Abstract
Background
Since there is no current international consensus on the optimal approach for pain management in acute pancreatitis (AP), analgesic practices may vary across different healthcare settings.
Objective
This study explored global disparities in analgesic use, in particular opioids, during admission and at discharge in hospitalised AP patients.
Methods
This was a post hoc analysis of the prospective PAINAP database, which included all admissions for AP between April and June 2022 with a 1‐month follow‐up. Demographic details, analgesic use, and clinical outcomes were recorded during admission and at discharge. Odds ratios (ORs) for opioid use during admission and at discharge were identified using multivariable regression analyses.
Results
Amongst the 1864 patients (52% males, median age 56 (interquartile range, 41–71)) across three different continents, simple analgesics were predominantly used as the primary analgesic (70%). Opioid use during admission was lowest in European centres (67%). Admission in Asian (OR, 2.53 (95% confidence interval (CI), 1.59–4.04), p < 0.001), and Australian (OR, 5.81 (95% CI, 3.19–10.56), p < 0.001) centres was associated with opioid administration during admission compared with European centres. Increased pain severity, longer pre‐admission pain duration, organ failure, and longer length of admission increased opioid use during admission. At discharge, Asian (OR, 2.01 (95% CI, 1.40–2.88), p < 0.001) and Australian (OR, 1.91 (95% CI, 1.28–2.85), p = 0.002) centres were associated with opioid prescription compared with European centres. Increased pain severity, longer pre‐admission pain duration, acute necrotic collections, and walled‐off necrosis also increased the likelihood of opioid prescription at discharge.
Conclusion
There are substantial intercontinental differences in opioid use for AP pain. Accordingly, there is a need for international guidelines on pain management in AP.
Keywords: abdominal pain, acute necrotic collections, acute pancreatitis, analgesia, opioids, walled‐off necrosis
Key summary.
Summarise the established knowledge on this subject
Managing severe epigastric pain remains a clinical challenge in patients with acute pancreatitis (AP)
In the absence of high‐quality recommendations for the management of AP pain, it is unknown whether analgesic practices, including opioid use, may vary worldwide and what factors influence opioid use for AP pain.
What are the significant and/or new findings of this study?
We described significant intercontinental differences in the administration of analgesics for AP pain, with the lowest opioid use during admission and on discharge in European centres.
Increased pain severity, longer pre‐admission pain duration, organ failure, and longer length of admission increased opioid use during admission.
Increased pain severity, longer pre‐admission pain duration, acute necrotic collections, and walled‐off necrosis increased the likelihood of opioid prescription at discharge.
INTRODUCTION
Acute pancreatitis (AP) is a frequent gastrointestinal disease increasing in prevalence in most Western countries. 1 Intense abdominal pain is the pre‐eminent presenting symptom, an essential diagnostic criterion, and a prognostic factor. 2 However, there is a lack of high‐level evidence‐based recommendations to guide the treatment of AP pain. Notably, the International Association of Pancreatology and the American Pancreatic Association guidelines for managing AP have no recommendations for pain relief. 3 The corresponding Japanese guidelines highlight the importance of obtaining adequate pain control in AP but acknowledge that there is a lack of consensus on the optimal approach. 4 The existing randomised controlled trials are limited by design, methodological and statistical heterogeneity. 5 This may account for significant disparities in the approach to analgesia in patients with AP in different centres around the world. 6 , 7
Without international consensus for the management of pain in AP, clinicians may have safety concerns regarding the use of opioids, and opioid‐sparing techniques are often recommended for these patients. 5 , 6 , 8 , 9 Previous findings suggest that opioids are often utilised for AP pain in European, Asian, and North American centres. 6 , 10 , 11 Single‐centre studies have suggested that opioid use in AP may be influenced by ethnicity, with Black and Hispanic patients being less likely to receive opioids compared to Whites and non‐Hispanics, respectively. 12 , 13 It was also suggested that physicians might be hesitant to prescribe opioids to younger patients due to addiction risk, but there was no data to support this claim. 13 Nevertheless, there is a lack of global multicentre studies on the use of opioids for AP pain.
Real‐world data delineating current analgesic and opioid practices around the world are important in guiding future high‐quality randomised controlled trials and guidelines for the management of AP pain. Such data would also explore the relationship between clinical, geographic, and pathological patient characteristics with the administration of pain modalities, in particular opioids. The primary aim of the present prospective multicentre cohort study was to describe intercontinental differences in the administration of analgesics, in particular opioids, in patients admitted with AP. Secondarily, we wished to determine associations between different clinical characteristics and the use of opioids during hospital admission and discharge.
METHODS
Study setting and participants
We performed a post hoc analysis of the prospective PAINAP database, an international multicentre cohort study recruiting patients admitted with an index episode of AP between April 1 and 30 June 2022, with a 1‐month follow‐up period, as previously described. 10 Adult patients (>18 years) fulfiling two or more revised Atlanta criteria for the diagnosis of AP 14 were eligible for the study. Patients with chronic pancreatitis, recurrent AP, or pregnancy were excluded. This original database included 2119 patients from 118 AP centres across 27 countries. For this study, we excluded patients with missing data on age, sex, or analgesic administration during admission. Continents with few patients included were excluded from the analysis due to low general representativeness, similar to previous studies exploring international practice. 11 Consequently, patients from South America (n = 8), North America (n = 25), and Africa (n = 24) were excluded from this study. Research ethics approval was not required for this study, and this was confirmed by the online National Research Ethics Service decision tool (http://www.hra‐decisiontools.org.uk/research/) according to the UK research ethics committee criteria. All centres in the study obtained local approval before data collection, and the study was conducted according to the principles of the Declaration of Helsinki. After the conclusion of data collection, data validation was performed on key variables (sex, smoking, aetiology, ERCP, epidural use, duration of pain before admission, analgesia side effects, the severity of AP according to the revised Atlanta criteria, and mortality) as previously described. 10 The original and validated data for most variables were well correlated (Spearman's correlation/Cohens kappa coefficient ≥ 0.69). However, for one variable—analgesia side effects—we found only a moderate correlation (Cohens kappa coefficient = 0.52) between the original and validated data. 10
Assessment variables
As part of the PAINAP study, variables regarding demographic details, ethnicity, clinical outcome of AP, and the administration of analgesics were collected. The site of inclusion was used to classify patients according to the continent (a full list of participating centres and the corresponding continent is shown in Supporting Information S2). Since both secondary and tertiary pancreatitis centres were invited to participate in the study, we created a variable classifying centre as secondary or tertiary. Demographic details included age, sex, ethnicity, and comorbidity (measured by the Charlson comorbidity index [CCI]). Regarding AP, the aetiology, baseline pain severity (measured using a numeric rating scale of 0–10), pain duration before admission, severity according to the revised Atlanta classification, 14 presence of organ failure or local complications, length of admission, and 30‐day mortality were recorded. Pre‐admission pain duration was classified as less than 12 h, 12–24 h, and more than 24 h before admission. Local complications included acute fluid collections, pseudocysts, acute necrotic collections, walled‐off necrosis, and thrombosis (portal/splenic vein). Analgesic administration was categorised into simple analgesics (paracetamol, non‐steroidal anti‐inflammatory drugs), weak opioids (codeine, dihydrocodeine or tramadol), strong opioids (morphine, diamorphine, oxycodone, hydromorphone, buprenorphine, fentanyl, tapentadol, pethidine, methadone, dezocine, or nalbuphine), adjuvant analgesics (gabapentinoids, antispasmodics, tricyclic antidepressant, or serotonin‐noradrenaline reuptake inhibitors), and other analgesics according to previous definitions. 10 The timing of analgesics was captured according to the temporal order of administration (primary, secondary, tertiary, or quaternary) and day of administration. Any analgesic prescriptions before admission and at discharge were recorded and classified as simple analgesics, weak opioids, strong opioids, adjuvant analgesics, or other analgesics, as described above. The use of acupuncture, epidural analgesia, and pain team assessments was also documented. Because of the focus on opioids in this study, we grouped patients based on whether they had any opioids (weak or strong) administered during admission and whether they had any prescription for opioids (weak or strong) at the time of discharge.
Statistical analysis
Categorical data were presented as numbers (%) and continuous data as mean ± standard deviation (SD) or median (interquartile range (IQR)) depending on data distributions. Missing data was handled by excluding patients with missing data on key variables (age, sex, analgesic use). For the remaining variables, the amount of missing data was reported for transparency. For the subsequent statistical analyses, our objective was to identify factors associated with opioid use during admission or opioid prescription at discharge, respectively. Prior to any statistical testing, patients were grouped based on opioid administration during admission (yes/no) and whether they had received an opioid prescription at discharge (yes/no), as described above. For the univariable analysis, we first compared groups based on opioid administration during admission using Student's t‐test, Wilcoxon Rank Sum test, Fisher's Exact test, or Chi‐squared test, depending on data type and distribution. Subsequently, we performed multivariable regression analysis using binary logistic regression in compliance with the TRIPOD guidelines. 15 In the multivariable model, we adjusted for all variables significantly associated with opioid administration during admission on univariable analysis, except for ethnicity, AP severity, and intensive care unit admission. These variables were left out of the model due to the risk of collinearity with continent (ethnicity) or organ failure and local complications (AP severity and intensive care unit admission). Accordingly, in this model, we adjusted for age, continent, centre type, aetiology, CCI, baseline pain severity, pre‐admission pain duration, organ failure, acute necrotic collections, walled‐off necrosis, splenic vein thrombosis, and length of admission. The results of the model were presented as Odds Ratios (ORs) with 95% Confidence Intervals (95% CIs). We also tested the performance of the model using the area under the curve of the receiver operating characteristics. The likelihood of an opioid prescription at discharge was analysed with the same approach as for the administration of opioids during admission as described above. In this model, we adjusted for age, continent, centre type, aetiology, CCI, baseline pain severity, pre‐admission pain duration, acute fluid collections, acute necrotic collections, walled‐off necrosis, portal vein thrombosis and splenic vein thrombosis. Because the proportion of patients included at secondary centres was higher among European centres, we repeated the multivariable models in a subgroup of patients recruited at tertiary centres. Statistical significance was set at p < 0.05. For statistical analysis, we used STATA software packages (StataCorp LP, version 17.0) and R (version 4.3).
RESULTS
Intercontinental differences in baseline and clinical characteristics
The cohort for this study consisted of 1864 patients (Figure 1). The demographic and clinical characteristics of the patients included are presented in Table 1. Within the total cohort of 1864 patients, 971 (52%) patients were male. Median age varied slightly across continents, with patients having the highest median age of 58 years (IQR, 45–73) in European centres and the lowest median age of 44 years (IQR, 32–56) in Asian centres. Biliary AP was the most frequent aetiology in centres across Europe (838 patients, 61%), Asia (157 patients, 50%), and Australia (86 patients, 46%). Pre‐admission pain duration was more than 24 h in 847 (46%) patients across the total cohort of patients, especially in patients from Asian centres (214 patients, 69%). The proportion of patients admitted to a secondary centre was higher among European centres (538 patients, 39%) as compared to Asian (16 patients, 5%) and Australian centres (12 patients, 6%). Severe AP was present in 149 (8%) patients, with an especially high prevalence of severe AP within centres from Asia (67 patients, 22%) compared to Europe and Australia. Correspondingly, organ failure rates were higher in Asian centres (79 patients, 25%). Similarly, local complications were more prevalent in centres in Asia. In Asia, 81 (26%) patients required intensive care unit admission compared with 90 (7%) patients in European centres and 8 (4%) in Australian centres. The length of admissions was the longest in Asian centres, with a median of 10 days (IQR, 6–17). However, mortality rates were comparable between centres across all three continents.
FIGURE 1.
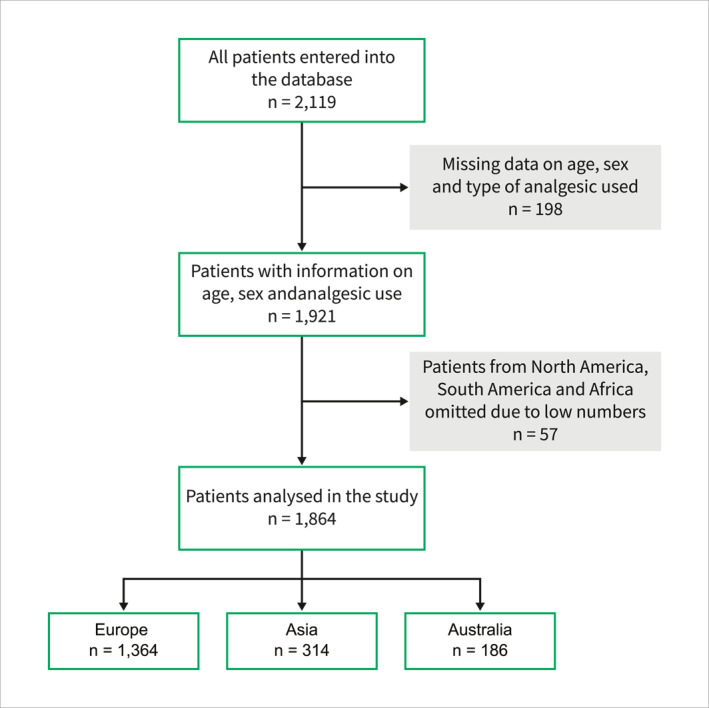
The flowchart for the patients included in the study was stratified according to continent.
TABLE 1.
Demographic and clinical characteristics of patients included in the study were stratified according to geography (n = 1864).
| Europe (n = 1364) | Asia (n = 314) | Australia (n = 186) | Missing data, n (%) | |
|---|---|---|---|---|
| Sex, n (%) | 0 (0) | |||
| Male | 665 (49) | 210 (67) | 96 (52) | |
| Female | 699 (51) | 104 (33) | 90 (48) | |
| Median age, [years] (IQR) | 58 (45–73) | 44 (32–56) | 53 (36–68) | 0 (0) |
| Age category, [years] n (%) | 0 (0) | |||
| <30 | 97 (7) | 57 (18) | 24 (13) | |
| 30–40 | 154 (11) | 70 (22) | 29 (15) | |
| 40–50 | 192 (14) | 66 (21) | 33 (18) | |
| 50–60 | 266 (20) | 51 (16) | 25 (13) | |
| 60–70 | 217 (16) | 37 (12) | 31 (17) | |
| 70–80 | 232 (17) | 21 (7) | 22 (12) | |
| >80 | 206 (15) | 12 (4) | 22 (12) | |
| Type of centre | 0 (0) | |||
| Secondary | 538 (39) | 16 (5) | 12 (6) | |
| Tertiary | 826 (61) | 298 (95) | 174 (94) | |
| Ethnicity | 37 (2) | |||
| White | 1201 (90) | 10 (3) | 75 (42) | |
| Asian | 83 (6) | 303 (97) | 13 (7) | |
| Black | 22 (2) | 0 (0) | 0 (0) | |
| Other | 27 (2) | 0 (0) | 93 (51) | |
| Aetiology, n (%) a | 0 (0) | |||
| Biliary | 838 (61) | 157 (50) | 86 (46) | |
| Alcohol | 243 (18) | 98 (31) | 40 (22) | |
| Post ERCP | 49 (4) | 11 (4) | 2 (1) | |
| Hypertriglyceridemia | 33 (2) | 20 (6) | 8 (4) | |
| Other | 258 (19) | 36 (11) | 59 (32) | |
| Median CCI‐score (IQR) | 2 (0–4) | 0 (0–2) | 1 (0–3) | 52 (3) |
| CCI category, n (%) | 52 (3) | |||
| 0 | 375 (29) | 179 (57) | 66 (35) | |
| 1–2 | 383 (29) | 82 (26) | 57 (31) | |
| >2 | 554 (42) | 53 (17) | 63 (34) | |
| Median baseline pain severity, [NRS] (IQR) | 7 (6–8) | 7 (6–8) | 7 (4–8) | 312 (17) |
| Pain duration, [hours] n (%) | 23 (1) | |||
| <12 | 389 (29) | 34 (11) | 45 (25) | |
| 12–24 | 423 (31) | 63 (20) | 40 (22) | |
| >24 | 537 (40) | 214 (69) | 96 (53) | |
| AP severity, n (%) b | 31 (2) | |||
| Mild AP | 1079 (81) | 125 (40) | 162 (89) | |
| Moderate AP | 186 (14) | 120 (38) | 12 (7) | |
| Severe AP | 73 (5) | 67 (22) | 9 (5) | |
| Organ failure, n (%) | 25 (1) | |||
| No organ failure | 1227 (91) | 231 (75) | 173 (93) | |
| Organ failure | 116 (9) | 79 (25) | 13 (7) | |
| Local complications, n (%) c | 15 (1) | |||
| Acute fluid collection | 124 (9) | 43 (14) | 6 (3) | |
| Pseudocyst | 22 (2) | 5 (2) | 3 (2) | |
| Acute necrotic collection | 71 (5) | 89 (30) | 6 (3) | |
| Walled‐off necrosis | 15 (1) | 36 (13) | 0 (0) | |
| Portal vein thrombosis | 13 (1) | 11 (4) | 0 (0) | |
| Splenic vein thrombosis | 16 (1) | 46 (16) | 0 (0) | |
| Intensive care unit admission, n (%) | 7 (0) | |||
| No | 1267 (93) | 233 (74) | 178 (96) | |
| Yes | 90 (7) | 81 (26) | 8 (4) | |
| Median length of admission, [days] (IQR) | 6 (4–10) | 10 (6–17) | 4 (3–6) | 19 (1) |
| 30‐day mortality, n (%) | 17 (1) | |||
| No | 1308 (97) | 300 (96) | 181 (98) | |
| Yes | 43 (3) | 12 (4) | 3 (2) |
Abbreviations: AP, acute pancreatitis; CCI, Charlson comorbidity index; ERCP, endoscopic retrograde cholangiopancreatography; IQR, interquartile range; NRS, numeric rating scale.
A subset of patients had overlapping aetiologies.
According to the Revised Atlanta classification.
A subset of patients had multiple local complications.
Intercontinental differences in analgesic administration
Details regarding the administration of analgesics are given in Table 2 and Figure 2 and presented below. Simple analgesics were most often administered as the primary analgesics in European (1069 patients, 78%), and Australian (116 patients, 62%) centres. In Asian centres, the most often used primary analgesics were simple analgesics (122 patients, 39%) and weak opioids (119 patients, 38%). Strong opioids were least often employed as primary analgesics in European centres (198 patients, 14%), whereas this proportion was higher in both Asian (66 patients, 21%) and Australian (62 patients, 33%) centres. Most patients were treated with an opioid (weak or strong) during admission across all continents, with the lowest proportion in European centres (918 patients, 67%) (Figure 3a). Pain team assessments were most common in Asian centres (97 patients, 31%) compared with European (115 patients, 8%) and Australian (19 patients, 10%) centres. Epidural analgesia use was rare and confined to centres in Europe and Asia. Acupuncture was exclusively used in Asian centres, accounting for merely 15 (5%) patients. European centres had the lowest opioid prescription rate at discharge (386 patients, 28%) compared to Asian (176 patients, 56%) and Australian (85 patients, 46%) centres (Figure 3b).
TABLE 2.
Administration of analgesics stratified according to geography (n = 1864).
| Europe (n = 1364) | Asia (n = 314) | Australia (n = 186) | Missing data n (%) | |
|---|---|---|---|---|
| Analgesics prior to admission, n (%) a | 0 (0) | |||
| No prescription | 1209 (89) | 295 (94) | 157 (84) | |
| Simple analgesics | 98 (7) | 17 (5) | 10 (5) | |
| Weak opioids | 46 (3) | 2 (1) | 5 (3) | |
| Strong opioids | 20 (1) | 1 (0) | 8 (4) | |
| Adjuvant analgesic | 37 (3) | 1 (0) | 16 (9) | |
| Other | 1 (0) | 1 (0) | 1 (1) | |
| Type of primary analgesia, n (%) | 0 (0) | |||
| Simple analgesics | 1069 (78) | 122 (39) | 116 (62) | |
| Weak opioids | 79 (6) | 119 (38) | 7 (4) | |
| Strong opioids | 198 (14) | 66 (21) | 62 (33) | |
| Adjuvant analgesic | 10 (1) | 7 (2) | 1 (1) | |
| Other | 8 (1) | 0 (0) | 0 (0) | |
| Type of secondary analgesia, n (%) | 0 (0) | |||
| No additional analgesia | 278 (20) | 58 (18) | 14 (7) | |
| Simple analgesics | 430 (32) | 119 (38) | 75 (40) | |
| Weak opioids | 342 (25) | 72 (23) | 13 (7) | |
| Strong opioids | 272 (20) | 59 (19) | 82 (44) | |
| Adjuvant analgesic | 20 (1) | 4 (1) | 1 (1) | |
| Other | 22 (2) | 2 (1) | 1 (1) | |
| Type of tertiary analgesia, n (%) | 0 (0) | |||
| No additional analgesia | 773 (57) | 175 (56) | 53 (28) | |
| Simple analgesics | 120 (9) | 33 (10) | 44 (24) | |
| Weak opioids | 134 (10) | 25 (8) | 20 (11) | |
| Strong opioids | 293 (21) | 77 (25) | 64 (34) | |
| Adjuvant analgesic | 31 (2) | 4 (1) | 4 (2) | |
| Other | 13 (1) | 0 (0) | 1 (1) | |
| Type of quaternary analgesia, n (%) | 0 (0) | |||
| No additional analgesia | 1170 (86) | 279 (89) | 90 (48) | |
| Simple analgesics | 39 (3) | 9 (3) | 36 (19) | |
| Weak opioids | 49 (3) | 2 (0) | 8 (4) | |
| Strong opioids | 78 (6) | 18 (6) | 50 (26) | |
| Adjuvant analgesic | 19 (1) | 6 (2) | 2 (1) | |
| Other | 9 (1) | 0 (0) | 0 (0) | |
| Epidural analgesia, n (%) | 200 (11) | |||
| No | 1169 (99) | 306 (99) | 179 (0) | |
| Yes | 7 (1) | 3 (1) | 0 (0) | |
| Acupuncture, n (%) | 218 (12) | |||
| No | 1157 (100) | 295 (95) | 179 (100) | |
| Yes | 0 (0) | 15 (5) | 0 (0) | |
| Pain team assessment, n (%) | 3 (0) | |||
| No | 1247 (92) | 217 (69) | 166 (90) | |
| Yes | 115 (8) | 97 (31) | 19 (10) | |
| Analgesic prescriptions at discharge, n (%) b | 0 (0) | |||
| No prescription | 581 (43) | 60 (19) | 53 (28) | |
| Simple analgesics | 653 (48) | 170 (54) | 117 (63) | |
| Weak opioids | 295 (22) | 119 (38) | 56 (30) | |
| Strong opioids | 108 (8) | 61 (19) | 32 (17) | |
| Adjuvant analgesic | 39 (3) | 8 (3) | 5 (3) | |
| Other | 11 (1) | 0 (0) | 0 (0) | |
| Opioid administration during admission, n (%) | 0 (0) | |||
| No | 446 (33) | 41 (13) | 28 (15) | |
| Yes | 918 (67) | 273 (87) | 158 (85) | |
| Opioid prescription at discharge, n (%) | 0 (0) | |||
| No | 978 (72) | 138 (44) | 101 (54) | |
| Yes | 386 (28) | 176 (56) | 85 (46) | |
| Median duration of opioid treatment [days] (IQR) | 2 (0–4) | 3 (1–5) | 2 (1–4) | 36 (2) |
Abbreviation: IQR, interquartile range.
A subset of patients had several analgesics prescribed prior to admission.
A subset of patients had several analgesics prescribed at discharge.
FIGURE 2.
Sankey diagram for the temporal order of analgesic administration in Europe (a), Asia (b) and Australia (c).
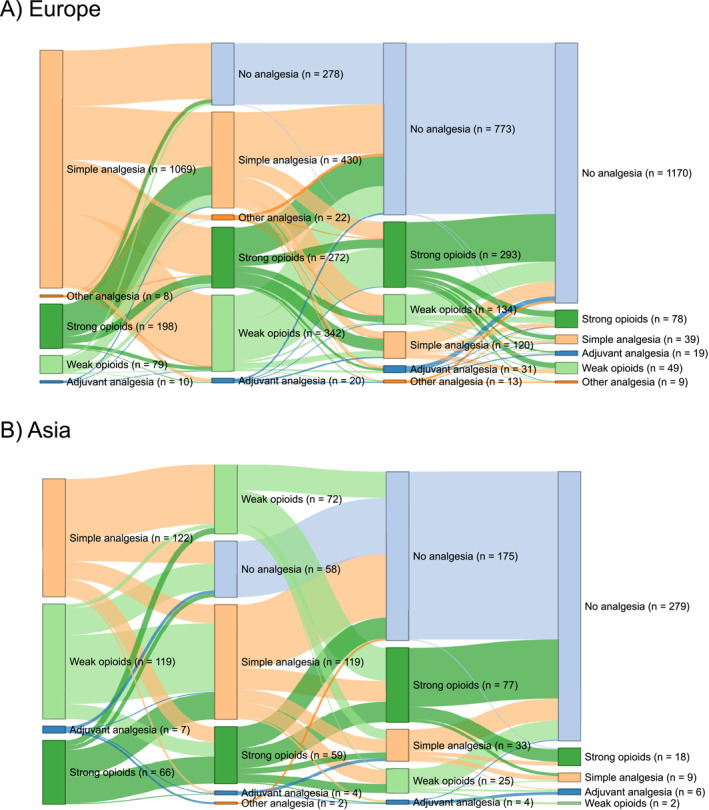
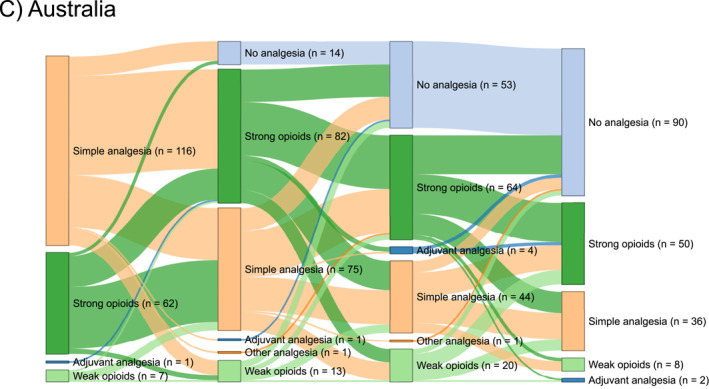
FIGURE 3.
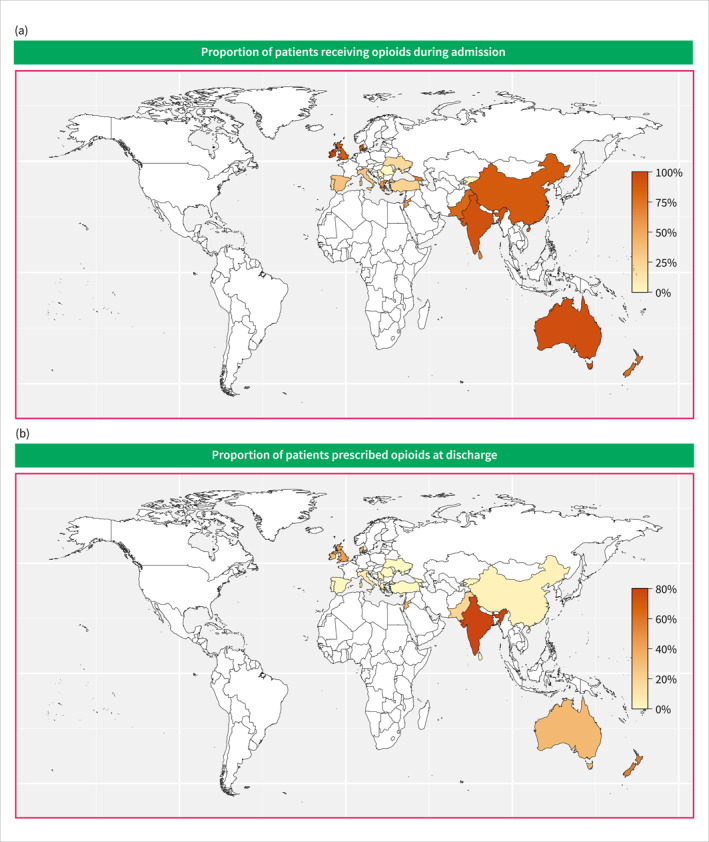
Heatmap of the proportion of patients receiving opioids during admission (a) or at discharge (b).
Variables associated with opioid administration during admission
Several variables were associated with opioid administration during admission on univariable analysis (Table S1). Results from the multivariable analysis are reported in Table S2 and Figure 4. Asian (OR, 2.53 (95% CI, 1.59–4.04), p < 0.001), and Australian (OR, 5.81 (95% CI, 3.19–10.56), p < 0.001) centres were associated with opioid administration during admission compared with European centres. Increased pain severity (OR, 1.21 (95% CI, 1.14–1.29), p < 0.001) and longer pre‐admission pain duration (OR, 1.55 (95% CI, 1.13–2.12), p = 0.007) were associated with opioid administration. Organ failure (OR, 1.75 (95% CI, 1.02–3.00), p = 0.04), and longer length of admission (OR, 1.03 (95% CI, 1.01–1.06), p = 0.002) were also associated with increased likelihood of opioid administration during admission. On the other hand, biliary AP compared to non‐biliary (OR, 0.69 (95% CI, 0.51–0.93), p = 0.02) and a CCI of 1‐2 compared to 0 (OR, 0.69 (95% CI, 0.47–1.01), p = 0.05) were associated with a decreased likelihood of opioid administration during admission.
FIGURE 4.
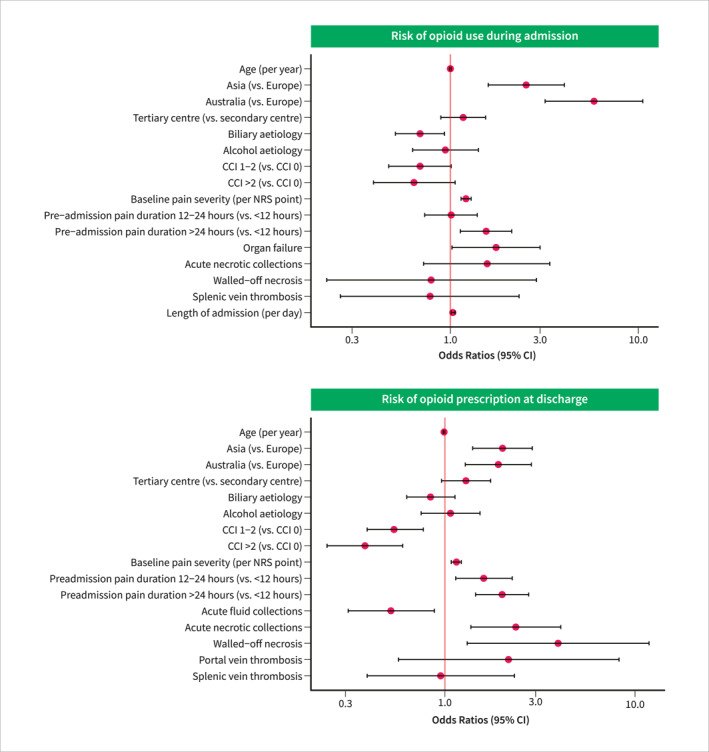
Forest plot illustrating the odds ratios for opioid use during admission or at discharge (multivariable analysis). CCI, Charlson comorbidity index; CI, confidence interval; NRS, numeric rating scale.
Variables associated with opioid prescription at discharge
Several variables were associated with opioid administration at hospital discharge on univariable analysis (Table S3). On multivariable analysis, Asian (OR, 2.01 (95% CI, 1.40–2.88), p < 0.001) and Australian (OR, 1.91 (95% CI, 1.28–2.85), p = 0.002) centres increased the likelihood of an opioid prescription at discharge compared with European centres (Table S4 and Figure 4). Increased severity of pain (OR, 1.15 (95% CI, 1.08–1.22), p < 0.001), longer pre‐admission pain duration (all p ≤ 0.007), acute necrotic collections (OR, 2.36 (95% CI, 1.37–4.07), p = 0.002) and walled‐off necrosis (OR, 3.94 (95% CI, 1.31–11.85), p = 0.02) also increased the likelihood of opioid prescription at discharge. Higher Charlson Comorbidity Indexes (all p ≤ 0.001) and acute fluid collections (OR, 0.52 (95% CI, 0.31–0.88), p = 0.02) decreased the likelihood of an opioid prescription at discharge. The results of the subgroup analyses, including only patients recruited at tertiary centres, were comparable to the results of the primary analyses (Tables S5 and S6).
DISCUSSION
Within this international multicentre, prospective cohort study of 1864 patients admitted with AP, we described significant intercontinental differences in the administration of analgesics for AP pain, with the lowest opioid use during admission and on discharge in European centres. Multivariate analysis revealed that increased pain severity on admission and longer pre‐admission pain duration were associated with increased opioid use during admission and on discharge. In contrast, biliary aetiology was associated with lower opioid use. Interestingly, organ failure and longer length of hospital admission were associated with increased opioid use during admission but not on discharge, whereas necrosis (acute collections and walled‐off) was associated with opioid prescriptions on discharge.
In this study, the most common primary pain medication was a simple analgesic across almost all continents, which, according to the WHO Ladder for cancer pain, is often recommended for pain management in AP. 8 , 16 The use of weak opioids as a primary analgesic was higher in Asia compared with other continents, and this is comparable to previous studies. 11 A study from California found that only 864 out of 4307 (∼20%) patients admitted with AP did not receive opioids during admission, which corresponds to our findings for opioid use during admission. 6 Our results are in comparison to a recent study by Matta et al., including 1612 AP patients from 22 centres in 4 continents, which found a similarly variable opioid use. 11 They also documented discharge opioid prescription rates, with Europe and Asia having much lower opioid prescriptions compared to our study. 11 This may be partly due to our larger European sample dominated by patients from the United Kingdom, where opioid prescriptions tend to be higher, 17 and our more diverse sample of Asian centres from six different countries as opposed to only centres from India.
The general use of opioids in the literature appears to vary based on geography. 17 , 18 The local organisation of the health care systems, including access to medical facilities and specialised treatment, may influence this difference. The attitude of the treating physicians may also influence the use of opioids, as indicated by previous evidence that opioid use differs between different doctors, even within the same department. 19 In an AP setting, this may also be attributed to differences in disease severity depending on geography. Correspondingly, our results and previous findings suggest that moderately severe or severe disease is more prevalent in an Asian hospital setting, potentially explaining the higher opioid use in Asian centres in this study. 11 Since pain severity upon admission and pre‐admission pain duration was associated with opioid use during admission and at discharge, it might also be that there are differences in pain tolerance across different continents and cultures, which was also raised in a previous study. 20 Pain severity was similar across continents in our study. However, it did appear that the pre‐admission pain duration was longer in patients treated at Asian centres, potentially contributing to higher use of opioids. Again, this may also be influenced by access to medical evaluation and treatment in different regions. 21
High‐quality evidence for the optimal modality for managing AP pain is lacking. Some randomised studies have found opioids superior to non‐opioids in reducing the need for rescue analgesia and increasing pain‐free periods with similar safety profiles. 22 , 23 , 24 On the other hand, another randomised study found that intravenous paracetamol, dexketoprofen, and tramadol were all equivalent in the management of AP pain. 25 This was supported by other randomised studies, which found no clinical differences between metamizole and morphine or diclofenac and tramadol for pain relief in AP patients. 26 , 27 Small numbers have limited these studies, and there is a scope for further studies into the efficacy and safety of different analgesic modalities in AP. An ongoing randomised, placebo‐controlled trial in AP using a peripheral restricted μ‐opioid receptor antagonist that reverses opioid effects in the gut but preserves analgesia may shed some light on the safety of opioid use in patients with severe AP pain. 28
Strengths and limitations
In this international multicentre study, we have gathered detailed information on the use of different analgesics over time during AP admission. Furthermore, we included multiple centres from several countries across continents, increasing the generalisability of our findings. The distribution between secondary and tertiary centres differed between Europe and Asia/Australia, potentially contributing to lower opioid use in European centres. However, our multivariable analysis of the effect of centre type on opioid use, and the subgroup analysis, including only patients recruited at tertiary centres, gave similar results. Recruitment time for this study was limited, which might affect results due to possible seasonal variance in AP. 29 , 30 , 31 However, these studies were based on very regional data, and it remains unknown how seasons affect AP across larger geographical areas. Since these data are observational, we could not infer causality from the results, and it remains unknown whether the observed differences may be attributed to local traditions, access to medical care, or differences based on severity or pain perception across different continents. Nevertheless, the data provide valuable insight into analgesic practices worldwide in AP from a large dataset with little missing data.
CONCLUSION
In conclusion, we observed substantial regional differences in the use of analgesics for AP pain management. There is an unmet need for international consensus on how to effectively treat AP pain and reduce disparities between different regions, taking into consideration regional prescription practices. Acknowledging this gap in the management of pain in AP, guidelines developed by the European Pancreatic Club and supported by the United European Gastroenterology 32 are currently in development to provide a standardised approach to pain management and promote consistency in care practices across diverse healthcare settings for patients with AP.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS APPROVAL
All centres in the study obtained local approval before data collection.
Registration
ISRCTN registration number ISRCTN10634411, https://www.isrctn.com/ISRCTN10634411.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGEMENTS
The full list of PAINAP collaborators is available in Supplementary File S1. We acknowledge the Newcastle Joint Research Office for developing and maintaining the REDCap database. Cecilie Siggaard Knoph was supported by a grant from the Novo Nordisk Foundation, #NNF19OC0057331.
Knoph CS, Lucocq J, Kamarajah SK, Olesen SS, Jones M, Samanta J, et al. Global trends in opioid use for pain management in acute pancreatitis: a multicentre prospective observational study. United European Gastroenterol J. 2024;12(8):1114–27. 10.1002/ueg2.12641
Contributor Information
Sanjay Pandanaboyana, Email: S.pandanaboyana@nhs.net.
PAINAP Collaborative:
Neil Cark, Riinu Pius, Eduardo Houghton, Mariano Gimenéz, Karla Uribe, Florencia Rodriguez, Justin Gundara, Thomas Mackay, Huynh Phan, Joel Lewin, Claire McElhatton, Mehan Siriwardhane, Russell Hodgson, Hassan Malik, Ryan Ward, Kerilee Young, Shaneel Bappayya, Benjamin Loveday, Jaswinder Samra, Tamara Gall, Anubhav Mittal, Ting Ting Chan, Vincent Wing‐ho Lo, Hui Liang, Cong Wang, Wei Huang, Tao Jin, Yongzi Wu, Qing Xia, Nikolaou Georgio, Nikolaos Koronakis, Line Davidsen, Emad Hamed, Salem Mohamed, Zaza Demetrashvili, Ana Tvaladze, Irakli Kachakhidze, Tea Zurabashvili, Orestis Ioannidis, Stylianos Kapiris, Eleni Mavrodimitraki, Maria Sotiropoulou, Nikolaos Machairas, Dimitrios Schizas, Athanasios Syllaios, Michail Vailas, Georgios Chlorakis, Evangelos Kalaitzakis, Maria Tsafaridou, Francesk Mulita, Georgios‐Ioannis Verras, Amit Gupta, Deepak Rajput, Oshin Sharma, Rajesh Goud, Misbah Unnisa, Lovenish Bains, Nishu Singh, Jahnvi Dhar, Mahmoud Abdelmoeti, Criostóir Ó Súilleabháin, Robert O'Connell, Marcello Calabro, Antonio La Terra, Andrea Muretore, Riccardo Brachet Contul, Margherita Diotallevi, Annamaria Mascaro, Paolo Millo, Santino Antonio Biondo, Carmelo Mazzeo, Eugenio Cucinotta, Francesco Fleres, AOUG Marinak, Veronica Brocco, Marco Ceresoli, Maria Rennis, Danilo Centonze, Coatanza Distefano, Massimiliano Veroux, Domenico Zerbo, Selene Bogoni, Alan Biloslavo, Velentina Bianchi, Marcello Candelli, Francesco Franceschi, Antonio Gasbarrini, Enrico Nista, Gabriele Sganga, Giuseppe Tropeano, Fondazione Policlinico, Caterina Altieri, Vincenza Dinuzzi, Matteo Marconi, Umberto Rivolta, Vitale Roberto Dameno, Mario V. Papa, Andrea Balla, Pasquale Lepiane, Federica Saraceno, Alberto Aiolfi, Davide Bona, Andrea Sozzi, Pasquale Cianci, Marco Varesano, Ivana Conversano, Roberta Abete, Raffaele D'Avino, Ester Marra, Gianpaolo Marte, Pasquale Tammaro, Davide Gobatti, Serena Marmaggi, Francesco Palmieri, Roberto Sampietro, Roberto Manca, Federica Pilla, Enrico Piras, Giusto Pignata, Ilaria Canfora, Jacopo Andreuccetti, Rossella D'Alessio, Claudia Armellin, Ugo Grossi, Marco Massani, Alessandro Pontin, Tommaso Stecca, Tiaizna Pilia, Adolfo Pisanu, Mauro Podda, Mario Giuffrida, Gennaro Perrone, Simone Guadagni, Luca Morelli, Alice Frontali, Francesca Basurto, Stefano D'Ugo, Farshad Manoochehri, Marcello Spampinato, Laura Apadula, Paoletta Preatoni, Lodovico Sartarelli, Osama Al‐Jaiuossi, Mairam Ernisova, Andrey Sopuev, Bruce Sua, Anthony Farfus, Keith Teo, Brittany Smith, Bathiya Ratnayake, Jayvee Buchanan, Elinor Clark, Saxon Connor, Todd Hore, Salman Attari, Bushra Kadir, Sadik Memon, Zaigham Abbas, Muhammad Ali Quadeer, Abeer Altaf, Pooja Ameet, Jalpa Devi, Nandlal Seerani, Ameer Afzal, Ali Akbar, Mohammad Sohail Asghar, Tiago Sa, Ana Lucia Barreira, Numo Carvalho, Brigitta Cismasiu, Susana Henriques, Francisco Vara Luiz, Andreea Draghici, Valentin Grigorean, Vlad Porojan, Alexandru‐Rares Stoian, Lucia Teaca, Dragana Arbutina, Vladica Cuk, Bojan Kovacevic, Luka Mandic, Glenn Bonney, Yujia Gao, Ning Qi Pang, Abdalla Bellil, John Devar, Zafar Khan, Vusi Khumalo, Martin Smith, Sergio Estevez‐Fernandez, Beatriz Romero Mosquera, Sergio Rodriguez, Guillermo Garcia‐Rayado, Jean Felix Piñerua‐Gonsalvez, M Lourdes Ruiz Rebollo, Jose M. Olmos, Javier Tejedor‐Tejada, Manuel Diez‐Alonso, Belen Matias‐Garcia, Fernando Mendoza Moreno, Cristina Vera‐Mansilla, Helena Salvador Roses, Diego Vázquez Gómez, Juan Rodriguez Oballe, Umesh Jayarajah, Malith Nandasena, Aloka Pathirana, Sami Galal‐Eldin, Shahab Hajibandeh, Hytham Hamid, Elif Colak, Larysa Sydorchuk, Ruslan Knut, Ksenia Voronyuk, Serge Chooklin, Vitalii Baryskyi, Ruslan Sydorchuk, Samrat Mukherjee, Maitreyi Patel, Amina Akhtar, Miriam Asarbakhsh, Frances Nolan, Nicholaas Schuijtvlot, Sandhya Prem, Anuradha Thrikandiyur, Millicent Morris, Thomas Mroczek, George Sgourakis, Asma Sultana, Rebecca Varley, Thomas Groot‐Wassink, Roland Labinoti, Brett Packham, Keving Seebah, Sophie Allen, Shiva Mokhtassi, Ajay Belgaumkar, Henry De'Ath, Amy Cook, Christopher Delaney, Roisin Johnson, Becky Olali Azibaodinami, Ashley Sartini, Mea Stanfield, Ivan Tomasi, Venkat Kanakala, Simon Mbarushimana, Mark McKeever, Mamata Batilli, Gakul Bhatta, Subash Rai, Giles Bond‐smith, Amr Elserafy, Mohamed Shams, Tareq Al Saoudi, Neil Bhardwaj, Wajith Hussain, Francesco Lancellotti, Greta Montagnini, George Cairns, Marianne Hollyman, Asef Rakin, Mishal Shahid, Fraser Barbour, Jake Hawkyard, Matthew McTeer, Sanjay Pandanaboyana, Ellie Taylor, Matta Kuzman, Sarah Dyer, James Hopkins, Dimitri Pournaras, Alexis Sudlow, SK Kumar, Avinash Aujayeb, Alex Leo, Fatima Lorenzana Senra, Josef Watfah, Jenifer Barrie, Chris Brown, Dhanny Gomez, Somaiah Aroori, Debora Ciprani, Rahi Karmarkar, Eyas Almomani, Keith Roberts, Madeleine Fale, Ajay Gupta, Max Marsden, Chris Seet, Lakshya Soni, Mohammed Hamdan, Rohan Sadera, Vikas Sud, Edith Chinnah, Davide Di Mauro, Antonio Manzelli, Amira Orabi, Roberto Presa, Alex Reece‐Smith, Shahjehan Wajed, Jacob Fingret, Nehal Shah, Jignesh Jatania, Arun Krishna, David Berry, Loukiani Kitsikosta, Jack Helliwell, Benjamin Huntley, James Pine, Jih‐Dar Yau, Shiela Lee, Kamal Mahawar, Neehar Shetty, Emily Britton, Alice Shaw, Stijn van Laarhoven, Sukhpreet Gahunia, Miguel Gargia Ortega, Adam Lee, Cho Ee Ng, Jihene El Kafsi, John Mason, Gauri Vithlani, Rami Benhmida, James Gunell, Chetan Parmar, Da‐Costa Dorkeh, Mohamed Elnagar, Jih Ian Lee, Ashrafun Nessa, Zhu Hui Yeap, Niroshini Hemadasa, Saria Javed, Sharuk Sami, Dimitrios Damaskos, Andrew Healey, Maria Soupashi, Tania Triantafyllou, Maria Coats, Benjamin Douglass, Brid Hendry, Yasmin Hussain, Zhara Javid, Mia Mantyla, Khaman Rajkumar, Carven Chin, Shahab Hajibandeh, Nagappan Kumar, Ioannis Gerogiannis, Panagiotis Kapsampelis, Farid Gerge, Gulsum Anderson, Vu Dinh, Anna Phillips, and Dhiraj Yadav
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta‐analysis. Gastroenterology. 2022;162(1):122–134. 10.1053/j.gastro.2021.09.043 [DOI] [PubMed] [Google Scholar]
- 2. Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, et al. Acute pancreatitis: diagnosis and treatment. Drugs. 2022;82(12):1251–1276. 10.1007/s40265-022-01766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Working Group IAP/APA Acute Pancreatitis Guidelines . IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–e15. [DOI] [PubMed] [Google Scholar]
- 4. Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22(6):405–432. 10.1002/jhbp.259 [DOI] [PubMed] [Google Scholar]
- 5. Thavanesan N, White S, Lee S, Ratnayake B, Oppong KW, Nayar MK, et al. Analgesia in the initial management of acute pancreatitis: a systematic review and meta‐analysis of randomised controlled trials. World J Surg. 2022;46(4):878–890. 10.1007/s00268-021-06420-w [DOI] [PubMed] [Google Scholar]
- 6. Wu BU, Butler RK, Chen W. Factors associated with opioid use in patients hospitalized for acute pancreatitis. JAMA Netw Open. 2019;2(4):e191827. 10.1001/jamanetworkopen.2019.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholten WK, Christensen A.‐E, Olesen AE, Drewes AM. Quantifying the adequacy of opioid analgesic consumption globally: an updated method and early findings. Am J Public Health. 2019;109(1):52–57. 10.2105/ajph.2018.304753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandanaboyana S, Huang W, Windsor JA, Drewes AM. Update on pain management in acute pancreatitis. Curr Opin Gastroenterol. 2022;38(5):487–494. 10.1097/mog.0000000000000861 [DOI] [PubMed] [Google Scholar]
- 9. Balbale SN, Keswani RN. Opioid use for acute pancreatitis—toward a research agenda to optimize patient safety. JAMA Netw Open. 2019;2(4):e191834. 10.1001/jamanetworkopen.2019.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pandanaboyana S, Knoph CS, Olesen SS, Jones M, Lucocq J, Samanta J, et al. Opioid analgesia and severity of acute pancreatitis: an international multicentre cohort study on pain management in acute pancreatitis. United Eur Gastroenterol J. 2024;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18(7):1567–1575.e2. 10.1016/j.cgh.2019.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McHenry N, Shah I, Ahmed A, Freedman SD, Kothari DJ, Sheth SG. Racial variations in pain management and outcomes in hospitalized patients with acute pancreatitis. Pancreas. 2022;51(9):1248–1250. 10.1097/mpa.0000000000002160 [DOI] [PubMed] [Google Scholar]
- 13. McHenry N, Ahmed A, Shah I, Freedman SD, Nee J, Lembo A, et al. Racial and ethnic disparities in opioid prescriptions in benign and malignant pancreatic disease in the United States. Pancreas. 2022;51(10):1359–1364. 10.1097/mpa.0000000000002180 [DOI] [PubMed] [Google Scholar]
- 14. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 15. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13(1):1–10. 10.1016/j.eururo.2014.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vargas‐Schaffer G. Is the WHO analgesic ladder still valid? Twenty‐four years of experience. Can Fam Physician. 2010;56(6):514–517. e202‐5. [PMC free article] [PubMed] [Google Scholar]
- 17. Jayawardana S, Forman R, Johnston‐Webber C, Campbell A, Berterame S, de Joncheere C, et al. Global consumption of prescription opioid analgesics between 2009‐2019: a country‐level observational study. EClinicalMedicine. 2021;42:101198. 10.1016/j.eclinm.2021.101198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–1579. 10.1016/s0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnett ML, Olenski AR, Jena AB. Opioid‐prescribing patterns of emergency physicians and risk of long‐term use. N Engl J Med. 2017;376(7):663–673. 10.1056/nejmsa1610524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawson A, List T. Comparison of pain thresholds and pain tolerance levels between Middle Easterners and Swedes and between genders. J Oral Rehabil. 2009;36(4):271–278. 10.1111/j.1365-2842.2009.01943.x [DOI] [PubMed] [Google Scholar]
- 21. Richards GC, Aronson JK, Mahtani KR, Heneghan C. Global, regional, and national consumption of controlled opioids: a cross‐sectional study of 214 countries and non‐metropolitan territories. Br J Pain. 2022;16(1):34–40. 10.1177/20494637211013052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahapatra SJ, Jain S, Bopanna S, Gupta S, Singh P, Trikha A, et al. Pentazocine, a kappa‐opioid agonist, is better than diclofenac for analgesia in acute pancreatitis: a randomized controlled trial. Am J Gastroenterol. 2019;114(5):813–821. 10.14309/ajg.0000000000000224 [DOI] [PubMed] [Google Scholar]
- 23. Saini M, Samanta J, Kumar A, Choudhury A, Dhar J, Jafra A, et al. Buprenorphine versus diclofenac for pain relief in acute pancreatitis: a double‐blinded randomized controlled trial. Clin Gastroenterol Hepatol. 2023. [DOI] [PubMed] [Google Scholar]
- 24. Jakobs R, Adamek Ac Von MU. Buprenorphine or procaine for pain relief in acute pancreatitis A prospective randomized study. Scand J Gastroenterol. 2000;35(12):1319–1323. 10.1080/003655200453692 [DOI] [PubMed] [Google Scholar]
- 25. Gülen B, Dur A, Serinken M, Karcioğlu Ö, Sönmez E. Pain treatment in patients with acute pancreatitis: a randomized controlled trial. Turkish J Gastroenterol. 2016;27(2):192–196. 10.5152/tjg.2015.150398 [DOI] [PubMed] [Google Scholar]
- 26. Peiró AM, Martínez J, Martinez E, Madaria E, Llorens P, Horga J, et al. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8(1):25–29. 10.1159/000114852 [DOI] [PubMed] [Google Scholar]
- 27. Kumar NS, Muktesh G, Samra T, Sarma P, Samanta J, Sinha SK, et al. Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: a randomized parallel group double blind active controlled pilot study. Eur J Pain. 2020;24(3):639–648. 10.1002/ejp.1515 [DOI] [PubMed] [Google Scholar]
- 28. Knoph CS, Cook ME, Fjelsted CA, Novovic S, Mortensen MB, Nielsen LBJ, et al. Effects of the peripherally acting μ‐opioid receptor antagonist methylnaltrexone on acute pancreatitis severity: study protocol for a multicentre double‐blind randomised placebo‐controlled interventional trial, the PAMORA‐AP trial. Trials. 2021;22(1):940. 10.1186/s13063-021-05885-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sirtl S, Hohmann E, Beyer G, Hamm J, Neesse A, Ammer‐Herrmenau C. The four seasons of pancreatitis—etiology of acute pancreatitis during the course of the year. Z Gastroenterol. 2024. [DOI] [PubMed] [Google Scholar]
- 30. Wu D, Tang M, Zhao Y, Zhou S, Xu X, Wang F, et al. Impact of seasons and festivals on the onset of acute pancreatitis in Shanghai, China. Pancreas. 2017;46(4):496–503. 10.1097/mpa.0000000000000795 [DOI] [PubMed] [Google Scholar]
- 31. Guarino M, Bologna A, Ursini F, De Giorgi A, Alfano F, Gambuti E, et al. Chronobiology of acute pancreatitis in a single Italian centre. Eur Rev Med Pharmacol Sci. 2020;24(4):1988–1994. [DOI] [PubMed] [Google Scholar]
- 32. European Pancreatic Club . European interdisciplinary guidelines on pain management in acute pancreatitis. https://www.europeanpancreaticclub.org/about‐us/diagnosis‐and‐treatment‐guidelines/european‐interdisciplinary‐guidelines‐on‐pain‐management‐in‐acute‐pancreatitis/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


