;85:179–188. doi: 10.1002/path.1700850117. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J. Protein synthesis and energy expenditure in relation to feeding. Int J Vitam Nutr Res. 1986;56(2):197–200. [PubMed] [Google Scholar]
- Gitlin D., Koch C. On the mechanisms of maternofetal transfer of human albumin and gamma-G globulin in the mouse. J Clin Invest. 1968 May;47(5):1204–1209. doi: 10.1172/JCI105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F., Kelly F. J. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J. 1984 Jan 15;217(2):507–516. doi: 10.1042/bj2170507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F., Lewis S. E., Kelly F. J. Protein synthesis during the developmental growth of the small and large intestine of the rat. Biochem J. 1984 Jan 15;217(2):527–534. doi: 10.1042/bj2170527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. L. Some aspects of changing body composition of mice during successive pregnancies and lactations. J Endocrinol. 1973 Jan;56(1):37–46. doi: 10.1677/joe.0.0560037. [DOI] [PubMed] [Google Scholar]
- Kanto U., Clawson A. J. Effect of energy intake during pregnancy and lactation on body composition in rats. J Nutr. 1980 Sep;110(9):1829–1839. doi: 10.1093/jn/110.9.1829. [DOI] [PubMed] [Google Scholar]
- Kernoff L. M., Pimstone B. L., Solomon J., Brock J. F. The effect of hypophysectomy and growth hormone replacement on albumin synthesis and catabolism in the rat. Biochem J. 1971 Sep;124(3):529–535. doi: 10.1042/bj1240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. E., Kelly F. J., Goldspink D. F. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J. 1984 Jan 15;217(2):517–526. doi: 10.1042/bj2170517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Bignold L. P., Morgan E. H. Plasma protein metabolism and transfer to the fetus during pregnancy in the rat. Am J Physiol. 1969 Apr;216(4):876–883. doi: 10.1152/ajplegacy.1969.216.4.876. [DOI] [PubMed] [Google Scholar]
- Mayel-Afshar S., Grimble R. F. Changes in protein turnover during gestation in the foetuses, placentas, liver, muscle and whole body of rats given a low-protein diet. Biochim Biophys Acta. 1983 Mar 31;756(2):182–190. doi: 10.1016/0304-4165(83)90090-9. [DOI] [PubMed] [Google Scholar]
- Mayel-Afshar S., Grimble R. F. Tyrosine oxidation and protein turnover in maternal tissues and the fetus during pregnancy in rats. Biochim Biophys Acta. 1982 May 27;716(2):201–207. doi: 10.1016/0304-4165(82)90269-0. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Garlick P. J. Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol. 1981 Sep;241(3):E238–E245. doi: 10.1152/ajpendo.1981.241.3.E238. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. J., Brasel J. A. One cycle of reproduction consisting of pregnancy, lactation or no lactation, and recovery: effects on carcass composition in ad libitum-fed and food-restricted rats. J Nutr. 1984 Sep;114(9):1548–1559. doi: 10.1093/jn/114.9.1548. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Peters T., Jr Intracellular aspects of transferrin synthesis and secretion in the rat. J Biol Chem. 1971 Jun 10;246(11):3508–3511. [PubMed] [Google Scholar]
- Naismith D. J., Morgan B. L. The biphasic nature of protein metabolism during pregnancy in the rat. Br J Nutr. 1976 Nov;36(3):563–566. doi: 10.1079/bjn19760109. [DOI] [PubMed] [Google Scholar]
- Naismith D. J., Richardson D. P., Pritchard A. E. The utilization of protein and energy during lactation in the rat, with particular regard to the use of fat accumulated in pregnancy. Br J Nutr. 1982 Sep;48(2):433–441. doi: 10.1079/bjn19820125. [DOI] [PubMed] [Google Scholar]
- Palmer M. F., Rolls B. A. Activities of some metabolic enzymes in the small intestinal mucosa during pregnancy and lactation in the rat. J Reprod Fertil. 1980 Sep;60(1):231–236. doi: 10.1530/jrf.0.0600231. [DOI] [PubMed] [Google Scholar]
- Razooki Hasan H., White D. A., Mayer R. J. Extensive destruction of newly synthesized casein in mammary explants in organ culture. Biochem J. 1982 Jan 15;202(1):133–138. doi: 10.1042/bj2020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remesar X., Arola L., Palou A., Alemany M. Body and organ size and composition during the breeding cycle of rats (Rattus norvegicus). Lab Anim Sci. 1981 Feb;31(1):67–70. [PubMed] [Google Scholar]
- Roberts S. B., Coward W. A. Lactation increases the efficiency of energy utilization in rats. J Nutr. 1984 Dec;114(12):2193–2200. doi: 10.1093/jn/114.12.2193. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Matusik R. J., Richards D. A., Gupta P., Rodgers J. R. Multihormonal regulation of casein gene expression at the transcriptional and posttransciptional levels in the mammary gland. Recent Prog Horm Res. 1980;36:157–193. doi: 10.1016/b978-0-12-571136-4.50011-5. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., O'Neal D. L., McHugh J. E., Comstock J. P. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978 Jan 24;17(2):290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Rosso P., Streeter M. R. Effects of food or protein restriction on plasma volume expansion in pregnant rats. J Nutr. 1979 Nov;109(11):1887–1892. doi: 10.1093/jn/109.11.1887. [DOI] [PubMed] [Google Scholar]
- SPRAY C. M. A study of some aspects of reproduction by means of chemical analysis. Br J Nutr. 1950;4(4):354–360. doi: 10.1079/bjn19500059. [DOI] [PubMed] [Google Scholar]
- Sainz R. D., Calvert C. C., Baldwin R. L. Relationships among dietary protein, feed intake and tissue protein turnover in lactating rats. J Nutr. 1986 Sep;116(9):1820–1829. doi: 10.1093/jn/116.9.1820. [DOI] [PubMed] [Google Scholar]
- Sampson D. A., Hunsaker H. A., Jansen G. R. Dietary protein quality, protein quantity and food intake: effects on lactation and on protein synthesis and tissue composition in mammary tissue and liver in rats. J Nutr. 1986 Mar;116(3):365–375. doi: 10.1093/jn/116.3.365. [DOI] [PubMed] [Google Scholar]
- Sampson D. A., Jansen G. R. Protein and energy nutrition during lactation. Annu Rev Nutr. 1984;4:43–67. doi: 10.1146/annurev.nu.04.070184.000355. [DOI] [PubMed] [Google Scholar]
- Sampson D. A., Jansen G. R. Protein synthesis during lactation: no circadian variation in mammary gland and liver of rats fed diets varying in protein quality and level of intake. J Nutr. 1984 Aug;114(8):1470–1478. doi: 10.1093/jn/114.8.1470. [DOI] [PubMed] [Google Scholar]
- Sampson D. A., Jansen G. R. The effect of dietary protein quality and feeding level on milk secretion and mammary protein synthesis in the rat. J Pediatr Gastroenterol Nutr. 1985 Apr;4(2):274–283. doi: 10.1097/00005176-198504000-00020. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Siebrits F., Martinez J. A., Buttery P. J. The effect of lactation on the fractional synthetic rate of protein in the liver and muscle of rats. Int J Biochem. 1985;17(6):731–732. doi: 10.1016/0020-711x(85)90374-x. [DOI] [PubMed] [Google Scholar]
- Vernon R. G., Flint D. J. Control of fatty acid synthesis in lactation. Proc Nutr Soc. 1983 Jun;42(2):315–331. doi: 10.1079/pns19830035. [DOI] [PubMed] [Google Scholar]


